Abstract
The aim of this paper is to provide a comprehensive review of the epidemiological evidence linking type 2 diabetes mellitus and its related conditions, including obesity, hyperinsulinemia, and metabolic syndrome, to Alzheimer’s disease (AD). Several mechanisms could help to explain this proposed link; however, our focus is on insulin resistance and deficiency. Studies have shown that insulin resistance and deficiency can interact with amyloid-β protein and tau protein phosphorylation, each leading to the onset and development of AD. Based on those epidemiological data and basic research, it was recently proposed that AD can be considered as “type 3 diabetes”. Special attention has been paid to determining whether antidiabetic agents might be effective in treating AD. There has been much research both experimental and clinical on this topic. We mainly discuss the clinical trials on insulin, metformin, thiazolidinediones, glucagon-like peptide-1 receptor agonists, and dipeptidyl peptidase-4 inhibitors in the treatment of AD. Although the results of these trials seem to be contradictory, this approach is also full of promise. It is worth mentioning that the therapeutic effects of these drugs are influenced by the apolipoprotein E (APOE)-ε4 genotype. Patients without the APOE-ε4 allele showed better treatment effects than those with this allele.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Type 2 diabetes mellitus (T2DM) is currently extremely common due to the prevalence of obesity, as well as the aging of the population.Citation1 Prevention and treatment strategies for the classical macrovascular and microvascular complications of diabetes mellitus have significantly improved. Therefore, people are living longer with diabetes mellitus, which might lead to the emergence of new complications. Dementia is one example of these emerging new complications.Citation2 Compared with the general population, the increased risk of dementia is 50%–150% in people with T2DM.Citation3–Citation5 Prince et alCitation6 predicted that people living with dementia worldwide would increase from 35.6 million in 2010 to 115.4 million in 2050. If current studies have correctly predicted the association between dementia and T2DM, then the future burden of dementia, eg, Alzheimer’s disease (AD) and vascular dementia, might be even greater than that estimated as the prevalence of diabetes mellitus continues to rise.Citation7 AD is the most common form and cause of dementia, accounting for 60%–80% of all cases.Citation8
Over the past three decades, numerous epidemiological studies have shown a clear association between T2DM and an increased risk of developing AD. In addition, T2DM-related conditions, including obesity,Citation9 hyperinsulinemia,Citation10 and metabolic syndrome, may also be risk factors for AD. The exact mechanisms with clinical relevance are unclear. Several mechanisms have been proposed, including insulin resistance and deficiency, impaired insulin receptor and impaired insulin growth factor (IGF) signaling, glucose toxicity, problems due to advanced glycation end products and their receptors, cerebrovascular injury, vascular inflammation, and others.Citation11–Citation13 In this review, we discuss insulin resistance and deficiency. Currently, the drugs available are able to slow worsening of symptoms for 6–12 months but are effective in only about half of the treated population.Citation14 Also, no effective drugs are expected to be approved soon, given that several promising new agents have failed in Phase III clinical trials.Citation15,Citation16 Therefore, it is important to accurately define the role of T2DM in the development of AD for preventing and treating the disease. In this review, we discuss the clinical trials on antidiabetic agents, ie, insulin, metformin, thiazolidinediones, glucagon-like peptide-1 receptor (GLP-1R) agonists, and dipeptidyl peptidase (DPP)-4 inhibitors, in the treatment of AD.
Alzheimer’s disease
Clinically, AD is manifested by progressive memory loss and a gradual decline in cognitive function, eventually leading to premature death of the individual, that occurs typically 3–9 years after diagnosis.Citation17 The neuropathological features associated with the disease include the presence of extracellular senile plaques containing amyloid-β (Aβ) protein, neurofibrillary tangles that consist mainly of intracellular and abnormally phosphorylated tau protein, and a dramatic loss of neurons and synapses, especially in the hippocampus and cortex.Citation18–Citation21 Considering these pathological changes, the “amyloid cascade hypothesis” is certainly the most popular current view. This hypothesis proposes that accumulation of Aβ, as either a consequence of increased production or decreased removal of Aβ, instigates all other downstream AD-associated phenomena and ultimately the disease itself.Citation22–Citation24 Despite the indistinguishable clinical symptoms of dementia, there are two different types of origin-based AD. In a small proportion (familial early-onset AD), the disease has a genetic origin and is caused by missense mutations in three genes, ie, Aβ protein precursor, presenilin-1, and presenilin-2.Citation25 These genes affect less than 5% of cases of AD, that usually happen in middle age.Citation26 The great majority of AD cases are sporadic in origin, with older age, being female, vascular disease, head trauma, family history of dementia, and genetic factors (eg, apolipoprotein E [APOE] ε4 allele) as the mainly immutable risk factors. Aside from these factors, there are several controllable risk factors. In a recent study, international experts reached an agreement that more than half of sporadic or late-onset AD cases were related to seven controllable risk factors, ie, depression, diabetes, smoking, and obesity in middle age, high blood pressure in midlife, lack of exercise, and a lower level of education.Citation27
T2DM and its related conditions
When considering the links between T2DM and AD, it is important to consider the natural history that leads to T2DM. There are two underlying mechanisms involved, ie, insulin resistance and inadequate insulin secretion from pancreatic β-cells.Citation28 Initially, pancreatic β-cells increase insulin secretion in response to insulin resistance, causing hyperinsulinemia, and are able to effectively maintain glucose levels below the T2DM range. When β-cell function begins to decline, insulin production is inadequate to overcome insulin resistance, and blood glucose levels rise, resulting in prediabetes and T2DM. Being overweight or obese is the major reason for insulin resistance.Citation29 This natural history is also part of the metabolic syndrome, which includes hypertension, dyslipidemia, and elevated systemic inflammation.Citation30 From a mechanistic standpoint, it is difficult to discern whether the main mechanism linking T2DM to AD is glycemia, hypertension, insulin resistance, or factors specifically related to adipose tissue. Because they are related sequentially and often occur simultaneously, understanding this relationship is fundamental to the study of the role of adiposity, hyperinsulinemia, metabolic syndrome, and diabetes in AD.
Epidemiological studies linking T2DM, obesity, hyperinsulinemia, and metabolic syndrome to AD
T2DM
In the 1990s, the Rotterdam Study, aiming to determine the influence of T2DM on the risk of dementia and AD, found that T2DM almost doubled the risk of dementia and AD.Citation31 In a longitudinal study of 1,138 subjects, they explored the relationship between the aggregation of vascular risk factors (hypertension, heart disease, current smoking) and AD and showed that diabetes and smoking were the strongest risk factors and the risk of AD associated with diabetes was stronger than previously reported (relative risk 3.8), independent of other vascular conditions.Citation32 So far, numerous prospective epidemiological studies have explored the relationship between diabetes and AD, and most have identified diabetes as a risk factor for ADCitation33 (). Studies specifically assessing the incidence of dementia in people with diabetes mellitus, adjusting for glycemic control, microvascular complications, and comorbidity (eg, hypertension and stroke), have also demonstrated an increased risk; eight of 13 longitudinal population-based studies were reviewed and an excess risk for AD in adults with diabetes was found, ranging from 50% to 100%.Citation3 In a comprehensive meta-analysis, with a total of 6,184 people with diabetes and 38,530 without diabetes, the aggregate relative risk of AD for people with diabetes was 1.5 (95% confidence interval [CI] 1.2–1.8).Citation34
Table 1 Summary of representative prospective epidemiological studies relating type 2 diabetes mellitus to Alzheimer’s disease
While the relationship between diabetes and the major types of dementia is controversial, T2DM is a complex metabolic disorder that is closely associated with other identified risk factors for dementia, including atherosclerotic vascular disease, the APOE-ε4 allele, and some indicators of diabetes, such as diabetes duration, high glucose level, and insulin treatment, may also be risk factors of dementia. A study in Japanese Americans found no association between diabetes in middle age and dementia;Citation35 however, both Whitmer et alCitation36 and Schnaider et alCitation37 found that diabetes in middle age increased the risk of dementia in the elderly when considering prospective studies with a large sample size. The causes of such differences are still not known. The Honolulu-Asia Aging Study also found that diabetes in old age was related to a higher risk of AD (relative risk 1.8) and AD pathology on autopsy, particularly in subjects with the APOE-ε4 allele.Citation38 Several other studies have shown that diabetes with the APOE-ε4 allele was associated with increased risk of dementia.Citation39–Citation41 One study in postmenopausal women found that the risk of mild cognitive impairment and dementia increased with each 1% elevation in glycosylated hemoglobin, a marker of glucose control, even in women without T2DM.Citation42 One study from Sweden analyzed 1,301 community dwellers aged 75 years and older and found that diabetes had no significant relationship with AD, but was related to vascular dementia.Citation43 This association was stronger in subjects with diabetes who reported treatment with insulin. In a meta-analysis, the aggregate relative risk of AD for people with diabetes was 1.5 (95% CI 1.2–1.8), while for vascular dementia the relative risk was 2.5 (95% CI 2.1–3.0).Citation34 Another meta-analysis found a similar result, ie, the relative risk of developing AD was 1.6 (95% CI 1.4–1.7) and vascular dementia was 2.3 (95% CI 2.0–2.7).Citation33 In general, it is clear that T2DM has a higher dementia risk, but the associations are stronger for vascular dementia compared with AD. The association is also influenced by many factors, including ethnicity, the APOE-ε4 allele, diabetes duration, glycemic level, and insulin treatment. Determining what factors and what percentages of these factors contribute to this link is also quite important.
T2DM-related conditions
Obesity
Many studies have explored the association between obesity and AD. Obesity, especially obesity in middle age, usually assessed by body mass index (BMI) or waist circumference, has a strong and independent association with an increased risk for AD. The Baltimore Longitudinal Study of Aging showed that the incidence of AD increased in men who gained weight between the ages of 30 and 45 years and in women with a BMI >30 at ages 30, 40, and 45 years.Citation9 A meta-analysis reported an increased risk (95% CI 1.59–2.62) of AD with obesity (BMI ≥30), and people with the APOE-ε4 allele had a higher level of risk.Citation44 The Honolulu-Asia Aging Study reported that groups with and without dementia showed no differences in weight from midlife to late life.Citation31 The explanation for this difference may be ethnicity, ie, Asians may be more susceptible to the effects of obesity compared with Europeans.Citation45 A meta-analysis of 16 papers found that many types of abnormal body weight in midlife, including underweight, overweight, and obesity, increase the risk of dementia.Citation46
The results of studies of late-life obesity with AD have been conflicting, and the following four conditions have been suggested: an increased risk, a reduced risk, no relationship, and a U-shaped relationship, with both high and low BMI related to an increased risk of AD. Higher BMI at ages 70, 75, and 79 years predicts a higher dementia risk;Citation47 however, an analysis of the Cardiovascular Health Study of subjects ≥65 years of age found a 40% decreased risk with BMI >30 compared with subjects of normal weight.Citation48 WhitmerCitation49 reported a decreased risk of dementia with increasing BMI in subjects ≥76 years of age, but a U-shaped association in subjects <76 years of age. They also found that a higher waist circumference is related to a higher AD risk in the younger elderly, but not in the oldest population. The causes for this paradox remain unclear. There may be weight decreases with aging and frailty.Citation50 It has been suggested that changes in body composition with age make BMI a poor measure of obesity in the elderly;Citation49 survival bias related to high obesity may be an important factor, and midlife studies had a much longer period of follow-up prior to diagnosis of dementia. The average length of follow-up for the late-life studies was 7.13 years, which would include the prodromal phase of AD in particular.Citation51 Further data are required from studies with longer durations of follow-up in late life, and more suitable weight indicators are needed for the elderly.
Hyperinsulinemia
Recent attention has turned to the question of whether hyperinsulinemia may directly increase the risk of cognitive decline. Two longitudinal studies, one in elderly Japanese Americans in HawaiiCitation10 and another in persons aged 65 years and older from northern Manhattan,Citation52 found that the risk of incident AD was higher in persons with hyperinsulinemia. These studies also found that the risk of AD related to hyperinsulinemia was higher among persons with the APOE-ε4 allele. Another study found that higher c-peptide levels, a measure of insulin secretion, may be related to worse cognition, even among those without diabetes.Citation53 The Nurses’ Health Study found that “young-old” women (mean age 64 years) without diabetes but with higher c-peptide levels showed cognitive decline approximately 10 years later.Citation54 In the Physicians’ Health Study II, older men aged 60–92 (mean 71.3) years showed similar results.Citation55 Therefore, hyperinsulinemia may be the reason for T2DM being associated with an increased risk for AD.
Metabolic syndrome
Metabolic syndrome, first described about 40 years ago,Citation56 has a cluster of risk factors including abdominal obesity, hypertension, lipid abnormalities, and impaired metabolism of glucose and insulin. Studies addressing the association between metabolic syndrome and AD are limited and the results are mixed. One study in 2,632 elderly black and white people found that the metabolic syndrome measured using National Cholesterol Education Program guidelines was associated with a higher risk of cognitive decline, particularly among those with high levels of inflammatory markers.Citation57 A population-based study of 980 elderly subjects aged 69–78 years found that the metabolic syndrome is significantly associated with AD.Citation58 However, this suggested association between metabolic syndrome and AD was not confirmed by four large, longitudinal, population-based studies, including the Honolulu-Asia Aging Study,Citation59 the Three-City Study,Citation60 the Italian Longitudinal Aging Study,Citation61 and a multiethnic elderly cohort in the USA.Citation62 However, the prevalence of metabolic syndrome depends on the studied population and the definition, except for the most commonly used definition, ie, the Third Adults Treatment Panel of the National Cholesterol Education Program criteria.Citation63 At least two other more recent sets of clinical criteria were also presented, ie, the National Heart, Lung and Blood Institute/American Heart Association criteriaCitation64 and the International Diabetes Federation criteria.Citation65 It is still unclear if one or two separate components of metabolic syndrome can drive the relationship with cognitive decline, or whether the individual components are additive or interact in some way.Citation66 Individual components of the metabolic syndrome should not be evaluated in isolation, and careful methodological approaches are needed to understand the timing and non-linear relationships between these components over time.Citation67
Insulin resistance and deficiency: potential mechanisms linking T2DM and its related conditions to AD
A large number of studies have shown that insulin resistance and deficiency, a marker of T2DM, play an important role in AD pathology. The first molecular clue as to how the brain might become insulin-resistant in AD came from studies demonstrating that Aβ oligomers bind to hippocampal neurons and trigger the removal of dendritic insulin receptor substrates (IRs) from the plasma membrane,Citation68 which was subsequently demonstrated in AD brains.Citation69 Lower levels and sensitivity of insulin, IGF, and IRs were observed in AD neuropathology.Citation70–Citation72 Greatly increased biomarkers of peripheral insulin resistance in the hippocampus of non-diabetic AD patients further implicated insulin resistance in AD.Citation73 In T2DM, tumor necrosis factor (TNF)-α signaling activates c-Jun N-terminal kinase,Citation74 resulting in IRs-1 serine phosphorylation and peripheral insulin resistance.Citation68 Similarly, Aβ oligomers cause abnormal activation of the TNF-α/c-Jun N-terminal kinase pathway and inhibition of IRs-1 in cultured hippocampal neurons.Citation75 Recently, it was even proposed that AD can be an “insulin-resistant brain state” or even a “type 3 diabetes”.Citation76 Insulin was found to modulate Aβ protein precursor expression and processing both in vivo and in vitro. Insulin and IGF-1 inhibited Aβ production through Akt-mediated phosphorylation/inactivation of glycogen synthase kinase-3βCitation77 and prevented abnormal intracellular accumulation of Aβ by increasing its extracellular secretion in the brain and accelerating its trafficking from the Golgi and trans-Golgi network to the plasma membrane.Citation69,Citation78 Insulin and IGF-1 also prevented accumulation of Aβ by promoting the transport of Aβ-binding carrier proteins, including transthyretin and albumin, into the brain.Citation79–Citation81 Devi et alCitation82 demonstrated that streptozotocin-induced insulin-deficient diabetes accelerates Aβ accumulation via the translational upregulation of the β-secretase enzyme, BACE1, and its substrate, amyloid precursor protein, in a transgenic mouse model of AD. Another potential mechanism could be the interference of insulin with extracellular proteolytic Aβ degradation occurs via the insulin-degrading enzyme, a metalloprotease that also catabolizes insulin and IGF-1.Citation69,Citation79,Citation80,Citation83,Citation84 Under insulin resistance conditions, insulin may competitively inhibit the insulin-degrading enzyme, thus impairing degradation of Aβ, increasing its neurotoxicity and promoting AD.Citation69,Citation79,Citation80,Citation83,Citation84 Besides Aβ, insulin resistance and deficiency also increases tau protein phosphorylation through activation of glycogen synthase kinase-3β.Citation85,Citation86
Considering the above, neurons in the T2DM brain could be more vulnerable to the toxicity of Aβ due to insulin resistance and deficiency.Citation87 Conversely, insulin resistance and deficiency could lead to increased production of Aβ and Aβ-induced oxidative damage at the mitochondria.Citation88 Therefore, the current hypothesis regarding insulin resistance and deficiency may represent a critical contributing factor in the acceleration of Aβ production during the progression of sporadic AD ( and ), and thus insulin resistance and deficiency may be an important therapeutic target in patients with AD.
Figure 1 Overview of the role of insulin resistance and insulin deficiency in the pathology of Alzheimer’s disease.
Abbreviations: BACE1, β-site amyloidogenic cleavage of precursor protein-cleaving enzyme 1; GSK-3β, glycogen synthase kinase-3β; IDE, insulin-degrading enzyme; IRs, insulin receptor substrates; TNF, tumor necrosis factor; JNK, c-Jun N-terminal kinase; APP, amyloid precursor protein; Aβ, amyloid-β; IGF, insulin growth factor.
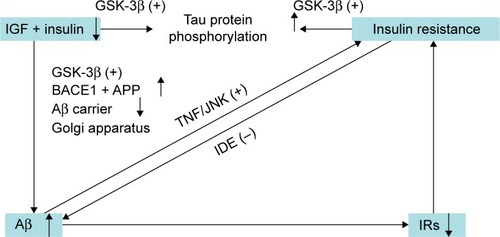
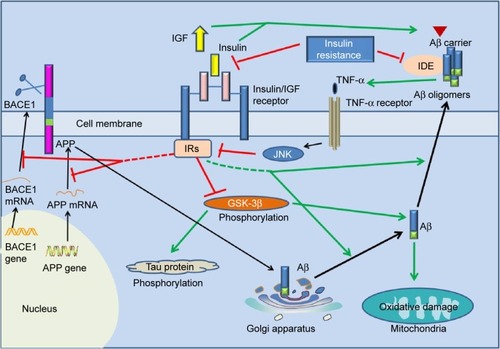
Figure 2 The underlying link between Alzheimer’s disease and type 2 diabetes mellitus.
Abbreviations: APP, amyloid precursor protein; Aβ, amyloid-β; IDE, insulin-degrading enzyme; BACE1, β-site amyloidogenic cleavage of precursor protein-cleaving enzyme 1; GSK-3β, glycogen synthase kinase-3β; IRs, insulin receptor substrates; TNF-α, tumor necrosis factor alpha; JNK, c-Jun N-terminal kinase; IGF-1, insulin-like growth factor 1.
Implications for treatment of AD with or without T2DM
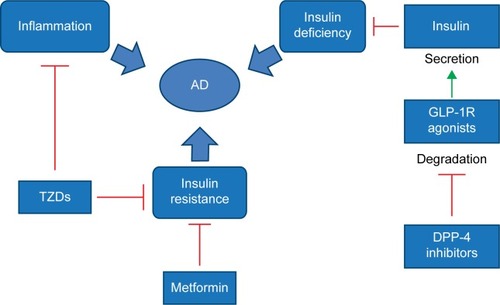
Given the role of insulin resistance and deficiency in the pathogenesis of AD, it could be possible that a drug currently approved for T2DM may also be useful for AD (). We now summarize some of the relevant clinical trials.
Figure 3 Possible mechanisms of antidiabetic drugs in the treatment of AD.
Abbreviations: AD, Alzheimer’s disease; DPP-4, dipeptidyl peptidase-4; TZDs, thiazolidinediones; GLP-1R, glucagon-like peptide-1 receptor.
Intranasal insulin
Insulin has also been studied in cognitively impaired patients, because intranasal administration can quickly deliver insulin to the central nervous system across olfactory and trigeminal perivascular channels and axonal pathways,Citation89 and there are fewer potential side effects, such as hypoglycemia, when compared with intravenous insulin infusions. Thus, intranasal delivery of insulin is a viable long-term therapy for AD. A 2008 study reported that intranasal insulin (20 IU, twice a day) for 21 days improved story recall, attention, and caregiver-rated functional status in cognitively impaired subjects or individuals with AD.Citation90 In another study, 40 individuals with AD and 64 with mild cognitive impairment received either placebo, 20 IU of insulin, or 40 IU of insulin administered via nasal spray over the course of 4 months. The between-subject comparison showed that intranasal insulin improved delayed memory and cognitive function. Based on positron emission tomography findings, the authors also provided evidence that both doses of insulin had a higher 18F fluorodeoxyglucose uptake in the parietotemporal, frontal, precuneus, and cuneus regions of the central nervous system following administration of intranasal insulin compared with placebo, linking the enhancement in function of those brain areas.Citation91 Given what is known about AD and diabetes, it is not surprising that intranasal insulin could have different effects depending on the APOE-ε4 genotype. In a study by Reger et alCitation92 the participants were further subdivided based on APOE-ε4 genotype (APOE-ε4 negative or positive allele), and cognitive function was measured 45 minutes after administration of intranasal treatment. Participants with the APOE-ε4 negative allele displayed significant cognitive improvement on the 20 IU and 40 IU doses when compared with the placebo group and the APOE-ε4 positive group, and patients in the APOE-ε4 negative group taking 40 IU of insulin performed better than patients in the other groups. In the APOE-ε4 positive group, cognitive function (working and verbal memory) was reduced with treatment. Currently, the sample size of clinical trials exploring insulin administration in AD patients seems to be relatively low, with a relatively short treatment period. Therefore, longer trials are needed, as well as APOE-ε4 allele related differences in insulin metabolism.
Metformin
Metformin is an orally active biguanide that lowers blood glucose levels by suppressing hepatic glucose output, increasing insulin-mediated glucose disposal, increasing intestinal glucose use, and decreasing fatty acid oxidation. It also reduces insulin levels,Citation93 inflammation and thrombosis,Citation94 and the risks of metabolic syndromeCitation95 and diabetesCitation96 in persons without diabetes. Long-term use of metformin is also associated with a lower risk of certain cancers.Citation97,Citation98 While the mechanisms of action are not completely understood for metformin, studies have shown that patients with T2DM and AD, and receiving antidiabetic drugs including metformin, have a lower rate of cognitive impairment than in untreated patients.Citation99 This result suggests that diabetic medication might somehow affect neuronal networks in the brain, leading to functional preservation or benefit in AD patients. In a large epidemiological study comparing individuals with T2DM, either taking antidiabetic drugs or not, metformin and sulfonylureas decreased the risk of dementia by 35% over 8 years in patients with T2DM.Citation100 Notably, another large epidemiological trial, based on the UK-based General Practice Research Database, including 7,086 individuals aged 65 years and older with an incident diagnosis of AD and the same number of matched controls without dementia, showed that patients with T2DM who were long-term users of metformin, had a slightly higher risk of AD than those who did not receive the drug.Citation101 The conflicting results of these studies point to more research being needed.
Thiazolidinediones
Thiazolidinediones are peroxisome proliferator-activated receptor-γ (PPARγ) agonists and potent insulin sensitizers.Citation102 Their mechanism involves stimulation of the action of PPARγ in response to changes in insulin, thereby triggering a drop in serum glucose.Citation103 The best characterized PPARγ agonists are pioglitazone and rosiglitazone. Thiazolidinediones also have potent anti-inflammatory properties.Citation104 Given the role of insulin resistance and inflammation in the pathogenesis of AD,Citation105,Citation106 these agents are being studied as a potential treatment for AD. One small study showed that persons receiving rosiglitazone 4 mg daily had improved memory and selective attention.Citation107 In a larger trial, more than 500 patients with mild to moderate AD were randomized to 6 months of treatment with placebo or rosiglitazone 2, 4, or 8 mg, resulting in significant improvement on the Alzheimer’s Disease Assessment Scale-cognitive subscale in APOE-ε4-negative patients on 8 mg rosiglitazone, while persons with the APOE-ε4 allele showed no benefit.Citation108 However, a Phase III trial of rosiglitazone (NCT0428090) in mild to moderate AD found no benefit.Citation109 Pioglitazone and rosiglitazone seem to have similar results. Researchers in one study reported improvement of cognition with pioglitazone in patients with both T2DM and AD, whereas another study showed no effect.Citation110 The major limitation of thiazolidinediones in the prevention of dementia is the side effects of edema and congestive heart failure. In the interests of safety, the USA and Europe have either partially or completely restricted the use of rosiglitazone for treatment of T2DM.Citation111 Therefore, solving these side effect issues is very important for the future application of thiazolidinediones.
GLP-1R agonists and DPP-IV inhibitors
GLP-1 is a gut-derived incretin hormone that enhances glucose-stimulated insulin secretion and suppresses glucagon secretion.Citation112 GLP-1 is rapidly degraded by DPP-4; however, administration of specific DPP-4 inhibitors can increase the half-life of endogenous GLP-1 and hence prolong the activation of GLP-1R in different cell types.Citation113,Citation114 Currently, GLP-1R agonists and DPP-4 inhibitors are routinely used to treat T2DM.Citation112,Citation114,Citation115 In agreement with the proposed role of insulin signaling declining with the development of AD, GLP-1R agonists are an attractive option because they activate pathways common to bypassing IRs and boost insulin-related signaling pathways through G protein-dependent signaling.Citation116 In fact, animal studies have revealed that GLP-1R plays an important role in the control of synaptic plasticity and in some forms of memory formation.Citation117,Citation118 Exendin-4 and liraglutide, two types of GLP-1R agonists, also restored impaired insulin signaling, exerting neuroprotective effects on neurons and synapses, improving cognition, and decreasing Aβ accumulation in the brain in a transgenic mouse model of AD.Citation75,Citation119 As recently suggested, an agent that chronically decreases Aβ levels should be beneficial in APOE-ε4 allele carriers.Citation120 If the beneficial effect of GLP-1R agonists is found to translate to primates, APOE-ε4 allele carriers may possibly benefit from the use of GLP-1R agonists. Similarly, the DPP-4 inhibitors, sitagliptin and vildagliptin, have been found to have beneficial effects on learning and memory in animal models.Citation121,Citation122 Unfortunately, no relevant clinical data are available. We are awaiting the results of two clinical trials, ie, a study of exendin-4 in 230 patients with mild cognitive impairment/early-stage AD (NCT1255163) and a large-scale Phase II clinical trial assessing the safety and efficacy of liraglutide in 206 patients with mild cognitive impairment (NCT1843075).
Summary
T2DM and AD have traditionally been treated as independent disorders. With extensive and indepth research on T2DM and AD, epidemiological associations and some common pathophysiological mechanisms have been found. If demonstrated to be true, common pharmacotherapy should be effective, and clinical trials testing the effectiveness of antidiabetic drugs in AD patients should be initiated. The results will not only be important for the treatment of AD patients, but will also be key to understanding the connection between these serious but seemingly unrelated disorders.
Acknowledgments
We thank LetPub (www.letpub.com) for its assistance with English language during preparation of this manuscript.
Disclosure
The author reports no conflicts of interest in this work.
References
- WildSRoglicGGreenASicreeRKingHGlobal prevalence of diabetes estimates for the year 2000 and projections for 2030Diabetes Care20042751047105315111519
- StrachanMWReynoldsRMMarioniREPriceJFCognitive function, dementia and type 2 diabetes mellitus in the elderlyNat Rev Endocrinol20117210811421263438
- BiesselsGJStaekenborgSBrunnerEBrayneCScheltensPRisk of dementia in diabetes mellitus: a systematic reviewLancet Neurol200651647416361024
- CukiermanTGersteinHWilliamsonJCognitive decline and dementia in diabetes – systematic overview of prospective observational studiesDiabetologia200548122460246916283246
- StrachanMWDearyIJEwingFMFrierBMIs type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studiesDiabetes Care19972034384459051402
- PrinceMBryceRAlbaneseEWimoARibeiroWFerriCPThe global prevalence of dementia: a systematic review and meta-analysisAlzheimers Dement201391637523305823
- StrachanMWPriceJFFrierBMDiabetes, cognitive impairment, and dementiaBMJ20083367634618174567
- Alzheimer’s Association2014 Alzheimer’s disease facts and figuresAlzheimers Dement2014102e47e9224818261
- BeydounMALhotskyAWangYAssociation of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer’s diseaseAm J Epidemiol2008168101179118918835864
- PeilaRRodriguezBLWhiteLRLaunerLJFasting insulin and incident dementia in an elderly population of Japanese–American menNeurology200463222823315277613
- SjoholmANystromTInflammation and the etiology of type 2 diabetesDiabetes Metab Res Rev200622141015991254
- LuchsingerJAType 2 diabetes and cognitive impairment: linking mechanismsJ Alzheimer’s Dis201230Suppl 2S185S19822433668
- de la MonteSMContributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s diseaseDrugs2012721496622191795
- LanctotKLRajaramRDHerrmannNTherapy for Alzheimer’s disease: how effective are current treatments?Ther Adv Neurol Disord20092316318021179526
- GreenRCSchneiderLSAmatoDAEffect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trialJAMA2009302232557256420009055
- QuinnJFRamanRThomasRGDocosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trialJAMA2010304171903191121045096
- QuerfurthHWLaFerlaFMAlzheimer’s diseaseN Engl J Med2010362432934420107219
- SelkoeDJAlzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-proteinJ Alzheimers Dis200131758012214075
- MoreiraPISantosMSSeicaROliveiraCRBrain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetesJ Neurol Sci20072571–220621417316694
- MoreiraPIHondaKZhuXBrain and brawn: parallels in oxidative strengthNeurology2006662 Suppl 1S97S10116432155
- GoedertMSpillantiniMGA century of Alzheimer’s diseaseScience2006314580077778117082447
- HardyJAllsopDAmyloid deposition as the central event in the aetiology of Alzheimer’s diseaseTrends Pharmacol Sci199112103833881763432
- HardyJSelkoeDJThe amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeuticsScience2002297558035335612130773
- HardyJAHigginsGAAlzheimer’s disease: the amyloid cascade hypothesisScience199225650541841851566067
- RocchiAPellegriniSSicilianoGMurriLCausative and susceptibility genes for Alzheimer’s disease: a reviewBrain Res Bull200361112412788204
- CummingsJLAlzheimer’s diseaseN Engl J Med20043511566715229308
- SmithADYaffeKDementia (including Alzheimer’s disease) can be prevented: statement supported by international expertsJ Alzheimers Disease201438469970324326609
- DeFronzoRALilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDMDiabetes19883766676873289989
- StefanNKantartzisKMachannJIdentification and characterization of metabolically benign obesity in humansArch Intern Med2008168151609161618695074
- LuchsingerJAGustafsonDRAdiposity, type 2 diabetes, and Alzheimer’s diseaseJ Alzheimers Disease200916469370419387106
- OttAStolkRPvan HarskampFPolsHAHofmanABretelerMMDiabetes mellitus and the risk of dementia: the Rotterdam StudyNeurology19995391937194210599761
- LuchsingerJReitzCHonigLSTangM-XSheaSMayeuxRAggregation of vascular risk factors and risk of incident Alzheimer diseaseNeurology200565454555116116114
- GudalaKBansalDSchifanoFBhansaliADiabetes mellitus and risk of dementia: a meta-analysis of prospective observational studiesJ Diabetes Investig201346640650
- ChengGHuangCDengHWangHDiabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studiesIntern Med J201242548449122372522
- CurbJDRodriguezBLAbbottRDLongitudinal association of vascular and Alzheimer’s dementias, diabetes, and glucose toleranceNeurology199952597197510102414
- WhitmerRASidneySSelbyJJohnstonSCYaffeKMidlife cardiovascular risk factors and risk of dementia in late lifeNeurology200564227728115668425
- Schnaider BeeriMGoldbourtUSilvermanJMDiabetes mellitus in midlife and the risk of dementia three decades laterNeurology200463101902190715557509
- PeilaRRodriguezBLLaunerLJType 2 diabetes, APOE gene, and the risk for dementia and related pathologies The Honolulu-Asia Aging StudyDiabetes20025141256126211916953
- TakedaMMartinezRKudoTApolipoprotein E and central nervous system disorders: reviews of clinical findingsPsychiatry Clin Neurosci201064659260721105952
- IrieFFitzpatrickALLopezOLEnhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the Cardiovascular Health Study Cognition StudyArch Neurol2008651899318195144
- DoreGAEliasMFRobbinsMAEliasPKNagyZPresence of the APOE epsilon4 allele modifies the relationship between type 2 diabetes and cognitive performance: the Maine-Syracuse StudyDiabetologia200952122551256019693485
- YaffeKBlackwellTWhitmerRAKruegerKBarrett ConnorEGlycosylated hemoglobin level and development of mild cognitive impairment or dementia in older womenJ Nutr Health Aging200610429329516886099
- XuWLQiuCXWahlinAWinbladBFratiglioniLDiabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up studyNeurology20046371181118615477535
- ProfennoLAPorsteinssonAPFaraoneSVMeta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disordersBiol Psychiatry201067650551219358976
- ReavenGMLawsAInsulin Resistance: The Metabolic Syndrome XClifton, NJ, USAHuman Press1999
- AnsteyKCherbuinNBudgeMYoungJBody mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studiesObes Rev2011125e426e43721348917
- GustafsonDRothenbergEBlennowKSteenBSkoogIAn 18-year follow-up of overweight and risk of Alzheimer diseaseArch Intern Med2003163131524152812860573
- FitzpatrickALKullerLHLopezOLMidlife and late-life obesity and the risk of dementia: cardiovascular health studyArch Neurol200966333634219273752
- WhitmerRAThe epidemiology of adiposity and dementiaCurr Alzheimer Res20074211712217430233
- MorleyJEAnorexia, sarcopenia, and agingNutrition200117766066311448592
- JohnsonDKWilkinsCHMorrisJCAccelerated weight loss may precede diagnosis in Alzheimer diseaseArch Neurol20066391312131716966511
- LuchsingerJATangM-XSheaSMayeuxRHyperinsulinemia and risk of Alzheimer diseaseNeurology20046371187119215477536
- OkerekeOHankinsonSEHuFBGrodsteinFPlasma C peptide level and cognitive function among older women without diabetes mellitusArch Intern Med2005165141651165616043685
- OkerekeOIPollakMNHuFBHankinsonSESelkoeDJGrodsteinFPlasma C-peptide levels and rates of cognitive decline in older, community-dwelling women without diabetesPsychoneuroendocrinology200833445546118261857
- OkerekeOIKurthTPollakMNGazianoJMGrodsteinFFasting plasma insulin, c-peptide and cognitive change in older men without diabetes: results from the Physicians’ Health Study IINeuroepidemiology201034420020720197703
- AvogaroPCrepaldiGEnziGTiengoAAssociation of hyperlipemia, diabetes mellitus and mild obesityActa Diabetol Lat19674572590
- YaffeKKanayaALindquistKThe metabolic syndrome, inflammation, and risk of cognitive declineJAMA2004292182237224215536110
- VanhanenMKoivistoKMoilanenLAssociation of metabolic syndrome with Alzheimer disease. A population-based studyNeurology200667584384716966548
- KalmijnSFoleyDWhiteLMetabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men: the Honolulu-Asia Aging StudyArterioscler Thromb Vasc Biol200020102255226011031212
- RaffaitinCGinHEmpanaJ-PMetabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the Three-City StudyDiabetes Care200932116917418945929
- SolfrizziVScafatoECapursoCMetabolic syndrome and the risk of vascular dementia: the Italian Longitudinal Study on AgeingJ Neurol Neurosurg Psychiatry201081443344019965842
- MullerMTangM-XSchupfNManlyJJMayeuxRLuchsingerJAMetabolic syndrome and dementia risk in a multiethnic elderly cohortDement Geriatr Cogn Disord200724318519217641531
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in AdultsExecutive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on Detection, Evaluation, and Treatment of high blood cholesterol in adults (Adult Treatment Panel III)JAMA2001285192486249711368702
- GrundySMCleemanJIDanielsSRDiagnosis and management of the metabolic syndrome an American Heart Association/National Heart, Lung, and Blood Institute scientific statementCirculation2005112172735275216157765
- AlbertiKGZimmetPShawJIDF Epidemiology Task Force Consensus GroupThe metabolic syndrome – a new worldwide definitionLancet200536694911059106216182882
- CrichtonGEEliasMFBuckleyJDMurphyKJBryanJFrisardiVMetabolic syndrome, cognitive performance, and dementiaJ Alzheimers Dis201230S77S8721971405
- WattsASLoskutovaNBurnsJMJohnsonDKMetabolic syndrome and cognitive decline in early Alzheimer’s disease and healthy older adultsJ Alzheimers Dis201335225326523388170
- ZhaoW-QDe FeliceFGFernandezSAmyloid beta oligomers induce impairment of neuronal insulin receptorsFASEB J200822124626017720802
- MoloneyAMGriffinRJTimmonsSO’ConnorRRavidRO’NeillCDefects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signallingNeurobiol Aging201031222424318479783
- SteenETerryBMRiveraEJImpaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease-is this type 3 diabetes?J Alzheimers Dis200571638015750215
- HoyerSNitschRCerebral excess release of neurotransmitter amino acids subsequent to reduced cerebral glucose metabolism in early-onset dementia of Alzheimer typeJ Neural Transm19897532272322926384
- CraftSAlzheimer disease: insulin resistance and AD – extending the translational pathNat Rev Neurol20128736036222710630
- TalbotKWangH-YKaziHDemonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive declineJ Clin Invest201212241316133822476197
- HirosumiJTuncmanGChangLA central role for JNK in obesity and insulin resistanceNature2002420691333333612447443
- BomfimTRForny-GermanoLSathlerLBAn anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease–associated Aβ oligomersJ Clin Invest201212241339135322476196
- RiveraEJGoldinAFulmerNTavaresRWandsJRde la MonteSMInsulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholineJ Alzheimers Dis20058324726816340083
- PhielCJWilsonCALeeVM-YKleinPSGSK-3α regulates production of Alzheimer’s disease amyloid-β peptidesNature2003423693843543912761548
- WadaAYokooHYanagitaTKobayashiHNew twist on neuronal insulin receptor signaling in health, disease, and therapeuticsJ Pharmacol Sci200599212814316210778
- CarroETrejoJLSpuchCBohlDHeardJMTorres-AlemanIBlockade of the insulin-like growth factor I receptor in the choroid plexus originates Alzheimer’s-like neuropathology in rodents: new cues into the human disease?Neurobiol Aging200627111618163116274856
- CarroETorres-AlemanIThe role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s diseaseEur J Pharmacol2004490112713315094079
- BakerLDCrossDJMinoshimaSBelongiaDWatsonGSCraftSInsulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetesArch Neurol2011681515720837822
- DeviLAlldredMJGinsbergSDOhnoMMechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer’s diseasePLoS One201273e3279222403710
- PlumLSchubertMBrüningJCThe role of insulin receptor signaling in the brainTrends Endocrinol Metab2005162596515734146
- GaspariniLNetzerWJGreengardPXuHDoes insulin dysfunction play a role in Alzheimer’s disease?Trends Pharmacol Sci200223628829312084635
- KremerALouisJVJaworskiTVan LeuvenFGSK3 and Alzheimer’s disease: facts and fictionFront Mol Neurosci201141721904524
- LiXLuFTianQYangYWangQWangJZActivation of glycogen synthase kinase-3 induces Alzheimer-like tau hyperphosphorylation in rat hippocampus slices in cultureJ Neural Transm200611319310215959856
- PiconePGiacomazzaDVetriVInsulin-activated Akt rescues Aβ oxidative stress-induced cell death by orchestrating molecular traffickingAging Cell201110583284321624038
- SuzanneMContributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s diseaseDrugs2012721496622191795
- ThorneRPronkGPadmanabhanVFreyWH2ndDelivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administrationNeuroscience2004127248149615262337
- RegerMWatsonGGreenPIntranasal insulin improves cognition and modulates β-amyloid in early ADNeurology200870644044817942819
- CraftSBakerLDMontineTJIntranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trialArch Neurol2012691293821911655
- RegerMWatsonGFreyWH2ndEffects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotypeNeurobiol Aging200627345145815964100
- KitabchiAETemprosaMKnowlerWCDiabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metforminDiabetes20055482404241416046308
- GoldbergRHortonEMarcovinaSIntensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose toleranceDiabetes20055451566157215855347
- OrchardTJTemprosaMGoldbergRThe effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trialAnn Intern Med2005142861161915838067
- KnowlerWCBarrett-ConnorEFowlerSEReduction in the incidence of type 2 diabetes with lifestyle intervention or metforminN Engl J Med2002346639340311832527
- EvansJMDonnellyLAEmslie-SmithAMAlessiDRMorrisADMetformin and reduced risk of cancer in diabetic patientsBMJ200533075031304130515849206
- CurrieCPooleCGaleEThe influence of glucose-lowering therapies on cancer risk in type 2 diabetesDiabetologia20095291766177719572116
- DomínguezROMarschoffERGonzálezSERepettoMGSerraJAType 2 diabetes and/or its treatment leads to less cognitive impairment in Alzheimer’s disease patientsDiabetes Res Clin Pract2012981687422658669
- HsuC-CWahlqvistMLLeeM-STsaiH-NIncidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metforminJ Alzheimers Dis201124348549321297276
- ImfeldPBodmerMJickSSMeierCRMetformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control studyJ Am Geriatr Soc201260591692122458300
- Yki-JärvinenHThiazolidinedionesN Engl J Med2004351111106111815356308
- MalinowskiJMBolestaSRosiglitazone in the treatment of type 2 diabetes mellitus: a critical reviewClin Ther200022101151116811110228
- NestoRC-reactive protein, its role in inflammation, type 2 diabetes and cardiovascular disease, and the effects of insulin-sensitizing treatment with thiazolidinedionesDiabet Med200421881081715270782
- TuppoEEAriasHRThe role of inflammation in Alzheimer’s diseaseInt J Biochem Cell Biol200537228930515474976
- CraftSInsulin resistance and Alzheimers disease pathogenesis: potential mechanisms and implications for treatmentCurr Alzheimer Res20074214715217430239
- WatsonGCholertonBARegerMAPreserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary studyAm J Geriatr Psychiatry2005131195095816286438
- RisnerMSaundersAAltmanJEfficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s diseasePharmacogenomics J20066424625416446752
- GoldMAldertonCZvartau-HindMRosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III studyDement Geriatr Cogn Disord201030213114620733306
- MillerBWWillettKCDesiletsARRosiglitazone and pioglitazone for the treatment of Alzheimer’s diseaseAnn Pharmacother201145111416142422028424
- CheungBMBehind the rosiglitazone controversyExpert Rev Clin Pharmacol20103672372522111775
- DeaconCFAhrénBPhysiology of incretins in health and diseaseRev Diabet Stud20118329322262068
- DruckerDJNauckMAThe incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetesLancet200636895481696170517098089
- DeaconCDipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative reviewDiabetes Obes Metab201113171821114598
- LovshinJADruckerDJIncretin-based therapies for type 2 diabetes mellitusNat Rev Endocrinol20095526226919444259
- VilsbøllTKrarupTMadsbadSHolstJJBoth GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjectsRegul Pept2003114211512112832099
- DuringMJCaoLZuzgaDSGlucagon-like peptide-1 receptor is involved in learning and neuroprotectionNat Med2003991173117912925848
- AbbasTFaivreEHölscherCImpairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: interaction between type 2 diabetes and Alzheimer’s diseaseBehav Brain Res2009205126527119573562
- McCleanPLParthsarathyVFaivreEHölscherCThe diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s diseaseJ Neurosci201131176587659421525299
- SelkoeDJPreventing Alzheimer’s diseaseScience201233761011488149222997326
- PipatpiboonNPintanaHPratchayasakulWChattipakornNChattipakornSCDPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumptionEur J Neurosci201337583984923240760
- PintanaHApaijaiNChattipakornNChattipakornSCDPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant ratsJ Endocrinol2013218111123591914
- TyasSLManfredaJStrainLAMontgomeryPRRisk factors for Alzheimer’s disease: a population-based, longitudinal study in Manitoba, CanadaInt J Epidemiol200130359059711416089
- MacKnightCRockwoodKAwaltEMcDowellIDiabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and AgingDement Geriatr Cogn Disord2002142778312145454
- HassingLBJohanssonBNilssonSEDiabetes mellitus is a risk factor for vascular dementia, but not for Alzheimer’s disease: a population-based study of the oldest oldInt Psychogeriatr200214323924812475085
- HonigLSTangMXAlbertSStroke and the risk of Alzheimer diseaseArch Neurol200360121707171214676044
- ArvanitakisZWilsonRSBieniasJLEvansDABennettDADiabetes mellitus and risk of Alzheimer disease and decline in cognitive functionArch Neurol200461566166615148141
- BorensteinARWuYMortimerJADevelopmental and vascular risk factors for Alzheimer’s diseaseNeurobiol Aging200526332533415639310
- HaydenKMZandiPPLyketsosCGVascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County studyAlzheimer Dis Assoc Disord20062029310016772744
- AkomolafeABeiserAMeigsJBDiabetes mellitus and risk of developing Alzheimer disease: results from the Framingham StudyArch Neurol200663111551155517101823
- AhtiluotoSPolvikoskiTPeltonenMDiabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic studyNeurology201075131195120220739645
- OharaTDoiYNinomiyaTGlucose tolerance status and risk of dementia in the community: the Hisayama studyNeurology201177121126113421931106
- ChengDNobleJTangMXSchupfNMayeuxRLuchsingerJAType 2 diabetes and late-onset Alzheimer’s diseaseDement Geriatr Cogn Disord201131642443021757907
- LiJWangYJZhangMVascular risk factors promote conversion from mild cognitive impairment to Alzheimer diseaseNeurology201176171485149121490316
