Abstract
Background
Loss of mechanical tension appears to be the major factor underlying decreased collagen synthesis in aged skin. Numerous in vitro studies have shown the impact of mechanical forces on fibroblasts through mechanotransduction, which consists of the conversion of mechanical signals to biochemical responses. Such responses are characterized by the modulation of gene expression coding not only for extracellular matrix components (collagens, elastin, etc.) but also for degradation enzymes (matrix metalloproteinases [MMPs]) and their inhibitors (tissue inhibitors of metalloproteinases [TIMPs]). A new device providing a mechanical stimulation of the cutaneous and subcutaneous tissue has been used in a simple, blinded, controlled, and randomized study.
Materials and methods
Thirty subjects (aged between 35 years and 50 years), with clinical signs of skin sagging, were randomly assigned to have a treatment on hemiface. After a total of 24 sessions with Mécano-Stimulation™, biopsies were performed on the treated side and control area for in vitro analysis (dosage of hyaluronic acid, elastin, type I collagen, MMP9; equivalent dermis retraction; GlaSbox®; n=10) and electron microscopy (n=10). Furthermore, before and after the treatment, clinical evaluations and self-assessment questionnaire were done.
Results
In vitro analysis showed increases in hyaluronic acid, elastin, type I collagen, and MMP9 content along with an improvement of the migratory capacity of the fibroblasts on the treated side. Electron microscopy evaluations showed a clear dermal remodeling in relation with the activation of fibroblast activity. A significant improvement of different clinical signs associated with skin aging and the satisfaction of the subjects were observed, correlated with an improvement of the sagging cheek.
Conclusion
Mécano-Stimulation is a noninvasive and safe technique delivered by flaps microbeats at various frequencies, which can significantly improve the skin trophicity. Results observed with objective measurements, ie, in vitro assessments and electron microscopy, confirm the firming and restructuring effect clinically observed.
Introduction
Fibroblasts are the most abundant cells in the dermis. They play a major role in synthesizing collagen and elastic fibers, glycoproteins, and proteoglycans in the extracellular matrix. They also develop contractile forces required for matrix remodeling and migration activities.
Skin aging is characterized by changes in the function and structure of the dermis. With aging, the skin tends to become thinner, drier, and less elastic. Fibroblastic dysfunction is one of the main markers of skin aging. This abnormal proliferation, differentiation, and synthesis of fibroblasts result in structural and functional skin changes. Phenotypic studies about wrinkle fibroblasts showed that the remarkable change in mechanical properties of the skin with aging is indeed due to reduced contractile capacity as well as reduced migration activity of wrinkle fibroblasts.Citation1
Early in 1911, Dr Lucien Jacquet observed that progressive gradual mechanical excitation of the skin (gradual progression in frequency and duration of treatment) is supposed to counteract the age-related loss of the firmness of the face, neck, and upper chest skin.Citation2
More recently, it has been shown that fibroblasts contain a molecular machinery to convert mechanical stimuli into responses that influence tissue remodeling. In vitro studies demonstrated that they are able to change their behavior and induce complex cascades of biochemical events in extracellular matrix.Citation3–Citation5 Thus, mechanical stimuli lead to a “synthetic” fibroblast phenotype characterized by the induction of connective tissue synthesis while simultaneously inhibiting matrix degradation; in mechanically “stressed” lattices, stress fiber and focal adhesion formation by fibroblasts have been observed.Citation6
Mechanical stimulation performed on the skin surface sends a signal to the distant cells such as keratinocytes, fibroblasts, and adipocytes and causes production of collagen, elastin, and activation of lipolysis. Loss of mechanical tension appears to be the major factor underlying decreased collagen synthesis in aged skin; an aging-related loss of fibroblast interaction with the surrounding fibrous matrix has also been shown, leading to the vicious cycle of diminished procollagen synthesis, disorganization of collagen bundles, and further loss of mechanical stimulation of fibroblasts. Myofibroblasts (active fibroblasts characterized by α-smooth muscle (SM)-actin protein expression) play a central role in scarring and cell migration and motility within the connective tissue.Citation7
Fibroblast and collagen fibers presented alignment along the stretching axis upon axial stimulation, compared to free-floating lattices.Citation8
A previous study using a technique based on mechanical stimulation of the cutaneous and subcutaneous tissues has shown that this technique is able to restart their natural production of collagen and elastin by delivering microbeats to the skin’s surface, which can stimulate cell rejuvenation in-depth.Citation9 However, the evaluations were performed on the arm skin, and it was not clear if these results could be generalized to the face as well.
In the present study, a mechanical stimulation has been applied on the facial skin for 24 sessions over 8 weeks. The primary aim was to assess the clinical and macroscopic effect of mechanical stimulation on the facial skin. The secondary aim was to investigate the mechanism of action of this technique at the cellular level by measuring contractile activity of the fibroblasts and quantifying the skin changes induced by fibroblastic activity.
Materials and methods
This is a monocentric, double-blinded, controlled, and randomized interventional study on the effects of a technique called Mécano-Stimulation™.
Volunteers
Thirty healthy subjects (20 females and 10 males) aged 35–50 years were enrolled in this study. The inclusion criteria were Fitzpatrick skin phototype I–IV,Citation10 with mild-to-moderate facial skin sagging (cutaneous sagging grade 1–3, according to Ezure et alCitation11) ().
Figure 1 Ezure scoring scale.
Exclusion criteria were sun or ultraviolet (UV) exposure during the last 15 days, any facial dermatologic pathologies such as rosacea, any chronic or acute systemic affections, any topical or systemic treatment that could interfere with the results of the study, and any contraindications to the Mécano-Stimulation (eg, herpes, vitiligo, acne: inflammatory and infective phase). All volunteers signed an informed consent form in agreement with the Declaration of Helsinki after having enough time to understand the modalities and the aim of the study. The study protocol was approved by the local ethical committee (CPP [Comités de Protection des Personnes] Est II) and the National French Health Authorities (ANSM [L’Agence nationale de sécurité du Médicament et des produits de santé]).
Treatment
The treatment was applied on one side of the face (randomized hemiface: chin, nasolabial fold, cheek, cheekbone, temple, forehead) during 15 minutes, three times per week for 8 weeks (24 sessions). In order to standardize the treatment, all treatments were performed by the same practitioner. The treatment was carried out via the Mécano-Stimulation system (LPG Systems, Valence, France), which transforms manual massage movements through mechanical procedures by means of different treatment heads. The treatment heads used in this study are composed of two motorized flaps inside a treatment chamber, which are dedicated to the treatment of the face or small areas. The treatment forces are exerted without any pinching or twisting of the skin but through combining both horizontal and vertical stimulation (). These heads contain a small engine allowing the flaps to vibrate in a regular repeated way, which is accompanied with aspiration.
Figure 2 Double stimulation.
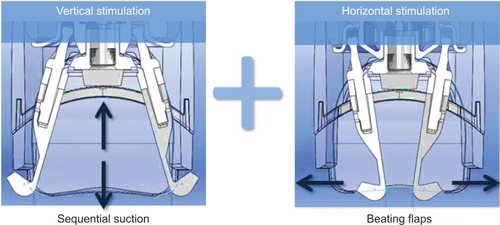
Different parameters (intensity and rhythm of the aspiration and beating frequency of the flaps) can be modulated at any time according to the individual sensitivity and tissue state (infiltrated, loosened, or wrinkled skin).
Lift heads used in this study were “motorized”: heads contain a small engine allowing the flaps to beat in a regular and permanent way, synchronized specifically with the sequential aspiration.
Measurement
Evaluations were performed before the treatment (T0), after 24 sessions (T1), and 1 month after the end of the treatment (T2). Volunteers were evaluated in the standard skin situation (last face washing the night before measuring sessions, without any cosmetic application until the measures [no water, soap, cream, or makeup]). During the study, they were also asked to not change their facial skin care habits, hygiene, and cosmetic products, to avoid sun exposure and UV treatment even occasionally, to avoid any medical and invasive treatment on the face, and to report systemically any medication consumption to the investigator. All evaluations and measurements were performed under standard conditions (constant temperature 20°C–23°C and hygrometry 40°C–60°C) after a 20-minute rest period.
The main evaluation criterion was clinical scoring for skin sagging using a photographic scale. The secondary outcomes were clinical scoring of skin relief and wrinkles, skin color and radiance, and subject self-assessments. The biopsies were taken from both sides of the face (treatment and control side) from 19 subjects, nine for histopathologic changes and ten for the in vitro evaluation of the fibroblasts for their contractile capacity.
Primary outcome
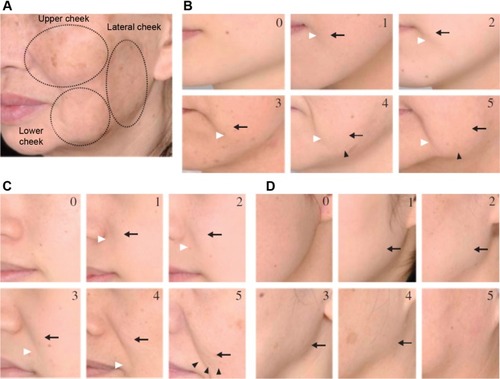
The primary outcome, measured at T0 and T1, was the facial skin sagging quotation performed by a blinded dermatologist, thanks to a clinical scoring based on the six-grade scale of Ezure et alCitation11 and carried out on three different locations: upper part of the cheek/nasolabial folds (B zone), lower part of the cheeks (C zone), and lateral part of the cheek (D zone), as described in .
Secondary outcomes
Clinical scoring
A blinded dermatologist evaluated the different signs of skin aging, with each criterion being assessed on both treated and nontreated side.
Skin radiance: A clinical scoring of skin radiance was calculated through a visual analysis scale (CLBT™) which includes seven descriptors. Actually, four different colors (pink red, olive, beige, light pink) between 10% and 100% lead to the final analysis of three different parameters (skin transparency, brightness, and luminosity) on a 0–10 scale.Citation12
Skin relief: An analogical 0–9 scale was used to evaluate the skin relief parameters. This allowed the calculation of a skin relief score defined as the sum of the following scores: surface irregularity score + wrinkles/lines/blemishes score + skin texture score + under-eye dark circles score + papules/microcysts score. The lower the score indicates lower skin relief, indicating better appearance.
Skin color: The same 0–9 scale was used to evaluate the skin color through the following parameters: heterogeneity, eye circle, redness/rosacea, skin spots, and papules/scars. The sum of these parameters present the global color score.
Self-assessment
The volunteers were asked to fill a 9-point questionnaire at T0 (before) and T1 (after 24 sessions of treatment) for the following parameters (absence/presence and the intensity):
wrinkles and fine lines;
roughness;
nasolabial folds;
elasticity;
firmness/softness;
radiance;
under-eye dark circles.
They were also asked to compare their skin with pretreatment status for firmness, elasticity, smoothness, wrinkles, sagging, and brightness. They were supposed to answer the satisfaction questionnaire as well.
Photography
At each visit, the front, left, and right lateral photographs were taken of the whole and lower face in normal daylight, with eyes closed (Canon EOS Rebel XS with a 50-mm lens and two flashes positioned on a Faraghan Medical System). All photos were standardized for light (using Gretag MacBeth Colour Checker Chart) and position (stereotaxic table; Eotech SA, Marcoussis, France).
In vitro study
Two 2 mm punch biopsies were taken from nine subjects (four males and five females) from both sides of the face (treatment and control side) at T1. The samples were placed in sterile gauze moistened with saline and immediately treated: Each biopsy was placed in a 25 cm2 culture dish and was allowed to adhere to the plastic surface for approximately 30 minutes at 37°C. Three milliliters of Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal calf serum, 40 mg/mL of gentamicin, and 2 mg/mL of fungizone (DMEMc) were added to each dish. The dishes were then incubated at 37°C in 5% CO2. Subculture of fibroblasts was established after 3–4 weeks of outgrowth by trypsination (trypsin-EDTA (ethylene diamine tetra acetique) [1x]; PAA laboratory, France) of explant cultures. The culture medium was changed two times a week. Fibroblasts cultivated in passages lower than ten were used.
Migratory capacity
Fibroblasts that were either subjected or not subjected to Mécano-Stimulation were embedded in three-dimensional hydrated collagen, using a modified version of the technique developed by Bell et alCitation13, to study their mechanical behavior by measuring their capacity to contract free-floating lattices. Collagen lattices were made by mixing six volumes of 1.76× concentrated medium, three volumes of rat tail type I collagen solution (2 mg/mL), and one volume of fibroblasts suspension (8×105 cells/mL). This mixture was poured into plastic Petri dishes (60 mm diameter). The gel polymerized in <30 minutes at 37°C. Culture medium was then added and changed every 48 hours. To measure the retracted lattice diameter, the culture dishes were placed on a transparent metric scale. The measurement of the lattice diameter was performed for 10 days. The evaluation of the migratory capacities of fibroblasts makes it possible to show the possible reorganization of extracellular matrix in the presence of Mécano-Stimulation.
Measurements of contractile forces developed by fibroblasts in GlaSbox® device (Growing Lattice Study box)
The cell chamber was composed of eight rectangular culture cavities in which the lattices developed. Two silicon beams were placed opposite to each other with strain gauges hanging apart, down into each cavity at 27 mm distance. The lattice was attached to this sensor through a grid directly etched onto the lower part of the beams. The output signal from the strain gauges was amplified, then converted, and collected by a computer, which included an acquisition card and a specific software giving the forces in real time. Fibroblasts were prepared as defined in the previous paragraph; the lattice mixture was poured into a rectangular culture cavity of the GlaSbox® and polymerized in <30 minutes at 37°C. Two milliliters of culture medium was added. The GlaSbox® was then placed into a humidified incubator at 37°C, and force measurements were started immediately and for 24 hours. The results were expressed as contractile forces (arbitrary unit) of the fibroblasts either subjected or not subjected to Mécano-Stimulation according to the time. These measurements quantify the contractile forces of fibroblasts and make it possible to know whether a treatment applied for 15 minutes, three times per week during 8 weeks (24 sessions) already shows a “lifting” effect at the scale of fibroblasts.
Synthesis studies
Fibroblasts either subjected or not subjected to Mécano-Stimulation were seeded in 12-cavity plates at 0.04×106 cells per cavity. The plates were incubated at 37°C. The next day, 500 μL of DMEMc was poured into each cavity and the plates were incubated at 37°C. After 48 hours, the supernatant part was placed in a protease inhibitor cocktail (10% v/v, Merck Millipore, Fontenay sous bois, France). The samples were stored at −80°C until analysis.
Collagen I, hyaluronic acid, matrix metalloproteinase-1, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 (TIMP-1) assay
The quantification was evaluated using an enzyme-linked immunosorbent assay kit (USCN, Life Science and RayBiotech®, Inc). Standards or samples were added into the precoated cavities with a specific biotin-conjugated antibody. After washing the samples, avidin (collagen I and hyaluronic acid) or streptavidin (matrix metalloproteinase [MMP]-1, MMP-9, and TIMP-1) conjugated to horseradish peroxidase were added to each cavity and then incubated. After adding the tetramethylbenzidine (TMB) substrate solution, only those cavities that contained study substances showed a color change. The enzyme–substrate reaction is terminated by the addition of sulfuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450 nm. The concentration is then determined by comparing the optical density of the samples to the standard curve.
Elastin assay
The total soluble elastin was assayed by a colorimetric method (Fastin™ Elastin Assay; Biocolor). The samples were precipitated with a solution of trichloroacetic acid and arginine, then the reagent 5,10,15,20-tetraphenyl-21,23-porphine tetra-sulfonate was added, which can particularly bind to elastin. The formed complex was dissociated by a solution of guanidine-HCl and propan-1-ol. The developed color was measured at 550 nm using a spectrophotometer (Multiscan EX; Thermo).
Ultrastructural studies
Two-millimeter punch biopsies were taken under local anesthesia (1% xylocain) from the jugular area of ten other subjects (five males and five females) at T1. Biopsies from the treated and nontreated sides were coded and examined pair by pair in a blinded manner.
Each biopsy was cut into small fragments, some of which were fixed in 3% paraformaldehyde in phosphate-buffered saline (PBS), the others in 2% glutaraldehyde in sodium cacodylate buffer at 4°C for 3 days. For standard morphological examination, the glutaraldehyde-fixed fragments were washed and then postfixed in 1% osmium tetroxide for 2 hours. After dehydration in graded ethanol series, the fragments were impregnated and embedded in epoxy resins (60°C for 3 days). Ultrafine sections were contrasted with uranyl acetate and lead citrate before observation.
The paraformaldehyde-fixed fragments, used for immunocytochemistry, were washed, dehydrated, and embedded in LR White resin (UVA polymerization at 4°C; EMS, Hatfield, PA, USA). Ultrafine sections on formvar-coated nickel grids (EMS) were exposed to the α-SM-actin antibody (diluted 1/50, overnight at 4°C), washed in PBS, and incubated for 1 hour with goat anti-mouse antibodies coupled to 10 nm colloidal gold (diluted 1/10; Amersham, Little Chalfont, UK). After final washes in PBS and distilled water, the sections were counterstained with uranyl acetate alone.
Transmission electron microscopy of all specimens was performed, and digital images were obtained and analyzed. Density and overall quality of the dermal fibrous structures, ie, collagen bundles and elastic fibers, were evaluated semiquantitatively. Also, the morphological signs of fibroblast metabolic and secretory activity as well as the intensity of the α-SM-actin expression were noted.
Statistical analysis
Data are presented as mean ± standard deviation. The normality of the data distribution was tested with a Shapiro–Wilk test. Differences between groups at baseline and at the end of the 24 sessions were tested with a Student’s t-test for paired values (or a Wilcoxon test in case of nonnormally distributed data).
Variance analysis with repeated measurements (analysis of variance) completed by a Student–Newman–Keuls test (normal distribution of parameters) or a Friedman test completed by a Dunn’s test (abnormal distribution of parameters) was applied to analyze the evolution of the studied parameters over the chosen time period.
Statistical significance was set at 5% and at 5%–10% for trends.
For the in vitro study, one-way variance analysis followed by a Fisher’s exact test (if needed) was performed for synthesis studies. Two-way analysis of variance (groups and time) followed by a Fisher’s exact test (if needed) was performed for the measurements of migratory capacities and contractile forces.
Results
Thirty subjects (20 females and 10 males) with a mean age of 44.5±4.6 years finished the 24 sessions of treatments. Among the 20 volunteers, 13 were under contraceptive treatments. Depression (n=4), seasonal allergies (n=2), hiatal hernia (n=1), hand eczema (n=1), familial hypercholesterolemia (n=1), epilepsy (n=1), allergy to nickel (n=1), allergy to tramadol (n=1), emphysema (n=1), and chronic bronchitis (n=1) were recorded as the past medical history of the volunteers.
Primary outcome: skin sagging
A clinical improvement of skin sagging of at least one parameter of the facial skin sagging (Ezure scale) was observed in 73% (n=22) of the subjects on the treated side versus in 53% (n=16) of the subjects for the nontreated area (P=0.108). The same calculation for the C and D zones revealed 70% (n=15) improvement on the treated side versus 50% (n=15) improvement for the control side (P=0.114) ().
Table 1 Results of the skin sagging scores
Secondary outcomes
Clinical scoring
CLBT color scale
At T0, the treated and untreated hemiface were similar for the clinical scores of skin complexion radiance evaluated with the CLBT scale.
Compared to the baseline, all scores were significantly improved on the treated hemiface (P<0.05). At T1, the color scores (skin transparency, brightness and luminosity) were significantly improved on the treated compared to the nontreated hemiface (, and ). Finally, the seven evaluated parameters presented a significant improvement in terms of time/treatment evolution.
Table 2 Results for CLBT™ scoring before (T0) and after (T1) the 24 treatment sessions
Figure 3 CLBT™ scoring before (T0) and after (T1) the 24 treatment sessions: results for the color components.
Notes: The visual analysis scale (CLBT scoringCitation12) follows seven descriptors that evaluate between 10% and 100% of four different colors: pink red (A), olive (B), beige (C), and light pink (D). All these color parameters presented a significant improvement (decrease) in terms of time/treatment evolution, in favor of the treated (blue) versus nontreated (red) side. *P<0.05.
Abbreviations: CLBT, Color, Luminosity, Brightness and Transparency Scoring; T, time.
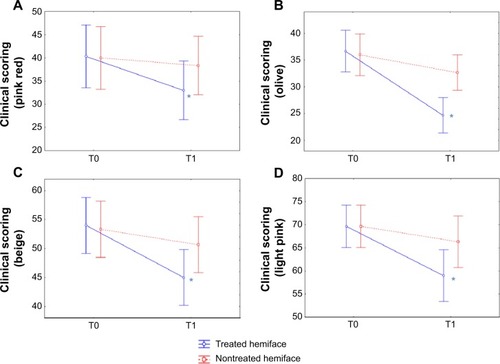
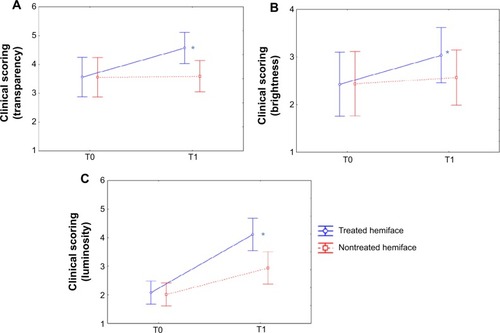
Figure 4 CLBT™ scoring before (T0) and after (T1) the 24 treatment sessions: results for transparency, brightness, and luminosity.
Abbreviations: CLBT, Color, Luminosity, Brightness and Transparency Scoring; T, time.
From a descriptive point of view, we can notice an improvement for the treated versus nontreated sides on all parameters:
“pink red”: 18.2% on the treated side versus 4.2% on the nontreated side;
“olive”: 32.7% versus 9.2%;
“beige”: 16.7% versus 5%;
“light pink”: 15.3% versus 4.8%;
transparency: 28.4% versus 1.1%;
brightness: 25.1% versus 5.3%;
luminosity: 97.6% versus 46.3%.
Relief
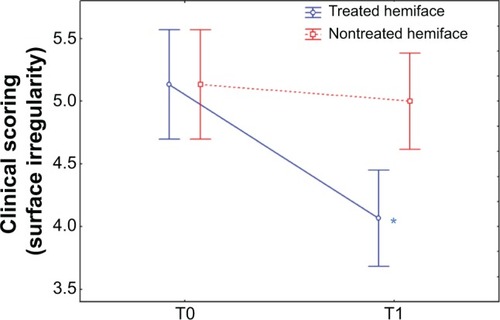
At baseline (T0), no significant differences were observed in surface irregularity, fine lines, wrinkles and blemishes, skin texture, under-eye dark circles, buttons/microcysts, and relief scores between the treated and the untreated hemiface. Compared to the baseline, at T1 all scores decreased on the treated hemiface (P<0.05) (except for buttons/microcysts) while no change was observed on the nontreated hemiface. A significant improvement in terms of time/treatment evolution was observed for all the evaluated parameters ( and ). An improvement was observed for treated versus nontreated side on the following parameters:
surface irregularity: 20.7% on the treated side versus 2.5% on the nontreated side;
fine lines, wrinkles, and blemishes: 21.1% versus 0.6%;
skin texture: 15% versus 6.37%;
under-eye dark circles: 14.2% versus 4.2%;
relief score: 19.2% versus 5.4%.
Table 3 Clinical scoring of skin radiance: evaluation through relief before (T0) and after (T1) the 24 treatment sessions
Figure 5 Clinical scoring of skin radiance: relief surface irregularity.
Abbreviation: T, time.
Color
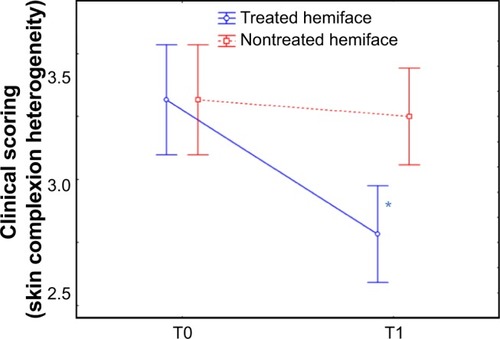
At T0, no difference between the treated and the untreated hemiface was observed for the clinical scores of skin color (except for buttons/spots, P=0.086). At T1 compared to baseline, heterogeneity, under-eye dark circles, papules/scars, and color score were significantly improved on the treated hemiface, while they were stable on the nontreated hemiface. A significant improvement in terms of time/treatment evolution was observed for all parameters (skin complexion heterogeneity, under-eye dark circles, redness, and color score) ( and ). An improvement for treated versus nontreated side was observed on the following parameters:
skin complexion heterogeneity: −13.8% on the treated versus +1.2% on the nontreated side;
eye circles: 15.1% versus 1.7%;
redness/rosacea: −9.4% versus +2.5%;
color score: 13.5% versus 1.7%.
Table 4 Clinical scoring of skin radiance: evaluation through skin color before (T0) and after (T1) the 24 treatment sessions
Figure 6 Clinical scoring of skin radiance: color skin complexion heterogeneity.
Abbreviation: T, time.
Subjects self-assessment
Subjects’ self-assessment evaluation revealed an improvement for 9 out of 10 parameters on the treated side while the skin status on the nontreated side was considered to show little improvement or even worsen compared to baseline. Differences at T1 between treated and untreated sides were significant for 7 of the 10 parameters and trend for 1. Significant evolution of treated side was noticed for roughness, firmness, radiance, and softness, compared to untreated hemiface. A significant improvement in terms of time/treatment evolution was observed for all parameters (skin complexion heterogeneity, under-eye dark circles, redness, and color score) ().
Table 5 Results of self-assessment evaluation
shows the results of the self-assessment questionnaire allowing the volunteers to rate their skin state at T1 and T2, compared to T0.
Table 6 Self-assessment of subjects skin at T1 (end of the treatment) and T2 (1 month after the end of the treatment), compared to T0
The results demonstrated a significant effect on the treated side compared to the nontreated side for all parameters (firmness, elasticity, smoothness, wrinkles, and sagging), except brightness at T1.
Satisfaction questionnaire
At T1, 90% of the volunteers were satisfied by the mechanostimulation while the satisfaction rate was 100% at T2. Of all the volunteers, 70% at T1 and 75% at T2 declared they wish to continue the treatment, and 77% of the volunteers judged the treatment as “very good” or “good” at T1. At T2, 87% of the subjects still felt the positive effects of the treatment.
Photographs
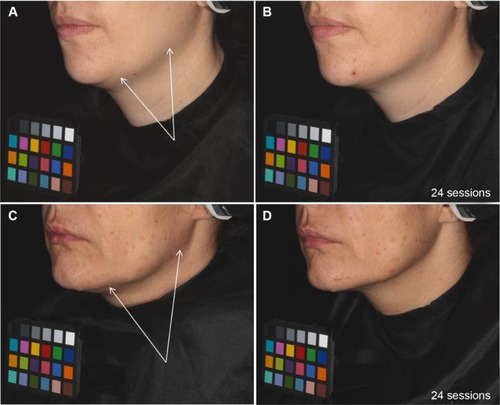
Standardized photographs illustrated an improvement of the oval face shape in 60% of the subjects on the treated area ().
Figure 7 Skin sagging evolution during the study: examples on two volunteers treated on the left side of the face before and after 24 sessions of treatment.
In vitro study
Migratory capacity
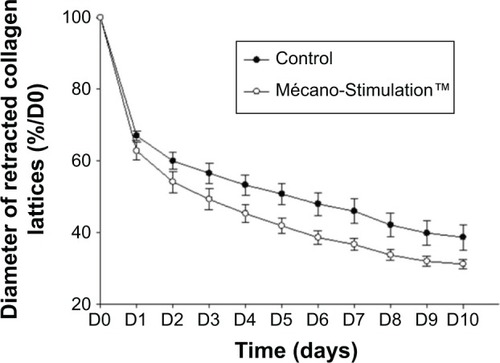
The migration of fibroblasts was evaluated through their capacity to retract free-floating collagen lattices. The retraction of collagen lattices obtained with fibroblasts subjected or not subjected to Mécano-Stimulation was time dependent. The retraction of collagen lattices obtained with fibroblasts subjected to Mécano-Stimulation was significantly increased compared to nonstimulated fibroblasts (P<0.001; ).
Figure 8 Effect of Mécano-Stimulation™ on fibroblast retraction according to time.
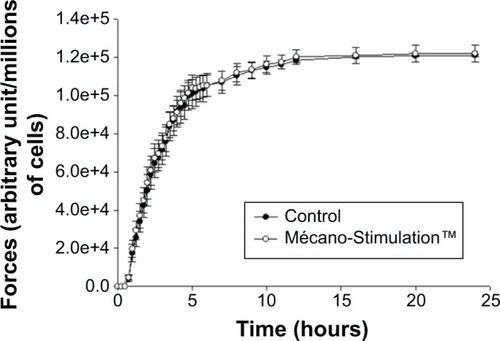
Measurements of contractile forces developed by fibroblasts
No significant difference of the development of contractile forces was observed between the Mécano-Stimulation group and the control group ().
Figure 9 Quantification of contractile forces developed by fibroblasts after the action of Mécano-Stimulation™ using GlaSbox® device.
Collagen I, elastin, and hyaluronic acid synthesis
For each product, synthesis results are expressed as % respective control.
At T1, a nonsignificant increase in the synthesis of collagen I was observed between the treated and untreated hemiface (107.8%±69.8% vs 100.0%±0.0%/control; ).
Figure 10 Collagen I, elastin, and hyaluronic acid synthesis of fibroblasts with and without the action of Mécano-Stimulation™.
Abbreviation: T, time.
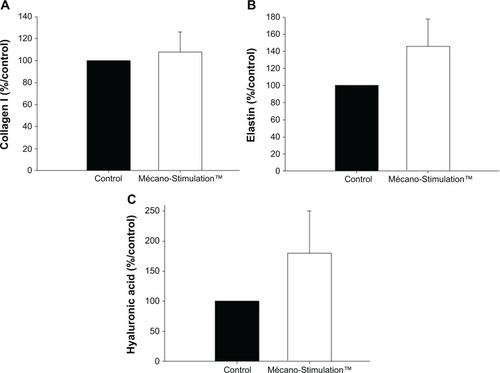
For elastin synthesis by the fibroblasts, a nonsignificant difference was observed on the treated side comparing to the untreated side (145.6%±32.1% vs 100.0%±0.0%/control for the treated and untreated hemiface, respectively, P=0.175; ). The same result was noticed for the synthesis of hyaluronic acid (180.2%±69.8% vs 100.0%±0.0%/control for the treated and untreated hemiface, respectively, P=0.268; ).
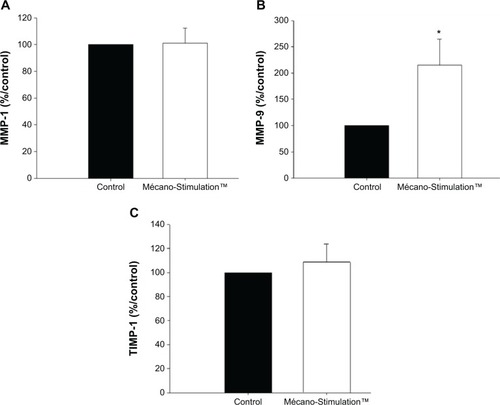
MMP-9, MMP-1, and TIMP-1 synthesis
For each MMP, results are expressed as % respective control.
A significant increase in the synthesis of MMP-9 (+115.4%, P<0.05 vs control group, P=0.033; ) was observed in fibroblasts from patients subjected to Mécano-Stimulation compared to control fibroblasts. No significant changes were observed in the synthesis of MMP-1 and TIMP-1 ().
Figure 11 MMP-1, MMP-9, and TIMP-1 synthesis of fibroblasts with and without the action of Mécano-Stimulation™.
Abbreviations: MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinases; T, time.
Ultrastructural results
After decoding of the samples, the treated side presented ultrastructural signs of improvement in four of the ten volunteers. These were characterized by:
changes in the fibroblast morphology comprising the enlargement of the intracellular network of ribosome-decorated rough endoplasmic reticulum, of the electron-lucent mitochondria with well-developed cristae, and of the presence of numerous small plasma membrane-bound vesicles suggestive of an increased secretory activity; none of these were observed in the nonresponders;
restructuring of the fragmented elastic fibers, ie, evidence of the elastic fiber recycling by “activated” fibroblasts;
increased α-SM-actin expression by the latter in 50% of the cases ().
Figure 12 Examples of ultrastructure observed when comparing the treated versus untreated side.
Abbreviations: EFs, elastic fibers; Fb, fibroblasts.
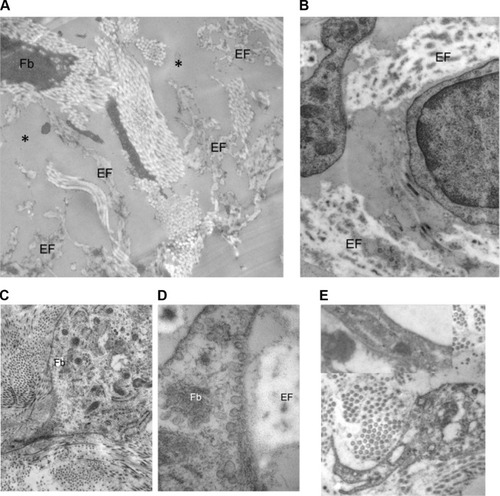
Facial skin of the control side showed changes related to the chronologic and actinic aging, such as elastic fiber fragmentation, diminished density and organization of the fibrous extracellular matrix, and quiescence of the dermal fibroblasts. These structural signs of tissue senescence were particularly accentuated in seven of the ten subjects.
Discussion
Cutaneous aging is a complex process that depends on intrinsic factors (chronologic aging, genetic basis) and on detrimental influences from the environment (mainly UV radiation).Citation14 The resulting effects such as skin laxity, sagging, and wrinkle formation are clinically obvious and concern mainly the superficial dermis.Citation15 Fibroblasts are the most abundant cells in the dermis, playing a major role in extracellular matrix components synthesis; matrix remodeling, and migration activities; because several in vitro studies have demonstrated that mechanical stimulation on fibroblasts could alter their behavior and induce complex cascades of biochemical events,Citation3,Citation4,Citation6 the idea of performing mechanical stimulation on the skin surface has emerged to positively affect the dermis.
With aging, the skin tends to become thinner, less elastic, and drier. Phenotypic studies about wrinkle fibroblasts showed that the mechanical properties of the skin change with aging: reduced contractile capacities as well as reduced migration activities were observed in wrinkle fibroblasts.Citation1
A major feature of aged skin is fragmentation of the dermal collagen matrix.Citation16 Fragmentation results from actions of specific enzymes (MMPs, collagenases, and elastases) and impairs the structural integrity of the dermis. Fibroblasts that produce and organize the collagen matrix cannot attach to fragmented collagen. Loss of attachment prevents fibroblasts from receiving mechanical information from their support, and they collapse. Stretch is critical for normal balanced production of collagen and collagen-degrading enzymes.Citation17 Then, in aged skin, collapsed fibroblasts produce low levels of collagen and high levels of collagen-degrading enzymes.Citation7
During the aging process, the proteoglycans such as hyaluronic acid, which has a high affinity for water, are less synthesized, and the contractile activity of fibroblasts decreases. Fibroblasts are losing their ability to express α-SM-actin and to differentiate into myofibroblasts. This induces lower forces of contraction on the extracellular matrix and a decrease of mechanical tension of the skin, producing a loss of elasticity and tonicity of the dermis.
Previous trials studied the effect of Mécano-Stimulation and showed its ability to reinduce natural production of collagen and elastin.Citation9
In the present study, 30 subjects underwent 24 sessions of treatment on the face with the Mécano-Stimulation in order to understand its effects through clinical, biological, and histological evaluations.
The results indicated that, while nonstatistically significant, a trend for a clinical improvement of skin sagging (the primary outcome) was observed, with an improvement of at least one parameter of the facial skin sagging clinical scoreCitation11 in 73% of subjects. The location where the mechanostimulation was less efficient was the upper part of the cheek/nasolabial fold, while a clinical improvement of the sagging was observed in 70% of subjects either on the lower part or the lateral part of the cheek. This result is in accordance with the fact that structural skin changes, such as sagging and wrinkling, clearly originate due to alterations in the dermis, which is a tissue with slow turnover.
Indeed, the macroscopic clinical signs of wrinkles and firmness did not show any particular change over a 6-month periodCitation18 while in the present study only 24 sessions of treatment over 8 weeks were performed. The wrinkle formation and sagging of the facial skin are long-term cumulative effects and would not be particularly affected by 8 weeks of treatment, so it is recommended to perform long-term studies in this field, as already reported in other studies.Citation19,Citation20 A longer treatment period could also enhance the visible changes over the treatment period.
In accordance with the latter observation, all the clinical scores for skin complexion radiance evaluated by the CLBT scaleCitation12 were significantly upgraded after treatment. Indeed, color transparency, brightness, and luminosity scores were significantly improved at T1 on the treated compared to the nontreated hemiface.
Such radiance improvement was observed after 6 months with the use of a topical treatment in aged human skin,Citation21 suggesting a very efficient impact of the Mécano-Stimulation on radiance.
Accordingly, clinical scores for surface irregularity, fine lines, wrinkles and blemishes, skin texture, under-eye dark circles, papules/microcysts, and relief were significantly improved compared to baseline on the treated side, while no significant change was observed on the nontreated hemiface.
The skin color evaluation focused on heterogeneity, under-eye dark circles, and global color score showed improvement on the treated side compared to the untreated side after treatment.
The evolution of both skin relief and color scores is along the same direction as other results. This could reinforce the skin global improvement of the subjects from a clinical point of view, considering that skin relief, color, radiance, and sagging are strongly interrelated in the aging process also.
Self-assessment questionnaires expressed significant results in efficiency of the treatment for parameters such as wrinkles, roughness, nasolabial folds, elasticity, firmness, skin radiance, softness, and under-eye dark circles. After 24 treatment sessions, the patients found their skin firmer, smoother, less wrinkled and less sagged, with stable effect 1 month after last treatment session.
These results, together with the fact that between 90% and 100% (T1 and T2) of the volunteers were satisfied by this technique and that between 70% and 75% (T1 and T2) of the volunteers declared their wish to continue the treatment underlined both positive objective and subjective efficacy of this method.
Beyond these aspects, the use of Mécano-Stimulation is considered to increase the synthesis of some extracellular matrix components, particularly MMP-9. The composition and organization of extracellular matrix components are governed by MMPs.Citation22 MMPs play major roles in tissue regeneration and remodeling. MMP-9 protein expression has been reported in the epidermal cells of acute wounds.Citation23 MMP-9 catalyzes the cleavage of denatured collagens of all types present in aged skin and the components of natural basement membrane as well.Citation24 Stimulation of MMP-9 synthesis explained no significant change in collagen I production in this study. No change in contractile forces developed by fibroblasts subjected to the action of Mécano-Stimulation was shown compared to control fibroblasts. These results indicate that the 24 sessions of Mécano-Stimulation are not enough in order to show a “lifting” effect at the scale of fibroblasts. However, the cell migration was faster due to the mechanical stimulations and could suggest a beneficial effect of Mécano-Stimulation on migratory and remodeling capacities. A significant increase in the synthesis of MMP-9 and the increase of fibroblasts migration suggest a beneficial early action of mechanical stimulation on the synthesis of extracellular matrix components. Mécano-Stimulation stimulates the decreased cell activity found in aged skin through cellular mechanotransduction.
The results suggest that a prolonged action of Mécano-Stimulation might provide a significant difference in the synthesis of extracellular matrix components such as collagen I, elastin, and hyaluronic acid. The expected changes include improvement of contraction forces and the components of the extracellular matrix.
Ultrastructural histopathologic positive changes observed in four out of ten study subjects correlated well with the clinical and biochemical findings. One of the most striking features observed with electron microscopy was apparent restructuring of the fragmented elastin fibers. Although it is difficult to differentiate them from newly synthesized elastic fibers or from the degradation of the fiber remnants, there is some evidence confirming that the mechanically stimulated skin could recycle this functionally important component of the extracellular matrix. However, the restructuring effects were less remarkable than those observed after long-term treatment with topical cream containing vitamin C.Citation25,Citation26
Formation of stress fibers and their attachment to focal adhesions via vinculin have been demonstrated as hallmarks of fibroblast organization in collagen lattices subjected to tension.Citation6 Accordingly, the overall increase in α-SM-actin expression on the treated side in half of the cases might support our clinical and biochemical results pointing to the morphological improvement. Yet, expression of actin stress fibers by dermal fibroblasts appeared to be a less reliable feature in the evaluation of the tissue response to mechanical constrains. Labeling of the α-SM-actin was observed in all biopsies, either from the treated or control sides, although the level of expression varied among the cells within the same biopsy and/or between the subjects.
Our results confirm then the restructuring potential of the physical stimulation procedure.
Conclusion
In conclusion, this original study allowed observing and demonstrating not only the clinical and visible immediate results but also the internal mechanisms of the skin through an external, noninvasive, and painless stimulation.
The study provides the evidence that the use of Mécano-Stimulation, a technique based on the application of mechanical stimuli of the cutaneous and subcutaneous tissue by delivering microbeats to the skin’s surface, is able to induce clinically identifiable improvement in facial skin appearance. This improvement is associated with a tendency to increase the synthesis of extracellular matrix components.
Acknowledgments
This research was made possible by the help and support of the volunteers, technicians, and research engineers at our center; we gratefully acknowledge Mrs Aurélie Durai, Vanessa Ecarnot, and Celine Thiebaud for their enthusiasm in carrying out the sensitive and accurate care and evaluation and Mrs Clélia Monteux and Mr Christian Gagnière for providing us with the device.
Disclosure
The authors certify that there is no conflict of interest with any financial organization regarding the research described in the article. The study was realized under the university auspices.
References
- Courderot-MasuyerCRobinSTauzinHHumbertPEvaluation of the behaviour of wrinkles fibroblasts and normal aged fibroblasts in the presence of poly-l-lactic acidJ Cosmet Dermatol Sci Appl201222027
- JacquetLLe massage plastique à double action dans le traitement des dermatoses [The double action plastic massage in the treatment of dermatosis]Paris Médical: la semaine du clinicien191101355358 French
- EastwoodMMacGroutherDABrownRAFibroblasts responses to mechanical forcesProc Inst Mech Eng19982128592
- ChiquetMRegulation of extracellular matrix gene expression by mechanical stressMatrix Biol19991841742610601729
- HarrisAKStopakDWildPFibroblast traction as a mechanism for collagen morphogenesisNature19812902492517207616
- KesslerDDethlefsenSHaaseIFibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotypeJ Biol Chem2001276365753658511468280
- VaraniJDameMKRittieLDecreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulationAm J Pathol200616861861186816723701
- HuJJHumphreyJDYehATCharacterization of engineered tissue development under biaxial stretch using nonlinear optical microscopyTissue Eng Part A20091571553156419063662
- RevuzJAdhouteHCesariniJPPoliFLacarriereCEmiliozziCClinical and histological effects of the Lift6® device used on facial skin ageingNouv. Dermatol200221335342
- FitzpatrickTBThe validity and practicality of sun-reactive skin type I through VI (Editorial)Arch Dermatol19881248698713377516
- EzureTHoshoiJAmanoSTsuchiyaTSagging of the cheek is related to skin elasticity, fat mass and mimetic muscle functionSkin Res Technol200915329930519624426
- MusnierCPiquemalPBeauPPitterJVisual evaluation in vivo of “complexion radiance” using the C.L.B.T. sensory methodologySkin Res Technol2004101505614731249
- BellEIvarssonGMerrillCProduction of tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitroProc Natl Acad Sci U S A19797612741278286310
- Mac MarySSainthillierJMJeudyAAssessment of cumulative exposure to UVA through the study of asymmetrical facial skin agingClin Interv Aging2010527728420924436
- GilchrestBASkin aging 2003: recent advances and current conceptsCutis20037251014533824
- FisherGJVaraniJVoorheesJJFibroblast collapse and therapeutic implicationArch Dermatol2008144566667218490597
- FisherGJQuanTPurohitTCollagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skinAm J Pathol200917410111419116368
- QiuHLongXYeJCInfluence of season on some skin properties: winter vs summer, as experienced by 354 Shanghainese women of various agesInt J Cosmet Sci201133437738321382055
- FanianFMac-MarySJeudyAEfficacy of micronutrient supplementation on skin aging and seasonal variation: a randomized, placebo-controlled, double-blind studyClin Interv Aging201381527153724255597
- WatsonREBowdenJJBastrillesJYLongSPGriffithsCEA cosmetic “anti-ageing” product improves photoaged skin: a double-blind, randomized controlled trialBr J Dermatol200916141942619438432
- HaftekMMac-MarySLe BitouxMAClinical, biometric and structural evaluation of the long-term effects of a topical treatment with ascorbic acid and madecassoside in photoaged human skinExp Dermatol20081794695218503551
- ChenWFuXGeSSunTShengZDifferential expression of matrix metalloproteinases and tissue-derived inhibitors of metalloproteinase in fetal and adult skinsInt J Biochem Cell Biol2007395997100517409012
- DasuMRSpiesMBarrowREHerndonDNMatrix metalloproteinases and their tissue inhibitors in severely burned childrenWound Repair Regen200311317718012753598
- MohanRChintalaSKJungJCMatrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regenerationJ Biol Chem200227732065207211689563
- NusgensBVHumbertPRougierATopically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase in the human dermisJ Invest Dermatol2001116685385911407971
- HumbertPHaftekMCreidiPTopical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: double-blind study vs. placeboExp Dermatol200312323724412823436
