Abstract
Aging is an inevitable and progressive biological process involving dysfunction and eventually destruction of every tissue and organ. This process is driven by a tightly regulated and complex interplay between genetic and acquired factors. Klotho is an antiaging gene encoding a single-pass transmembrane protein, klotho, which serves as an aging suppressor through a wide variety of mechanisms, such as antioxidation, antisenescence, antiautophagy, and modulation of many signaling pathways, including insulin-like growth factor and Wnt. Klotho deficiency activates Wnt expression and activity contributing to senescence and depletion of stem cells, which consequently triggers tissue atrophy and fibrosis. In contrast, the klotho protein was shown to suppress Wnt-signaling transduction, and inhibit cell senescence and preserve stem cells. A better understanding of the potential effects of klotho on stem cells could offer novel insights into the cellular and molecular mechanisms of klotho deficiency-related aging and disease. The klotho protein may be a promising therapeutic agent for aging and aging-related disorders.
Keywords:
Introduction
Klotho, the gene encoding the antiaging protein called klotho, was discovered in 1997 when mice developed multiple organ failure and shortened life span resembling human premature aging after this gene was serendipitously silenced.Citation1 The klotho-deficient phenotype can be rescued by overexpressing klotho via genetic manipulationCitation2 or viral delivery.Citation3 Subsequently, klotho genes in humans and rats were cloned (). The chromosomal localization of mouse klotho is on 13q12, encompassing 50 kb and consisting of five exons ().Citation1,Citation4 Two transcripts that arise from a single klotho gene through alternative RNA splicing were identified, and are predicted to encode a membrane and a secreted protein. The membrane protein consists of 1,014 amino acids, with an extracellular domain of twofold internal repeats (termed KL1 and KL2 domains), each about 450 amino acids long with 20%–40% homology to β-glucosidases. The secreted protein derived from the alternative transcript is only 550 amino acids long without the transmembrane domainCitation1,Citation4 ().
Figure 1 Schematic diagram of membrane klotho and secreted klotho.
Abbreviation: mRNA, messenger RNA.
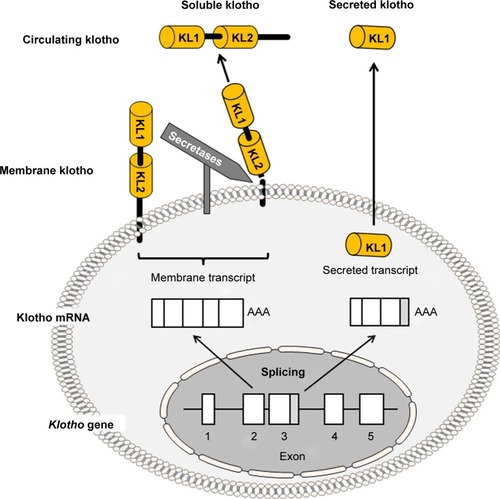
The chromosomal localization of the rat klotho gene is on 12q12. The rat membrane klotho protein is also 1,014 amino acids long and is 94% and 85% homologous to those of mouse and human klotho proteins, respectively.Citation5 The human klotho gene is localized on chromosome 13q12, encompassing 50 kb and consisting of five exons. Analogous to the mouse, two transcripts also arise from a single human klotho gene through alternative RNA splicing and encode a membrane (1,012 amino acids) or secreted (549 amino acids) protein.Citation6
Klotho is highly expressed in the kidney, brain, and to a lesser extent in other organs.Citation1,Citation7 The extracellular domain of membrane klotho can be cleaved and shed by secretases.Citation8–Citation10 This released extracellular domain is referred as “soluble klotho” in this manuscript to distinguish it from another short klotho protein containing only a KL1 domain called secreted klotho, which is directly encoded by secreted klotho transcript through alternative splicing (). Soluble klotho is the main functional form present in the circulation.Citation2,Citation11–Citation13 It is also present in the cerebrospinal fluidCitation13,Citation14 and urine of mammals.Citation11,Citation12,Citation15,Citation16 As a circulating substance, soluble klotho exerts biological actions on distant organs and multiple systems.Citation17–Citation20
Soluble klotho can function as a β-glucuronidaseCitation12,Citation21–Citation23 or sialidaseCitation24–Citation26 to regulate sodium dependent phosphate cotransporters (NaPi), organic cation transporters, renal outer medullary K+ channel 1, and calcium-channel transient receptor potential vanilloid 5 in the kidney and maintain mineral homeostasis. Klotho deficiency may also decrease extracellular volume through downregulation of the Na-K-2Cl cotransporter in the loop of Henle, with consequent increases in antidiuretic hormone and aldosterone hormonal responses,Citation27 both linked to the premature aging phenotype mediated by dehydration observed in klotho-deficient mice.Citation28,Citation29 Outside the kidney, klotho can also function as β-glucuronidase to enhance creatine transporter-protein activity and maintain neuronal function and survival,Citation30 and to increase several K+ channel expression and activities: 1) KCNQ1/KCNE1, required for proper hearing and cardiac repolarization;Citation31 2) voltage-gated K+ channel (Kv1.3),Citation32 expressed in many tissues to regulate a wide variety of cellular functions, including excitability, cell proliferation, apoptosis, immune response, insulin sensitivity, and platelet function; 3) the cardiac K+ channel,Citation33 a key channel for cardiac repolarization and deranged excitation following cardiac hypertrophy.Citation34 Furthermore, soluble klotho can also modulate the IGF-1Citation2 and WntCitation18,Citation35 signaling pathways, playing a key role in antiaging, anti-tumor growth, and antifibrosis. Importantly, soluble klotho can suppress apoptosis and protect cells against a variety of insults, including hypoxia, hyperoxia, oxidative stress, and cytotoxic drugs.Citation36,Citation37
Aging is an inevitable and progressive biological process resulting in dysfunction and destruction of almost all tissues and organs. This is driven by a tightly regulated and complex interplay between genetic and acquired factors. Aging is typically characterized by an increase in senescence, a quantitative and qualitative decrease in stem cells, and abnormal structure at tissue levels. The final outcome of aging is death. In this context, klotho plays an important role in suppressing aging.
There are significant mineral-metabolism disturbances in klotho deficiency, and mineral imbalance per se can induce premature aging induced by klotho deficiency. Indeed, some of the premature aging features and early death seen in klotho-deficient mice can be rescued by reducing plasma phosphate via genetic deletion of NaPi2a in the kidneyCitation38 or a low-phosphate diet.Citation39 Reduction of 1,25-(OH)2-vitamin D and its signaling pathway also rescued phenotypes in klotho-deficient mice.Citation40,Citation41 However, there may be additional mineral-independent effects of klotho that deserve some attention. There are sparse but definite data about the potential effects of the klotho protein on stem cells, a key player in aging and tissue-regeneration processes. This manuscript summarizes current data on how klotho deficiency induces stem cell senescence and depletion, and provides novel insights into the cellular and molecular mechanisms of how the klotho protein affects stem cells and aging. We propose the klotho protein as a potential candidate therapeutic agent to halt aging and aging-associated diseases.
Klotho deficiency induces premature aging
Klotho deficiency in hypomorphic klotho mice or silencing of the klotho gene led to similar phenotypes of premature aging and short life span.Citation1 In addition, global or renal-specific conditional knockout of klotho also led to a similar phenotype.Citation1,Citation42 These experiments confirmed that destruction of the klotho gene or loss of klotho function leads to an accelerated aging and eventually results in death at 2–3 months, a survival of only a tenth of normal mouse life span, regardless of how klotho deficiency was induced.
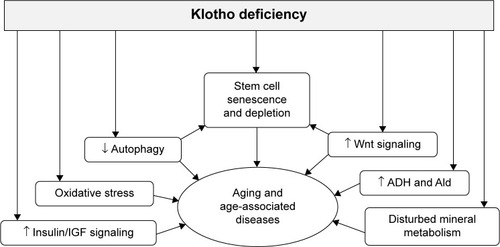
In addition to shortened life span, klotho-deficient mice demonstrated growth retardation; decreased physical activity; premature thymic involution; ectopic calcification; arteriosclerosis; pulmonary emphysema; osteoporosis; atrophy of skin, intestine, spleen, and gonads; and lipodystrophy.Citation1 Early sudden death may result from cardiac arrhythmia or failure to respond properly to stress, possibly because of sinoatrial node dysfunction.Citation43 Disturbed mineral metabolism is a prominent abnormal feature, including hypercalcemia, hyperphosphatemia, and hypervitaminosis D.Citation1,Citation12 The correction of phosphate and vitamin D levels rescues most of the premature aging phenotypes observed in klotho-deficient mice,Citation40,Citation41 indicating that klotho may suppress aging in mammals in large part through maintenance of mineral homeostasis or that mineral dysregulation (phosphate toxicity) may also play a causal role in the premature aging process (). Interestingly, chronic kidney disease (CKD) in both human and animals has low levels of renal and circulating klotho and shares many clinical manifestations with klotho-deficient mice,Citation44–Citation46 suggesting that CKD may be an accelerated aging syndrome.Citation47
Figure 2 Potential effects of klotho deficiency on stem cell depletion and the aging process.
Klotho deficiency and cellular senescence
Cell senescence refers to physiological, structural, biochemical, and molecular changes that occur progressively during aging, culminating in the permanent cessation of cell division. Senescence is characterized by altered cellular morphology, increased activity of senescence-associated β-galactosidase, increased formation of senescence-associated heterochromatin foci and p16, permanent DNA damage, and chromosomal instability.Citation48–Citation52 As a state of permanent inhibition of cell proliferation, cellular senescence initiates and promotes chronic inflammation in multiple age-related chronic diseases, such as obesity, diabetes, atherosclerosis, Alzheimer’s disease, cancer, kidney disease, and degenerative disease.Citation53–Citation57
It is recognized that oxidative and genotoxic stress and mitochondrial dysfunction activate the senescence response by stimulating two pathways: p53/p21 and p16 pathways.Citation51,Citation52 The p53 protein is a tumor suppressor and can be activated by the ataxia telangiectasis-mutated kinase, and in turn activates p21 that effectively arrests cell proliferation and induces irreversible cell-cycle arrest in the G1- to S-phase transition. On the other hand, p16 is a tumor suppressor and another master regulator of cellular senescenceCitation58 that can be activated through p38 MAPK, enhancing senescence at later times.Citation49,Citation51,Citation59,Citation60 Klotho deficiency upregulates p53/p21 expression and increases the number of senescent cells,Citation61 which can be attenuated by either knockdown of p53 or p21. Supplementation of klotho reduces H2O2-induced cell senescence and apoptosis through suppression of the p53/p21 signaling pathway.Citation62 Klotho deficiency-induced cell senescence exacerbates endothelial damage and kidney-cell apoptosis triggered by oxidative stress.Citation62,Citation63
Klotho deficiency and autophagy
Autophagy is a “self-eating” process to maintain homeostasis. This process involves the sequestration of cytoplasmic components in double-membrane autophagosomes. Autophagy dysfunction is implicated in a variety of physiological and pathological processes, such as infection, cancer, metabolic and neurodegenerative disorders, and cardiovascular and pulmonary diseases, as well as physical exercise and aging.Citation64–Citation66 Whether klotho deficiency affects autophagy activity is a very important subject to be addressed.
In klotho-deficient mice, there is severe atrophy of skeletal muscle and activation of the autophagic-lysosomal pathway. Moreover, the signaling activity of mTOR, a suppressor of autophagy, is suppressed, presumably due to deficiency of essential amino acids in the klotho-deficient mice.Citation67 Consistent with these findings in muscle, augmentation of autophagic markers, cleavage of light chain 3 (LC3), and autophagic ultrastructural alterations were found in the brain of klotho-deficient mice, similarly to those found in aged wild-type animals.Citation68
In contrast, some experiments have shown stimulatory effects of klotho on autophagy in tumor cells.Citation69,Citation70 Tumor cells have low abundance of klotho expression and downregulation of autophagy. The restoration of klotho regulates IGF-1 receptor phosphorylation, activates downstream Akt-p70S6K and ERK signaling and autophagy, and subsequently suppresses tumor-cell proliferation and induces apoptosis.Citation69 We recently found that transgenic mice overexpressing klotho had more cleaved LC3 and low levels of p62 in the kidney (unpublished data). Furthermore, an in vitro experiment showed that klotho induced puncta patterns of LC3 in cultured kidney cells, suggesting that klotho could activate autophagy flux in vivo and in vitro. It has been shown that defective autophagy predisposes the kidney to readily develop AKI after an ischemic insult and to more severe kidney impairment in senior versus general populations. Therefore, klotho-upregulated autophagy flux may be attributable to klotho-mediated renoprotection.
Aging of stem cells
Stem cells exist in most mammalian organs or tissues to maintain tissue homeostasis and participate in tissue repair or regeneration.Citation71,Citation72 Stem cells exhibit functional age-related changes, which manifest the declined homeostatic and regenerative activities of aging tissues.Citation73–Citation75 While some of the functional changes of stem cells arise intrinsically, others are imposed by age-related changes in the microenvironment or niche. The intrinsic changes have been observed at genomic, epigenomic, and proteomic levels. Some of the changes are potentially reversible, which may result in the rejuvenation of aged stem cells.
With aging, stem cells exhibit a diminished capacity of self-renewal and proliferation, which results in increased apoptosis or senescence in the stem cell compartment and depletion of functional stem cells.Citation76 Furthermore, stem cells show changes in lineage commitment. The cell fate is determined largely by the epigenome and mediated by various signaling pathways.Citation77–Citation83 Aging can alter the epigenome and pathways, which may lead to aberrant lineage specification of stem cell progeny, as demonstrated in such tissues as the skeletal muscle, tendon, and hematopoietic system.Citation84–Citation87 Accumulation of these abnormal progeny contributes to the gradual deterioration of tissue structure and function associated with aging. On the other hand, accumulation of DNA mutations is a common feature of stem cell aging. Elevated levels of DNA damage have been reported in aging epidermal stem cells and hematopoietic stem cells.Citation88,Citation89
With aging, the number of stem cells declines significantly in some cases.Citation90,Citation91 For example, depletion of melanocyte stem cells in the hair follicles and the appearance of mature pigmented melanocytes in the stem cell niche have been reported in both aged mice and humans,Citation92,Citation93 leading to one of the most visible phenotypic changes during aging – hair graying. Aging or genotoxic stress induces the accumulation of DNA damage in melanocyte stem cells that results in the loss of stem cell self-renewal.Citation92 Depletion of neural stem cells, possibly also related to a specific loss of capacity for self-renewal, appears to be responsible for declining neurogenesis with age.Citation94–Citation96 However, in other cases, stem cells do not show a significant depletion with age.Citation97,Citation98
As a self-renewing cell population to assure proper function and normal tissue homeostasis across the life span, stem cells are more resistant to the same factors that lead to age-related changes in their replicative or postmitotic progeny.Citation73,Citation99,Citation100 During DNA replication, aging-related mechanisms, such as telomere shortening, chromosome rearrangements, and single-base mutations,Citation101 can occur and ultimately lead to cellular senescence.Citation102 Stem cells possess defense and repair mechanisms that are relevant to both highly proliferative cells and to long-lived postmitotic cells.Citation100 It has been observed that adult stem cells, particularly those in continuously renewing tissues, undergo many rounds of cell division to maintain normal tissue homeostasis.Citation77 It has also been observed that telomeres in old stem cells are still longer than those in the other somatic cells in these tissues,Citation103,Citation104 as observed in the skin, small intestine, cornea, testis, and brain.Citation104 These observations suggest that stem cells divide at a much slower rate than their proliferative progeny or that they have evolved mechanisms to protect against telomere shortening.
A distinction among intrinsic irreversible changes (eg, genomic mutations), intrinsic reversible changes (eg, epigenomic alterations), and extrinsic influences from the microenvironment or niche in stem cells is important in studying stem cell aging. A mechanistic understanding of stem cell aging will contribute greatly to stem cell therapeutics for diseases and disorders of aging.
Klotho deficiency induces stem cell senescence and depletion
As discussed earlier, adult tissue stem cells have the ability to adjust to environmental changes, affect the proliferation of neighboring cells, and consequently participate in tissue maintenance and regeneration. Aging impedes stem cell renewal and proliferation. Any factor that interferes with the capacity of stem cells to self-renew, proliferate, differentiate, and replace in adult tissues could accelerate aging ().Citation16,Citation105–Citation111 Therefore, dysfunction and depletion of stem cells and progenitor cells contribute to aging. Emerging data showed that some aging-related characteristics in klotho-deficient mice may result from senescence and/or depletion of stem cells or differentiation of stem cells to promote fibrosis.Citation18,Citation85
In the premature aging observed in klotho-deficient mice, skin atrophy is one of the gross phenotypes.Citation1 The klotho-deficient mice had sparser hair than control mice. Histological examinations of the skin revealed a reduction in the number of hair follicles, and reduced dermal and epidermal thickness. The subcutaneous fat was barely detectable. Furthermore, there were a decreased number of stem cells, increased progenitor-cell senescence, and dramatic augmentation of Wnt protein and signaling activity in the skin,Citation18 indicating that klotho is required for maintenance of both stem cell number and function. A coimmunoprecipitation study indicated that soluble klotho binds to various Wnt family members, including Wnt1, Wnt3, Wnt4, and Wnt5a, suppresses Wnt transcription, and inhibits Wnt biological activity in the skin. An overexpression of klotho effectively antagonizes the activity of endogenous and exogenous Wnt, which induces accelerated cell senescence both in vitro and in vivo.Citation18 Therefore, overexpression of Wnt proteins may be one of the pathogenic factors to be implicated in aging, and klotho is a secreted Wnt antagonist. In the same context of Wnt effect on skin atrophy, enhanced Wnt activity is present in degenerative skeletal muscle of aged mice, which may have affected regeneration of skeletal muscle, impaired repair, and consequently increased tissue fibrosis. All of those alterations are associated with differentiation of muscular stem cells (satellite cells) from a myogenic to a fibrogenic lineage, whose conversion results from the activation of the canonical Wnt signaling pathway in aged myogenic progenitors and can be suppressed by Wnt inhibitors, including sFRP3 and DKK1. Therefore, the Wnt signaling pathway promotes muscular stem cell aging and increased tissue fibrosis.Citation85 As a Wnt signaling antagonist, klotho conceivably rescues myogenic stem cells, improves muscle repair, and suppresses fibrosis. Therefore, klotho may be a promising therapeutic target for muscle regeneration and muscular dystrophies.
In contrast, a mouse model of X-linked hypophosphatemia with deficient Wnt coreceptor low-density lipoprotein receptor-related protein 6 and consequent reduced Wnt signaling did not alter FGF23-induced phosphaturia or reduced mineralization of the bone, suggesting a potential Wnt-independent pathway of phosphate homeostasis.Citation112
Klotho replacement as a possible antiaging strategy
Given that changes of functionality and a decreased number of stem cells contribute to or accelerate aging, implantation of stem cells to replenish new functional stem cells would be one means to attenuate age-associated disease by rebuilding the tissue or organ. This has been shown to be effective in preclinical and clinical trials in some diseases, including multiple sclerosis,Citation113 myocardial infarction,Citation114 ischemic stroke,Citation115 and cancer,Citation116 and even for patients undergoing plastic surgery.Citation108 However, long-term side effects of stem cell implantation are not fully recognized, and should be a concern in most cases in which stem cells are permanently injected into patients.Citation108,Citation115 For example, recipients of genetically altered bone marrow transplants developed leukemia years after their allegedly successful transplants had cured their severe combined immunodeficiency. Despite potential side effects, recent advances in stem cell research and technology have shown promise.
On the other hand, activation or stimulation of endogenous or resident stem cells is another strategy to abate aging and age-associated disease. Current data from animal and in vitro cell-culture studies clearly demonstrated that klotho deficiency is associated with stem cell senescence and depletion. Furthermore, klotho deficiency may not only be a trigger for aging but also a pathogenic intermediate for accelerated aging and development of age-associated diseases, including Alzheimer’s disease, hypertension, osteoporosis, cardiovascular disease, and CKD. Conceivably, any therapy that restores or stimulates endogenous klotho or administration of exogenous klotho might provide a novel treatment strategy for aging and age-associated diseases.
Administration of exogenous klotho as a therapeutic agent
To date, klotho gene delivery is shown to effectively rescue many phenotypes observed in klotho-deficient mice, prolonging life span,Citation117 attenuating the progression of hypertension and kidney damage in spontaneous hypertensive rats,Citation118,Citation119 ameliorating angiotensin II-induced kidney injury,Citation120 improving endothelial function,Citation121 and protecting from uremic cardiomyopathy.Citation122 Although gene therapy is effective in animal studies, its safety is still questionable, and clinical application is not in proximity. There are few clinical trials testing gene therapy in specific diseases, usually genetic diseases, such as X-linked severe combined immunodeficiency,Citation123 cancer treatment, or delivery of vaccines.Citation124
Compared to viral delivery of the klotho gene in animals, administration of exogenous klotho protein is a safer, easier, and more direct modality to restore endocrine klotho deficiency. Similarly to the use of erythropoietin or erythropoiesis-stimulating agents to correct anemia in CKD patients and insulin to maintain normal glucose metabolism in type I diabetes, the administration of exogenous klotho protein may be a viable and effective option in the near future to dwindle aging. Klotho protein can potentially reverse or retard stem cell depletion and abate age-associated pathological processes.
To date, no studies of klotho protein administration in humans have been reported. In contrast, animal studies have already provided convincing and encouraging data to support the proof of concept that soluble klotho protein administration is safe and effective.Citation11 We showed that soluble klotho protein attenuates kidney damage and preserves kidney function in an ischemia–reperfusion injury model causing acute kidney injury, which is a state of acute klotho deficiency.Citation11 Furthermore, klotho protein inhibited renal fibrosis in a unilateral ureteral obstruction kidney-injury model, which is also a state of low klotho expression in the kidney.Citation125 Interestingly, intraperitoneal injection every other day of soluble klotho protein effectively extended the life span of homozygous klotho-deficient mice, ameliorated premature aging-related phenotypes, such as growth retardation, premature thymus involution, and vascular calcification, and effectively reduced cellular senescence.Citation126 Therefore, the preclinical data clearly support the therapeutic potential of soluble klotho protein for age-related disorders and klotho deficiency-associated diseases.
Activation of endogenous klotho expression
In the context of acute kidney injury, the renal tubules, where endogenous klotho is produced, are not fully destroyed, but are suppressed in their ability to produce klotho protein. In this context, strategies to increase endogenous production will be of therapeutic benefit. In particular, while klotho gene delivery is not yet implemented and klotho protein not yet available for clinical use, upregulation of endogenous klotho expression is of high clinical relevance.
To date, several categories of drugs in the market, including a PPARγ agonist,Citation127–Citation130 angiotensin II type I receptor antagonist (losartan),Citation131–Citation133 HMG-CoA reductase inhibitors (statin),Citation134 and vitamin D active derivatives,Citation135–Citation138 have been shown to be effective in upregulating klotho expression in vivo and in vitro. The effect of upregulating klotho is definitely not associated with those drugs well-identified original pharmacologic targets.
Antioxidants and free radical scavengers may not directly play a role in the modulation of klotho expression. However, they can ameliorate oxidative stress, which is linked to aging and suppression of klotho expression in the kidney and cultured kidney cells.Citation139,Citation140 Klotho deficiency in turn increases oxidative stress and makes cells more susceptible to oxidative stress. Therefore, antioxidants are potentially useful in interrupting this spiral deterioration by upregulating klotho production and thus exerting antioxidant properties.Citation141,Citation142
Conclusion and future directions
Since klotho was serendipitously identified in 1997, our understanding of it as an aging suppressor has been continuously growing. Klotho protein has pleiotropic actions on many organs and tissues in mammals. However, very limited and premature data about klotho effects on stem cells are available. A better understanding of the effects of klotho on stem cells not only provides novel insights into the role of stem cells in antiaging processes but could also make a significant contribution to the advancement of regenerative medicine clinical practice.
Animal models have clearly demonstrated that klotho deficiency induces shortened life span and accelerated aging.Citation1 Epidemiological data have also shown that soluble klotho is lower in elder than young adults, and that levels of soluble klotho are inversely correlated with age,Citation143–Citation145 indicating that aging is associated with klotho decline. Nonetheless, we do not know whether low soluble klotho is a prognostic biomarker for risk of earlier death in humans. Although we still do not fully understand why klotho is reduced with aging, maintenance of klotho levels by stimulation of endogenous klotho production or administration of exogenous klotho protein could be considered a potential therapeutic target to retard aging and attenuate age-associated diseases.
Thus far, animal experiments and in vitro cell-culture studies have shown the effects of soluble klotho protein on abating skin atrophy and skeletal muscle dystrophy during aging. It is anticipated that soluble klotho may play a pivotal role in regenerative medicine by preservation and activation of stem cells, particularly in heart tissue, where stem cells are very scarce or have low ability to replicate after injury. Therefore, if soluble klotho can activate stem cells or induce the replication of stem cells, klotho protein could be used as a promising therapeutic strategy for tissue repair and organ regeneration.
Acknowledgments
The authors acknowledge Drs Orson W Moe and Makoto Kuro-o for long-term collaborative work and support. MCH is in part supported by the NIH (R01-DK091392, R01-DK092461) and the Charles and Jane Pak Research Foundation, AB is in part supported by a Visiting Scholar Award from the National Natural Science Foundation of China (81170660H0509, 81270408H0220) and the Provincial Natural Science Foundation of Jiangsu, People’s Republic of China (BK2011849), and JAN is in part supported by the Ben J Lipps Research Fellowship Program of the American Society of Nephrology Foundation for Kidney Research and the Truelson Fellowship Fund at the Charles and Jane Pak Center of Mineral Metabolism and Clinical Research.
Disclosure
The authors report no conflicts of interest in this work.
References
- Kuro-oMMatsumuraYAizawaHMutation of the mouse klotho gene leads to a syndrome resembling ageingNature1997390665545519363890
- KurosuHYamamotoMClarkJDSuppression of aging in mice by the hormone klothoScience200530957421829183316123266
- MasudaHChikudaHSugaTKawaguchiHKuro-oMRegulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant miceMech Ageing Dev2005126121274128316144705
- Shiraki-IidaTAizawaHMatsumuraYStructure of the mouse klotho gene and its two transcripts encoding membrane and secreted proteinFEBS Lett19984241–26109537505
- OhyamaYKurabayashiMMasudaHMolecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stressBiochem Biophys Res Commun199825139209259791011
- MatsumuraYAizawaHShiraki-IidaTNagaiRKuro-oMNabeshimaYIdentification of the human klotho gene and its two transcripts encoding membrane and secreted klotho proteinBiochem Biophys Res Commun199824236266309464267
- KatoYArakawaEKinoshitaSEstablishment of the anti-klotho monoclonal antibodies and detection of klotho protein in kidneysBiochem Biophys Res Commun2000267259760210631108
- ChenCDTungTYLiangJIdentification of cleavage sites leading to the shed form of the anti-aging protein klothoBiochemistry201453345579558725110992
- ChenCDPodvinSGillespieELeemanSEAbrahamCRInsulin stimulates the cleavage and release of the extracellular domain of klotho by ADAM10 and ADAM17Proc Natl Acad Sci U S A200710450197961980118056631
- BlochLSineshchekovaOReichenbachDKlotho is a substrate for α-, β- and γ-secretaseFEBS Lett2009583193221322419737556
- HuMCShiMZhangJQuinonesHKuro-oMMoeOWKlotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protectiveKidney Int201078121240125120861825
- HuMCShiMZhangJKlotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubuleFASEB J20102493438345020466874
- ImuraAIwanoATohyamaOSecreted klotho protein in sera and CSF: implication for post-translational cleavage in release of klotho protein from cell membraneFEBS Lett20045651–314314715135068
- SembaRDMoghekarARHuJKlotho in the cerebrospinal fluid of adults with and without Alzheimer’s diseaseNeurosci Lett2014558374024211693
- HuMCShiMZhangJKlotho deficiency causes vascular calcification in chronic kidney diseaseJ Am Soc Nephrol201122112413621115613
- BarkerSLPastorJCarranzaDThe demonstration of αklotho deficiency in human chronic kidney disease with a novel synthetic antibodyNephrol Dial Transplant201530222323325324355
- HuangCLRegulation of ion channels by secreted klotho: mechanisms and implicationsKidney Int2010771085586020375979
- LiuHFergussonMMCastilhoRMAugmented Wnt signaling in a mammalian model of accelerated agingScience2007317583980380617690294
- GoetzRNakadaYHuMCIsolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-klotho complex formationProc Natl Acad Sci U S A2010107140741219966287
- CarpenterTOInsognaKLZhangJHCirculating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid statusJ Clin Endocrinol Metab20109511E352E35720685863
- ChangQHoefsSvan der KempAWTopalaCNBindelsRJHoenderopJGThe β-glucuronidase klotho hydrolyzes and activates the TRPV5 channelScience2005310574749049316239475
- TohyamaOImuraAIwanoAKlotho is a novel β-glucuronidase capable of hydrolyzing steroid beta-glucuronidesJ Biol Chem2004279119777978414701853
- LuPBorosSChangQBindelsRJHoenderopJGThe β-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6Nephrol Dial Transplant200823113397340218495742
- WolfMTAnSWNieMBalMSHuangCLKlotho up-regulates renal calcium channel transient receptor potential vanilloid 5 (TRPV5) by intra- and extracellular N-glycosylation-dependent mechanismsJ Biol Chem201428952358493585725378396
- ChaSKOrtegaBKurosuHRosenblattKPKuroOMHuangCLRemoval of sialic acid involving klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1Proc Natl Acad Sci U S A2008105289805981018606998
- ChaSKHuMCKurosuHKuro-oMMoeOHuangCLRegulation of renal outer medullary potassium channel and renal K(+) excretion by klothoMol Pharmacol2009761384619349416
- FischerSSKempeDSLeibrockCBHyperaldosteronism in klotho-deficient miceAm J Physiol Renal Physiol20102995F1171F117720719979
- TammaGGoswamiNReichmuthJDe SantoNGValentiGAquaporins, vasopressin, and aging: current perspectivesEndocrinology2015156377778825514088
- TangCPathareGMichaelDFajolAEichenmüllerMLangFDownregulation of klotho expression by dehydrationAm J Physiol Renal Physiol20113014F745F75021734097
- AlmilajiASopjaniMElviraBUpregulation of the creatine transporter Slc6A8 by klothoKidney Blood Press Res201439651652525531216
- AlmilajiAPakladokTMuñozCElviraBSopjaniMLangFUpregulation of KCNQ1/KCNE1 K+ channels by klothoChannels (Austin)20148322222924457979
- AlmilajiAHonischSLiuGRegulation of the voltage gated k channel kv1.3 by recombinant human klotho proteinKidney Blood Press Res201439660962225571875
- MunozCPakladokTAlmilajiAKlotho sensitivity of the hERG channelFEBS Lett2013587111663166823603386
- VandenbergJIPerryMDPerrinMJMannSAKeYHillAPhERG K(+) channels: structure, function, and clinical significancePhysiol Rev20129231393147822988594
- SatohMNagasuHMoritaYYamaguchiTPKanwarYSKashiharaNKlotho protects against mouse renal fibrosis by inhibiting Wnt signalingAm J Physiol Renal Physiol201230312F1641F165123034937
- RavikumarPYeJZhangJα-Klotho protects against oxidative damage in pulmonary epitheliaAm J Physiol Lung Cell Mol Physiol20143077L566L57525063799
- PanessoMCShiMChoHJKlotho has dual protective effects on cisplatin-induced acute kidney injuryKidney Int201485485587024304882
- OhnishiMRazzaqueMSDietary and genetic evidence for phosphate toxicity accelerating mammalian agingFASEB J20102493562357120418498
- MorishitaKShiraiAKubotaMThe progression of aging in klotho mutant mice can be modified by dietary phosphorus and zincJ Nutr2001131123182318811739863
- OhnishiMNakataniTLanskeBRazzaqueMSReversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1α-hydroxylaseKidney Int200975111166117219225558
- RazzaqueMSLanskeBHypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant miceTrends Mol Med200612729830516731043
- LindbergKAminRMoeOWThe kidney is the principal organ mediating klotho effectsJ Am Soc Nephrol201425102169217524854271
- TakeshitaKFujimoriTKurotakiYSinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expressionCirculation2004109141776178215037532
- HuMCShiizakiKKuro-oMMoeOWFibroblast growth factor 23 and klotho: physiology and pathophysiology of an endocrine network of mineral metabolismAnnu Rev Physiol20137550353323398153
- HuMCKuro-oMMoeOWSecreted klotho and chronic kidney diseaseAdv Exp Med Biol201272812615722396167
- HuMCKuro-oMMoeOWKlotho and chronic kidney diseaseContrib Nephrol2013180476323652549
- StenvinkelPLarssonTEChronic kidney disease: a clinical model of premature agingAm J Kidney Dis201362233935123357108
- BeltramiAPCesselliDBeltramiCAStem cell senescence and regenerative paradigmsClin Pharmacol Ther2012911212922089268
- QianYChenXSenescence regulation by the p53 protein familyMethods Mol Biol2013965376123296650
- SalamaRSadaieMHoareMNaritaMCellular senescence and its effector programsGenes Dev20142829911424449267
- BittoACroweEPLernerCTorresCSellCThe senescence arrest program and the cell cycleMethods Mol Biol2014117014515424906313
- Muñoz-EspínDSerranoMCellular senescence: from physiology to pathologyNat Rev Mol Cell Biol201415748249624954210
- CampisiJAndersenJKKapahiPMelovSCellular senescence: a link between cancer and age-related degenerative disease?Semin Cancer Biol201121635435921925603
- OlivieriFRecchioniRMarcheselliFCellular senescence in cardiovascular diseases: potential age-related mechanisms and implications for treatmentCurr Pharm Des20131991710171923061728
- CampisiJRobertLCell senescence: role in aging and age-related diseasesInterdiscip Top Gerontol201439456124862014
- TianXLLiYEndothelial cell senescence and age-related vascular diseasesJ Genet Genomics201441948549525269674
- ZhuYArmstrongJLTchkoniaTKirklandJLCellular senescence and the senescent secretory phenotype in age-related chronic diseasesCurr Opin Clin Nutr Metab Care201417432432824848532
- LiQZhangYFuJFOXA1 mediates p16(INK4a) activation during cellular senescenceEMBO J201332685887323443045
- PrieurABesnardEBabledALemaitreJMp53 and p16(INK4A) independent induction of senescence by chromatin-dependent alteration of S-phase progressionNat Commun2011247321915115
- LeontievaOVBlagosklonnyMVCDK4/6-inhibiting drug substitutes for p21 and p16 in senescence: duration of cell cycle arrest and MTOR activity determine geroconversionCell Cycle201312183063306923974099
- de OliveiraRMKlotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathwayFEBS Lett2006580245753575817014852
- IkushimaMRakugiHIshikawaKAnti-apoptotic and anti-senescence effects of klotho on vascular endothelial cellsBiochem Biophys Res Commun2006339382783216325773
- MaekawaYOhishiMIkushimaMKlotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathwayGeriatr Gerontol Int201111451051621518171
- SchneiderJLCuervoAMLiver autophagy: much more than just taking out the trashNat Rev Gastroenterol Hepatol201411318720024192609
- CuervoAMWongEChaperone-mediated autophagy: roles in disease and agingCell Res20142419210424281265
- ChoiAMRyterSWLevineBAutophagy in human health and diseaseN Engl J Med2013368765166223406030
- IidaRHKankoSSugaTMoritoMYamaneAAutophagic-lysosomal pathway functions in the masseter and tongue muscles in the klotho mouse, a mouse model for agingMol Cell Biochem20113481–2899821082218
- ShiozakiMYoshimuraKShibataMMorphological and biochemical signs of age-related neurodegenerative changes in klotho mutant miceNeuroscience2008152492494118343589
- ShuGXieBRenFRestoration of klotho expression induces apoptosis and autophagy in hepatocellular carcinoma cellsCell Oncol (Dordr)201336212112923248036
- XieBZhouJShuGRestoration of klotho gene expression induces apoptosis and autophagy in gastric cancer cells: tumor suppressive role of klotho in gastric cancerCancer Cell Int20131311823432957
- LiLCleversHCoexistence of quiescent and active adult stem cells in mammalsScience2010327596554254520110496
- WeissmanILStem cells: units of development, units of regeneration, and units in evolutionCell2000100115716810647940
- JonesDLRandoTAEmerging models and paradigms for stem cell ageingNat Cell Biol201113550651221540846
- DorshkindKMontecino-RodriguezESignerRAThe ageing immune system: is it ever too old to become young again?Nat Rev Immunol200991576219104499
- BellDRVan ZantGStem cells, aging, and cancer: inevitabilities and outcomesOncogene200423437290729615378089
- RuzankinaYPinzon-GuzmanCAsareADeletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell lossCell Stem Cell20071111312618371340
- van der FlierLGCleversHStem cells, self-renewal, and differentiation in the intestinal epitheliumAnnu Rev Physiol20097124126018808327
- RittiéLStollSWKangSVoorheesJJFisherGJHedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skinAging Cell20098673875120050020
- BrackASConboyIMConboyMJShenJRandoTAA temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesisCell Stem Cell200821505918371421
- BergerSLThe complex language of chromatin regulation during transcriptionNature2007447714340741217522673
- HembergerMDeanWReikWEpigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canalNat Rev Mol Cell Biol200910852653719603040
- MikkelsenTSKuMJaffeDBGenome-wide maps of chromatin state in pluripotent and lineage-committed cellsNature2007448715355356017603471
- BernsteinBEMikkelsenTSXieXA bivalent chromatin structure marks key developmental genes in embryonic stem cellsCell2006125231532616630819
- ZhouZAkinbiyiTXuLTendon-derived stem/progenitor cell aging: defective self-renewal and altered fateAging Cell20109591191520569237
- BrackASConboyMJRoySIncreased Wnt signaling during aging alters muscle stem cell fate and increases fibrosisScience2007317583980781017690295
- Taylor-JonesJMMcGeheeRERandoTALecka-CzernikBLipschitzDAPetersonCAActivation of an adipogenic program in adult myoblasts with ageMech Ageing Dev2002123664966111850028
- SudoKEmaHMoritaYNakauchiHAge-associated characteristics of murine hematopoietic stem cellsJ Exp Med200019291273128011067876
- SotiropoulouPACandiAMascréGBcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell deathNat Cell Biol201012657258220473297
- RossiDJBryderDSeitaJNussenzweigAHoeijmakersJWeissmanILDeficiencies in DNA damage repair limit the function of haematopoietic stem cells with ageNature2007447714572572917554309
- MaslovAYBaroneTAPlunkettRJPruittSCNeural stem cell detection, characterization, and age-related changes in the subventricular zone of miceJ Neurosci20042471726173314973255
- KuhnHGDickinson-AnsonHGageFHNeurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferationJ Neurosci1996166202720338604047
- InomataKAotoTBinhNTGenotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiationCell200913761088109919524511
- NishimuraEKGranterSRFisherDEMechanisms of hair graying: incomplete melanocyte stem cell maintenance in the nicheScience2005307571072072415618488
- RenaultVMRafalskiVAMorganAAFoxO3 regulates neural stem cell homeostasisCell Stem Cell20095552753919896443
- NishinoJKimIChadaKMorrisonSJHmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf expressionCell2008135222723918957199
- MolofskyAVSlutskySGJosephNMIncreasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageingNature2006443711044845216957738
- GiangrecoAQinMPintarJEWattFMEpidermal stem cells are retained in vivo throughout skin agingAging Cell20087225025918221414
- BrackASRandoTAIntrinsic changes and extrinsic influences of myogenic stem cell function during agingStem Cell Rev20073322623717917136
- SharplessNEDePinhoRAHow stem cells age and why this makes us grow oldNat Rev Mol Cell Biol20078970371317717515
- RandoTAStem cells, ageing and the quest for immortalityNature200644170971080108616810243
- Ben-PorathIWeinbergRAThe signals and pathways activating cellular senescenceInt J Biochem Cell Biol200537596197615743671
- CampisiJd’Adda di FagagnaFCellular senescence: when bad things happen to good cellsNat Rev Mol Cell Biol20078972974017667954
- WangCJurkDMaddickMNelsonGMartin-RuizCvon ZglinickiTDNA damage response and cellular senescence in tissues of aging miceAging Cell20098331132319627270
- FloresICanelaAVeraETejeraACotsarelisGBlascoMAThe longest telomeres: a general signature of adult stem cell compartmentsGenes Dev200822565466718283121
- BakerNBoyetteLBTuanRSCharacterization of bone marrow-derived mesenchymal stem cells in agingBone201570374725445445
- PengYXuanMLeungVYChengBStem cells and aberrant signaling of molecular systems in skin agingAgeing Res Rev20151982125446806
- FryCSLeeJDMulaJInducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopeniaNat Med2015211768025501907
- SalibianAAWidgerowADAbroukMEvansGRStem cells in plastic surgery: a review of current clinical and translational applicationsArch Plast Surg201340666667524286038
- FlorianCLangmannTWeberBHMorsczeckCMurine Müller cells are progenitor cells for neuronal cells and fibrous tissue cellsBiochem Biophys Res Commun2008374218719118619415
- GopinathSDRandoTAStem cell review series: aging of the skeletal muscle stem cell nicheAging Cell20087459059818462272
- MazzoccoliGTevyMFBorghesanMDelle VerginiMRVinciguerraMCaloric restriction and aging stem cells: the stick and the carrot?Exp Gerontol20145013714824211426
- UchihashiKNakataniTGoetzRMohammadiMHeXRazzaqueMSFGF23-induced hypophosphatemia persists in Hyp mice deficient in the WNT coreceptor Lrp6Contrib Nephrol201318012413723652555
- DerwenskusJLublinFDFuture treatment approaches to multiple sclerosisHandb Clin Neurol201412256357724507535
- LeeSBick-ForresterJMakkarRRForresterJSStem-cell repair of infarcted myocardium: ready for clinical application?Am Heart Hosp J20042210010615604853
- QiaoLYHuangFJZhaoMA two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patientsCell Transplant201423Suppl 1S65S7225333752
- RahmanMHohBKohlerNDunbarEMMuradGJThe future of glioma treatment: stem cells, nanotechnology and personalized medicineFuture Oncol2012891149115623030489
- Shiraki-IidaTIidaANabeshimaYImprovement of multiple pathophysiological phenotypes of klotho (kl/kl) mice by adenovirus-mediated expression of the klotho geneJ Gene Med20002423324210953914
- WangYSunZAntiaging gene klotho regulates endothelin-1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive ratsJ Hypertens20143281629163624979306
- WangYSunZKlotho gene delivery prevents the progression of spontaneous hypertension and renal damageHypertension200954481081719635988
- MitaniHIshizakaNAizawaTIn vivo klotho gene transfer ameliorates angiotensin II-induced renal damageHypertension200239483884311967236
- SaitoYNakamuraTOhyamaYIn vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndromeBiochem Biophys Res Commun2000276276777211027545
- XieJYoonJAnSWKuro-oOMHuangCLSoluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphateJ Am Soc Nephrol20152651150116025475745
- WilliamsDACuring genetic disease with gene therapyTrans Am Clin Climatol Assoc201412512212825125725
- HellerRHellerLCGene electrotransfer clinical trialsAdv Genet20158923526225620013
- DoiSZouYTogaoOKlotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in miceJ Biol Chem2011286108655866521209102
- ChenTHKuroOMChenCHThe secreted klotho protein restores phosphate retention and suppresses accelerated aging in klotho mutant miceEur J Pharmacol20136981–3677323041151
- YangHCDeleuzeSZuoYPotthoffSAMaLJFogoABThe PPARγ agonist pioglitazone ameliorates aging-related progressive renal injuryJ Am Soc Nephrol200920112380238819797472
- ZhangHLiYFanYKlotho is a target gene of PPAR-γKidney Int200874673273918547997
- ZhangRZhengFPPAR-γ and aging: one link through klotho?Kidney Int200874670270418756295
- ChenLJChengMFKuPMLinJWRosiglitazone increases cerebral klotho expression to reverse baroreflex in type 1-like diabetic ratsBiomed Res Int2014201430915124683546
- ZhouQLinSTangRVeeraragooPPengWWuRRole of fosinopril and valsartan on klotho gene expression induced by angiotensin II in rat renal tubular epithelial cellsKidney Blood Press Res201033318619220571281
- YoonHEGheeJYPiaoSAngiotensin II blockade upregulates the expression of klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathyNephrol Dial Transplant201126380081320813770
- KarallieddeJMalteseGHillBVibertiGGnudiLEffect of renin-angiotensin system blockade on soluble klotho in patients with type 2 diabetes, systolic hypertension, and albuminuriaClin J Am Soc Nephrol20138111899190523929932
- NarumiyaHSasakiSKuwaharaNHMG-CoA reductase inhibitors up-regulate anti-aging klotho mRNA via RhoA inactivation in IMCD3 cellsCardiovasc Res200464233133615485693
- LimKLuTSMolostvovGVascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23Circulation2012125182243225522492635
- LauWLLeafEMHuMCVitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate dietKidney Int201282121261127022932118
- ForsterREJurutkaPWHsiehJCVitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cellsBiochem Biophys Res Commun2011414355756221982773
- de BorstMHVervloetMGter WeePMNavisGCross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney diseaseJ Am Soc Nephrol20112291603160921852584
- MitobeMYoshidaTSugiuraHShirotaSTsuchiyaKNiheiHOxidative stress decreases klotho expression in a mouse kidney cell lineNephron Exp Nephrol20051012e67e7415976510
- OhHJNamBYLeeMJDecreased circulating klotho levels in patients undergoing dialysis and relationship to oxidative stress and inflammationPerit Dial Int2015351435124497597
- YamamotoMClarkJDPastorJVRegulation of oxidative stress by the anti-aging hormone klothoJ Biol Chem200528045380293803416186101
- Kuro-oMKlotho as a regulator of oxidative stress and senescenceBiol Chem389323324118177265
- ScholzeALiuYPedersenLSoluble α-klotho and its relation to kidney function and fibroblast growth factor-23J Clin Endocrinol Metab2014995E855E86124606097
- PedersenLPedersenSMBrasenCLRasmussenLMSoluble serum klotho levels in healthy subjects. Comparison of two different immunoassaysClin Biochem201346121079108323707222
- YamazakiYImuraAUrakawaIEstablishment of sandwich ELISA for soluble α-klotho measurement: age-dependent change of soluble α-klotho levels in healthy subjectsBiochem Biophys Res Commun2010398351351820599764
