Abstract
Alzheimer’s disease (AD) is the most common form of dementia. Mutations in the genes encoding presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein have been identified as the main genetic causes of familial AD. To date, more than 200 mutations have been described worldwide in PSEN1, which is highly homologous with PSEN2, while mutations in PSEN2 have been rarely reported. We performed a systematic review of studies describing the mutations identified in PSEN2. Most PSEN2 mutations were detected in European and in African populations. Only two were found in Korean populations. Interestingly, PSEN2 mutations appeared not only in AD patients but also in patients with other disorders, including frontotemporal dementia, dementia with Lewy bodies, breast cancer, dilated cardiomyopathy, and Parkinson’s disease with dementia. Here, we have summarized the PSEN2 mutations and the potential implications of these mutations in dementia-associated disorders.
Introduction
Alzheimer’s disease (AD) is the most common form of neurodegenerative disease of the brain. Pathological hallmarks of AD include intraneuronal accumulation of paired helical filaments composed of abnormal tau proteins and extracellular deposits of β-amyloid peptide (Aβ) in neuritic plaques.Citation1 Clinically, AD can be categorized into two phenotypes based on the ages of onset: early-onset AD (EOAD; <65 years) and late-onset AD (LOAD; >65 years), of which LOAD is the more common form worldwide. The proportion of EOAD in all AD cases is between 5% and 10%.Citation2 Presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein (APP) are mostly associated with autosomal dominant forms of EOAD.Citation3 Apart from genetic factors, mutations are environmentally related. Genetic–environmental interactions may be caused by variation in the age of onset, neuropathological patterns, and disease duration.Citation4 To date, more than 200 mutations have been described in PSEN1 throughout the world, but mutations in PSEN2 are extremely rare. Less than 40 mutations in PSEN2 have been identified.Citation5 From those, two PSEN2 mutations were detected in Korean patients. Unlike PSEN1, AD patients with PSEN2 mutations have a wide range in the age of onset, from 40 to 80 years.Citation6 Interestingly, some reports have suggested that the inherited mode of AD was autosomal inheritance with variable penetrance, which suggests that other environmental factors might also be significant for AD pathogenesis.Citation7 In addition, mutations in PSEN2 are also closely involved in other diseases, including EOAD, LOAD, frontotemporal dementia (FTD), dementia with Lewy bodies (DLBs), breast cancer, dilated cardiomyopathy (DCM). In this review, we studied and summarized PSEN2, in particular, the known PSEN2 mutations and the potential implications of PSEN2 in AD and in other disorders.
PSEN2 gene
In 1995, PSEN2 was initially reported as a causative gene for AD, following the identification of APP and PSEN1.Citation8 The gene was localized to chromosome lq42.13. It consists of 12 exons, of which exon 1 and exon 2 contain the untranslated regions.
PSEN2 transcription
Transcriptional regulation
PSEN2 is driven by two separate promoter elements, P1 and P2, which are located in exon 1 and exon 2, respectively. The upstream P1 is a housekeeping promoter. PSEN2-P1 activity depends on a stimulating protein 1 binding site at the most 5′ initiation site. The downstream P2 is induced by Egr-1, which represses PSEN2-P1 activity.Citation9 Interestingly, a study showed that Egr-1 cannot regulate the PSEN2 promoter in mouse.Citation10 APP influences the expression of Egr-1 by enhancing histone H4 acetylation of the Egr-1 promoter.Citation11
Splice variant
The two isoforms of PSEN2 protein are produced by alternative splicing. An aberrant splice variant of PSEN2 lacks exon 5, which results in the insertion of five amino acids, SSMAG, into the protein variant, and which introduces a premature stop codon in exon 6.Citation12 Aggregation of the PSEN2 variant protein was detected in the hippocampus and cerebral cortex of patients with sporadic AD.Citation13 The protein variant also was detected in sporadic AD patients, in the frontal lobe of patients with bipolar disorder, and in patients with schizophrenia.Citation14,Citation15 The PSEN2 variant is upregulated under hypoxic conditions in cell culture, and a study has shown that the PSEN2 variant influences the conformation of tau protein in human neuroblastoma cells.Citation12,Citation16
PSEN2 protein
Structure
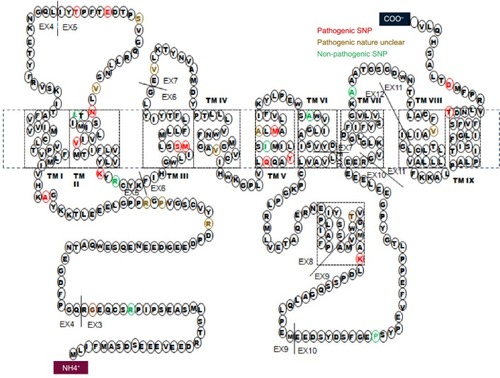
PSEN2 is located on chromosome 1, and it encodes the PSEN2 protein. PSEN2 is a transmembrane protein with 448 amino acids and a molecular weight of 55 Da.Citation17,Citation18 It is predicted to span the lipid bilayer nine times.Citation19 PSEN2 and PSEN1 are homologous, with a similarity of 67%.Citation20 The two proteins differ at the N-terminus and at the hydrophilic loop, while the hydrophobic region is highly conserved. PSEN2 is an unstable holoprotein. It undergoes autocatalytic endoproteolysis within the large cytoplasmic loop domain, to form a stable and biologically active heterodimer. In PSEN2, two aspartyl residues–D263 and D366 found in the adjacent transmembrane regions Transmembrane domain (TM)-VI and TM-VII–are the active sites of the γ-secretase complex.
Location
PSEN2 has two isoforms. Isoform 1 is found in the placenta, skeletal muscle and heart, while isoform 2, which lacks amino acids 263–296, is found in the brain, heart, placenta, liver, skeletal muscle, and kidney. Presenilin proteins that are localized in neurons reside in the endoplasmic reticulum and Golgi.Citation21
Function
Presenilin, an aspartyl protease, is a subunit of γ-secretase. γ-Secretase participates in the cleavage of APP, which can produce different lengths of β-amyloid peptide (Aβ). The Aβ42 form aggregates easier than the Aβ40 form. The accumulation of Aβ in the brain is a pathological characteristic of AD.Citation22 The process of Aβ aggregation is shown in . PSEN2 mutation might increase γ-secretase activity. Cell-based studies and mouse models have shown that some PSEN2 mutations cause an increased production of Aβ42, which is a major hallmark in the brains of patients with AD. Presenilin mutations are a major risk factor for AD.Citation23 Several studies have indicated that AD-related presenilin mutations can alter intracellular calcium signaling, which leads to Aβ aggregation to form brain plaques and neuronal cell death.Citation24,Citation25
Figure 1 The process of Aβ aggregation.
Abbreviations: AICD, APP intracellular domain; APP, amyloid precursor protein; APH-1, anterior pharynx-defective 1; CTFα, C-terminal fragment α; CTFβ, C-terminal fragment β; sAPP, soluble APP; PEN-2, presenilin enhancer 2.
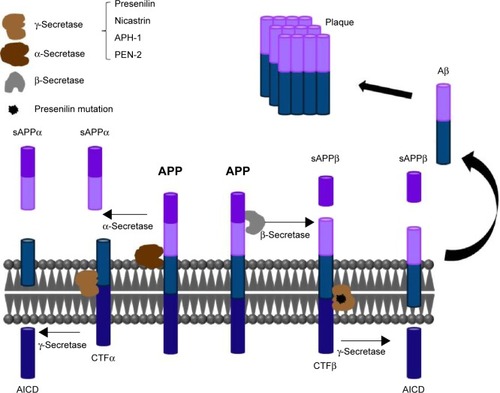
γ-Secretase catalyzes the intramembrane cleavage of integral membrane proteins. It plays an important role in intracellular signaling, including Notch signaling and APP processing. In separate studies published in 1996, Vito et alCitation26 and Wolozin et alCitation27 proposed that PSEN2 is involved in apoptosis. A study demonstrated that wild-type and mutant N141I-PSEN2 trigger p53-dependent apoptosis in HEK293 human cells and in murine neurons.Citation28 In primary rat cortical neurons, PSEN2, overexpression significantly increased susceptibility to staurosporine-induced apoptosis. PSEN2 mutations can promote apoptosis. Bcl-2 can down regulate pro-apoptotic activities, which are induced by PSEN2.Citation29 A recent study suggested that overexpression of human mutant PSEN2 induces changes in glucose metabolism, which is accompanied by a decrease in insulin levels.Citation30
PSEN2 mutations
Mutations in the presenilin genes are the main causes of familial EOAD. Similar to APP, mutant presenilins can enhance Aβ production and contribute to AD development, whereas PSEN2 plays less of a role than PSEN1. An extensive literature search for mutations in PSEN2 was conducted. As of date, 38 mutations have been reported. The number of mutations identified in PSEN1 is greater than five times this number.Citation31 Two PSEN2 mutations, Glu126fs and Lys306fs, are frameshift mutations, and the others are nonsynonymous substitutions (). PSEN2 mutations are associated with variable penetrance and a wide range in the age of disease onset, from 45 to 88.Citation32,Citation33 PSEN2 mutations are associated with both EOAD and LOAD. Only 17 of the 38 are predicted to be disease-causing mutations (). Ten of the mutations are not pathogenic and the others are still unclear. Sixteen mutations are located within transmembrane domains. Cell-based studies suggest that four of these mutations, T122P, N141I, M239I, and M239V, cause an increase in the amount of Aβ peptide.Citation34 The mutations T122R, S130L, and M239I were found to alter calcium signaling.Citation35–Citation37 Most of these mutations were discovered in European and African populations. Until now, only four missense mutations were described in Asian populations: Asn141Tyr was associated with EOAD in a Chinese Han family;Citation37 Gly34Ser was found in a Japanese patient;Citation39 and Arg62Cys and Val214Leu were described in the Korean patients.Citation6
Table 1 PSEN2 mutations
Related diseases
It was well-known that some mutations in PSEN2 cause familial AD, while some PSEN2 mutations are associated with other disorders, including DLB, FTD, breast cancer, DCM, and Parkinson’s disease with dementia (PDD).
Dementia with Lewy body
DLB is a progressive degenerative disease, accounting for 10%–20% of all dementias. The core clinical features of DLB are fluctuating cognition, recurrent visual hallucinations, and motor features of Parkinson’s disease.Citation40 Lewy bodies, an abnormal aggregation of protein, are found throughout the brain of DLB patients and in patients with other brain disorders, including AD and PDD. In 2008, a PSEN2 missense mutation, a C-to-T substitution at the second position of codon 85 leading to an alanine to valine substitution in the transcribed protein, was found in a proband with the clinical phenotype of Lewy body dementia. Neuropathological examination of the proband showed a mass of cortical Lewy bodies and hallmark lesions of AD. In his family, this mutation was identified in six carriers across two generations, with variable clinical presentation. Except for a young family member that was still asymptomatic, all carriers of the A85V mutation developed AD, DLB, or both. None of the patients carried other mutations in AD-related genes. The pathological PSEN1 mutation, A79V, is homologous to the A85V mutation in PSEN2.Citation85 Sequence phylogenetic analysis suggested that the A85 residue is highly conserved. The mutation is located on the N-terminal, cytoplasmic side, adjacent to the TM-I domain that might be critical for the protein function. Overall, it was predicted that the A85V mutation is pathogenic. In all family members with PSEN2 A85V, the genotype of apolipoprotein E (ApoE) was ε3/ε3, which suggests that α-synuclein pathological structures are linked to PSEN2 A85V without affecting the ApoE ε4 allele.Citation52 A PSEN2 mutation, R71W, was reported in a 73-year-old European patient with cognitive impairment and extrapyramidal symptoms, which was likely undiagnosed for DLB. One of the proband’s brothers also carried the R71W mutation and suffered an unspecified type of dementia. The other brother was healthy and did not have a PSEN2 mutation. The R71W mutation was previously identified in AD patients predicted to be possible pathogenic.Citation70 A PSEN2 mutation, R62H, presented in a DLB patient, with no history of neurological diseases, who showed extrapyramidal signs was characterized by a slight left arm rest tremor, bilateral upper limb postural tremor, and bradykinesia on the left side.Citation86 This mutation, located in the N-terminal of PSEN2, is conserved between PSEN1 and PSEN2. Walker et al showed that the R62H mutation did not affect Aβ42 levels or the Aβ42/Aβ40 ratio.Citation34 Guerreiro et al used PolyPhen-2 to show that the R62H variant is likely benign.Citation41 Based on these data, it is highly probably that PSEN2 R62H can be characterized as “not pathogenic”. Since the age of onset in carriers of the R62H mutation is significantly earlier than in affected non-carriers even after correcting for ApoE genotype, the R62H mutation may function as a disease modifier.Citation48
Breast cancer
Breast cancer is the most common malignancy among women in Europe and the US. Two PSEN2 mutations, R62H and R71W, have been identified in patients with breast cancer. The mutations are located in the hydrophilic, N-terminal domain. In HEK293 cells, the R62H and R71W mutations did not affect the levels of the PSEN2-CTF and PSEN2-NTF proteolytic products or the Aβ(42)/Aβ(40) ratio, but did influence PSEN2 stability. Full-length PSEN2 degenerated rapidly. In a study using transgenic Caenorhabditis elegans, the R62H and R71W mutations compromised PSEN2 function in Notch signaling.Citation49 PSEN2 has several potential roles in cancer. Deng et al and Wolozin et al reported that PSEN2 has pro-apoptotic activity.Citation27,Citation87 A study also has shown that PSEN2 can also adjust β-catenin levels and act in a p53-dependent mechanism to regulate cell growth.Citation49 In 2013, a study suggested a significant role for γ-secretase in breast cancer.Citation88
Frontotemporal dementia
FTD, a clinical phenotype of frontotemporal lobar degeneration, is the second most common form of early-onset (<65 years) neurodegenerative disease after AD.Citation89 It is mainly characterized by deterioration of behavior, personality, and language abilities.Citation89,Citation90 The prevalence of FTD is between 10% and 30% of all presenile dementia.Citation91–Citation96 FTD has a number of clinical phenotypes and pathological subtypes.Citation3,Citation97,Citation98 Clinical and molecular overlaps between AD and FTD or FTD-like phenotypes have been reported.Citation99 To date, at least four PSEN2 mutations have been found in FTD patients. In 2010, PSEN2 R62H was found in a 31-year-old patient. The patient’s healthy mother also carried this mutation. The interaction of the H1 MAPT haplotype and the ApoE ε2 allele might function as a protective modifier against FTD, while the H1 MAPT haplotype unaccompanied by the ApoE ε2 might be a risk enhancer for FTD.Citation43,Citation100 These possibilities imply that modifier, suppressor, and enhancer effects of multiple genes may be crucial for genetic analysis.
Dilated cardiomyopathy
DCM is a heart muscle disease in which the heart becomes enlarged and cannot pump blood efficiently. DCM usually leads to heart failure. The causative factor for DCM has not been determined, but DCM in families is genetically linked. In 2006, the PSEN2 S130L mutation was identified in two Caucasian families. It is highly conserved. Several family members with this mutation suffered DCM and heart failure.Citation36 Presenilin is expressed in multiple tissues, including in the heart, and it is required for cardiac development.Citation101–Citation104 Calcium signaling was altered in cultured skin fibroblasts from carriers of the mutation. The PSEN1 D333G also was identified in a DCM patient. Compared to the phenotypes seen in carriers of PSEN1 D333G, the phenotypes are milder in carriers of PSEN2 S130L, and PSEN2 S130L is not associated with heart failure as often. Currently, it is not clear whether γ-secretase activity is related to DCM. The Notch family of proteins is one of the major transcriptional regulators of cardiac growth and development.Citation105 Disordered Notch signaling is associated with valvular abnormalities, syndromic cardiovascular disease, congenital heart disease, and myocyte dysfunction.Citation106 PSEN2 knockout (PS2KO) mice grow normally without cardiac hypertrophy and fibrosis, while cardiac contractility improved.Citation107 PSEN2 plays an important role in cardiac systolic function by modulating Ca2+ signaling.
Parkinson disease with dementia
Parkinson’s disease (PD) was first described by James Parkinson in 1817. PD is a chronic, progressive, neurological disease that results from the destruction of nerve cells in the basal ganglia. The disease mainly affects movement, but as the neurological damage progresses, the disease often affects mental functions. PDD is an impairment in thinking and reasoning that eventually affects many people with PD. A 77-year-old carrier of PSEN2 V191E showed the PDD phenotype characterized by cognitive decline, visual hallucinations, and confusion during the final years of the PD. This PSEN2 mutation is located at a highly conserved amino acid residue in the protein. In a study by Bram Meeus, the V191E mutation did not exist in more than 1,200 control individuals, so he predicted that V191E is a damaging mutation.Citation70 A PSEN2 R163H variant has been reported in a Swedish PD family in who were also found a de novo α-synuclein A53T mutation. The proband’s mother also carried the mutation PSEN2 R163H, but she was healthy. Nevertheless, this mutation cannot be excluded with certainty as a cause of PD when in combination with α-synuclein.Citation67 PSEN2 S130L was identified in a patient with of LOAD, and his two siblings were diagnosed with PD. Unfortunately, the genetic results from the siblings are not available. The S130L mutation was also detected in the proband’s two unaffected children, but the segregation of the disease could not be determined. The correlation between PD and AD is not clear.
Conclusion
This review described mutations in PSEN2 from diverse disorders. Mutations in PSEN2 were shown to be a rare cause of familial AD. Pathogenic mutations in the PSEN1, PSEN2, and APP gene account for 18%–50% of familial EOAD cases with autosomal dominant pattern of inheritance.Citation108 PSEN genetic testing results could provide genetic counseling for patient’s family members. There is a considerable interest in the application of this genetic information in medical practice through genetic testing and counseling. PSEN2 mutations are involved in not only AD but also in other disorders, including FTD, DLB, PDD, breast cancer, and DCM. Why are PSEN2 mutations found in multiple diseases? Are these diseases related? Until now, the answer to this question has been unclear. There are several possible reasons that PSEN is associated with multiple diseases. PSEN2 is a transmembrane protein that is a component of γ-secretase intramembrane protease. γ-Secretase is required to process several types of integral membrane proteins, and is involved in different signaling pathways. Mutations in PSEN2 may disrupt the normal pathways and lead to different disorders. Thus, it can be hypothesized that these disorders might share underlying genetic factors. On the other hand, different neurodegenerative diseases show slightly different behavioral, language, and motor symptoms. Sometimes it is difficult to distinguish them clearly by clinical diagnosis. Many patients with both PDD and DLB have hallmark changes in the brain, including plaques and tangles that are associated with AD. These observations suggest that there may be a common pathogenetic mechanism in the formation of aggregated proteins. Therefore, mutations in PSEN2 might play a role in Aβ, α-synuclein, and tau aggregation.
Overall, genetic studies have already indicated that PSEN2 may affect people with FTD, PDD, LBD, breast cancer, and DCM. How presenilin 2 is implicated in the pathogenesis of these diseases is still unclear. This question needs to be further explored.
Acknowledgments
This manuscript was supported by grants from the Gachon University Gil Medical Center (grant number 2013-30) and the Korea Health Technology R&D Project (HI14C3331) through the Korea Health Industry Development Institute (KHIDI), Ministry of Health & Welfare, Republic of Korea.
Disclosure
The authors report no conflicts of interest in this work.
References
- HardyJA hundred years of Alzheimer’s disease researchNeuron200652131317015223
- BekrisLMYuCEBirdTDTsuangDWGenetics of Alzheimer diseaseJ Geriatr Psychiatry Neurol201023421322721045163
- ŻekanowskiCStyczyńskaMPepłońskaBMutations in presenilin 1, presenilin 2 and amyloid precursor protein genes in patients with early-onset Alzheimer’s disease in PolandExp Neurol2003184299199614769392
- BagyinszkyEYounYCAnSSKimSYThe genetics of Alzheimer’s diseaseClin Interv Aging2014953555124729694
- Alzheimer Disease and Frontotemporal Dementia Mutation Database [homepage on the Internet] Available from: http://www.molgen.ua.ac.be/admutationsAccessed February 16, 2015
- YounYCBagyinszkyEKimHChoiBOAnSSKimSProbable novel PSEN2 Val214Leu mutation in Alzheimer’s disease supported by structural predictionBMC Neurol201414110524885952
- Ertekin-TanerNGenetics of Alzheimer’s disease: a centennial reviewNeurol Clin200725361166717659183
- Levy-LahadEWascoWPoorkajPCandidate gene for the chromosome 1 familial Alzheimer’s disease locusScience199526952269739777638622
- RenbaumPBeeriRGabaiEEgr-1 upregulates the Alzheimer’s disease presenilin-2 gene in neuronal cellsGene200331811312414585504
- Ounallah-SaadHBeeriRGoshenIYirmiyaRRenbaumPLevy-LahadTranscriptional regulation of the murine Presenilin-2 gene reveals similarities and differences to its human orthologueGene20094462818919573580
- HendrickxAPierrotNTasiauxBEpigenetic induction of EGR-1 expression by the amyloid precursor protein during exposure to noveltyPLoS One201389e7430524066134
- SatoNHoriOYamaguchiAA novel presenilin-2 splice variant in human Alzheimer’s disease brain tissueJ Neurochem19997262498250510349860
- SatoNImaizumiKManabeTIncreased production of beta-amyloid and vulnerability to endoplasmic reticulum stress by an aberrant spliced form of presenilin 2J Biol Chem200127632108211411031265
- SmithMJSharplesRAEvinGExpression of truncated presenilin 2 splice variant in Alzheimer’s disease, bipolar disorder, and schizophrenia brain cortexMol Brain Res2004127112813515306129
- SatoHTakeuchiTSakaiKLTemporal cortex activation during speech recognition: an optical topography studyCognition1999733B55B6610585521
- NishikawaAManabeTKatayamaTNovel function of PS2V: change in conformation of tau proteinsBiochem Biophys Res Commun2004318243543815120619
- De StrooperBBeullensMContrerasBPhosphorylation, subcellular localization, and membrane orientation of the Alzheimer’s disease-associated presenilinsJ Biol Chem19972726359035989013610
- LiJXuMZhouHAlzheimer presenilins in the nuclear membrane, interphase kinetochores, and centrosomes suggest a role in chromosome segregationCell19979059179279298903
- LiXDangSYanCStructure of a presenilin family intramembrane aspartate proteaseNature20134937430566123254940
- RademakersRCrutsMVan BroeckhovenCGenetics of early-onset Alzheimer dementiaSci World J20033497519
- AnnaertWGLevesqueLCraessaertsKPresenilin 1 controls γ-secretase processing of amyloid precursor protein in pre-Golgi compartments of hippocampal neuronsJ Cell Biol1999147227729410525535
- De StrooperBIwatsuboTWolfeMSPresenilins and γ-secretase: structure, function, and role in Alzheimer diseaseCold Spring Harb Perspect Med201221a00630422315713
- CitronMWestawayDXiaWMutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic miceNature Med19973167728986743
- LeissringMAYamasakiTRWascoWCalsenilin reverses presenilin-mediated enhancement of calcium signalingProc Natl Acad Sci U S A200097158590859310900016
- LeissringMALaFerlaFMCallamarasNSubcellular mechanisms of presenilin-mediated enhancement of calcium signalingNeurobiol Dis20018346947811442355
- VitoPWolozinBGanjeiJKRequirement of the Familial Alzheimer’s Disease Gene PS2 for Apoptosis OPPOSING EFFECT OF ALG-3Journal of Biological Chemistry19962714931025310288940094
- WolozinBIwasakiKVitoPParticipation of presenilin 2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutationScience19962745293171017138939861
- Da CostaCAPaitelEMattsonMPWild-type and mutated presenilins 2 trigger p53-dependent apoptosis and down-regulate presenilin 1 expression in HEK293 human cells and in murine neuronsProc Natl Acad Sci U S A20029964043404811904448
- ArakiWYuasaKTakedaSPro-apoptotic effect of presenilin 2(PS2) overexpression is associated with down-regulation of Bcl-2 in cultured neuronsJ Neurochem20017961161116811752057
- LeeYJKimJEHwangISAlzheimer’s phenotypes induced by overexpression of human presenilin 2 mutant proteins stimulate significant changes in key factors of glucose metabolism[J]Mol Med Rep2013751571157823546527
- LarnerAJPresenilin-1 mutation Alzheimer’s disease: a genetic epilepsy syndrome?Epilepsy & Behavior2011211202221501974
- BirdTDLevy-LahadEPoorkajPWide range in age of onset for chromosome 1-related familial Alzheimer’s diseaseAnn Neurol19964069329369007102
- SherringtonRFroelichSSorbiSAlzheimer’s disease associated with mutations in presenilin 2 is rare and variably penetrantHum Mol Gen1996579859888817335
- WalkerESMartinezMBrunkanALPresenilin 2 familial Alzheimer’s disease mutations result in partial loss of function and dramatic changes in Aβ 42/40 ratiosJ Neurochem200592229430115663477
- ZattiGBurgoAGiacomelloMPresenilin mutations linked to familial Alzheimer’s disease reduce endoplasmic reticulum and Golgi apparatus calcium levelsCell Calcium200639653955016620965
- LiDParksSBKushnerJDMutations of presenilin genes in dilated cardiomyopathy and heart failureAm J Hum Genet20067961030103917186461
- ZattiGGhidoniRBarbieroLThe presenilin 2 M239I mutation associated with familial Alzheimer’s disease reduces Ca2+ release from intracellular storesNeurobiol Dis200415226927815006697
- NiuFYuSZhangZA novel mutation in the PSEN2 gene (N141Y) associated with early-onset autosomal dominant Alzheimer’s disease in a Chinese Han familyNeurobiol Aging201435102420.e12420.e524838186
- BaiYFTianJQuanWXMaedaKAssociation of mutations of presenilin-2 gene and sporadic Alzheimer’s diseaseJ Chin Med Univ201140357363
- KimTHDiagnosis and management of dementia with Lewy bodiesJ Korean Geriatr Psychiatry20121627581
- GuerreiroRJBaqueroMBlesaRGenetic screening of Alzheimer’s disease genes in Iberian and African samples yields novel mutations in presenilins and APPNeurobiol Aging201031572573118667258
- SleegersKRoksGTheunsJFamilial clustering and genetic risk for dementia in a genetically isolated Dutch populationBrain200412771641164915130954
- BrouwersNSleegersKVan BroeckhovenCMolecular genetics of Alzheimer’s disease: an updateAnn Med200840856258318608129
- Ertekin-TanerNYounkinLHYagerDMPlasma amyloid β protein is elevated in late-onset Alzheimer disease familiesNeurology200870859660617914065
- CrutsMVan DuijnCMBackhovensHEstimation of the genetic contribution of presenilin-1 and -2 mutations in a population-based study of presenile Alzheimer diseaseHum Mol Genet19987143519384602
- GalloMTomainoCPuccioGNovel MAPT Val75Ala mutation and PSEN2 Arg62Hys in two siblings with frontotemporal dementiaNeurol Sci2010311657019768372
- LohmannEGuerreiroRJErginel-UnaltunaNIdentification of PSEN1 and PSEN2 gene mutations and variants in Turkish dementia patientsNeurobiol Aging20123381850.e171850.e2722503161
- CruchagaCChakravertySMayoKRare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer’s disease familiesPLoS One201272e3103922312439
- ToMDGokgozNDoyleTGFunctional characterization of novel presenilin-2 variants identified in human breast cancersOncogene200625253557356416474849
- DobricicVStefanovaEJankovicMGenetic testing in familial and young-onset Alzheimer’s disease: mutation spectrum in a Serbian cohortNeurobiol Aging20123371481.e71481.e1222221884
- WallonDRousseauSRovelet-LecruxAThe French series of autosomal dominant early onset Alzheimer’s disease cases: mutation spectrum and cerebrospinal fluid biomarkersJ Alzheimers Dis201230484785622475797
- PiscopoPMarconGPirasMRA novel PSEN2 mutation associated with a peculiar phenotypeNeurology200870171549155418427071
- FinckhUMüller-ThomsenTMannUHigh prevalence of pathogenic mutations in patients with early-onset dementia detected by sequence analyses of four different genesAm J Hum Genet200066111011710631141
- FinckhUKuschelCAnagnostouliMNovel mutations and repeated findings of mutations in familial Alzheimer diseaseNeurogenetics200562858915776278
- BinettiGSignoriniSSquittiRAtypical dementia associated with a novel presenilin-2 mutationAnn Neurol200354683283614681895
- GiacomelloMBarbieroLZattiGReduction of Ca2+ stores and capacitative Ca2+ entry is associated with the familial Alzheimer’s disease presenilin-2 T122R mutation and anticipates the onset of dementiaNeurobiol Dis200518363864815755689
- El KadmiriNZaidNZaidYNovel presenilin mutations within Moroccan patients with early-onset Alzheimer’s diseaseNeuroscience201426921522224704512
- MüllerUWinterPBolenderCPreviously unrecognized missense mutation E126K of PSEN2 segregates with early onset Alzheimer’s disease in a familyJ Alzheimers Dis201442110911324844686
- TeddeANacmiasBCiantelliMIdentification of new presenilin gene mutations in early-onset familial Alzheimer diseaseArch Neurol200360111541154414623725
- SorbiSTeddeANacmiasBNovel presenilin 1 and presenilin 2 mutations in early-onset Alzheimer’s disease familiesNeurobiol Aging2002231SS312
- TomainoCBernardiLAnfossiMPresenilin 2 Ser130Leu mutation in a case of late-onset “sporadic” Alzheimer’s diseaseJ Neurol2007254339139317345043
- SassiCGuerreiroRGibbsRExome sequencing identifies 2 novel presenilin 1 mutations (p. L166V and p. S230R) in British early-onset Alzheimer’s diseaseNeurobiol Aging201435102422.e132422.e1624880964
- BernardiLTomainoCAnfossiMLate onset familial Alzheim er’s disease: novel presenilin 2 mutation and PS1 E318G polymorphismJ Neurol2008255460460618350357
- RogaevEISherringtonRRogaevaEAFamilial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 geneNature199537665437757787651536
- JayadevSLeverenzJBSteinbartEAlzheimer’s disease phenotypes and genotypes associated with mutations in presenilin 2Brain201013341143115420375137
- LaoJIBeyerKFernández-NovoaLA novel mutation in the predicted TM2 domain of the presenilin 2 gene in a Spanish patient with late-onset Alzheimer’s diseaseNeurogenetics19981429329610732806
- PuschmannARossOAVilariño-GüellCA Swedish family with de novo α-synuclein A53T mutation: Evidence for early cortical dysfunctionParkinsonism Relat Disord200915962763219632874
- ClarimónJGuerreiroRLleóAGenetic screening in a large cohort of early-onset Alzheimer’s disease patients from Spain: novel mutations in the amyloid precursor protein and presenilinesAlzheimers Dement200844T583
- PiscopoPTalaricoGCrestiniAA novel mutation in the predicted TMIII domain of the PSEN2 gene in an Italian pedigree with atypical Alzheimer’s diseaseJ Alzheimers Dis2010201434720164579
- MeeusBVerstraetenACrosiersDDLB and PDD: a role for mutations in dementia and Parkinson disease genes?Neurobiol Aging2012333629.e5629.e1822118943
- MarconGDi FedeGGiacconeGA novel Italian presenilin 2 gene mutation with prevalent behavioral phenotypeJ Alzheimers Dis200916350951119276543
- LeeJHKahnAChengRDisease-related mutations among Caribbean Hispanics with familial dementiaMolecular Genet Genomic Med201425430437
- SassiCGuerreiroRGibbsRInvestigating the role of rare coding variability in Mendelian dementia genes (APP, PSEN1, PSEN2, GRN, MAPT, and PRNP) in late-onset Alzheimer’s diseaseNeurobiol Aging201435122881.e12881.e625104557
- FinckhUAlbericiAAntoniazziMVariable expression of familial Alzheimer disease associated with presenilin 2 mutation M239INeurology200054102006200810822446
- TestiSFabriziGMPompaninSAutosomal dominant Alzheimer’s disease with early frontal lobe involvement associated with the Met239Ile mutation of Presenilin 2 geneJ Alzheimers Dis201231171122531416
- TremolizzoLSusaniEMapelliCFirst report of PSEN2 mutation presenting as posterior cortical atrophyAlzheimer Dis Assoc Disord Epub201479
- MarconGGiacconeGCupidiCNeuropathological and clinical phenotype of an Italian Alzheimer family with M239V mutation of presenilin 2 geneJ Neuropath Exp Neurol200463319920915055444
- CroesEATheunsJHouwing-DuistermaatJJOctapeptide repeat insertions in the prion protein gene and early onset dementiaJ Neurol Neurosurg Psychiatry20047581166117015258222
- LleóACastellvíMBlesaRUncommon polymorphism in the presenilin genes in human familial Alzheimer’s disease: not to be mistaken with a pathogenic mutationNeurosci Lett2002318316616811803125
- LindquistSGHasholtLBahlJMCA novel presenilin 2 mutation (V393M) in early-onset dementia with profound language impairmentEur J Neurol200815101135113918727676
- LindquistSGSchwartzMBatbayliMGenetic testing in familial AD and FTD: mutation and phenotype spectrum in a Danish cohortClin Genet200976220520919659892
- LleóABlesaRQueraltRFrequency of mutations in the presenilin and amyloid precursor protein genes in early-onset Alzheimer disease in SpainArch Neurol200259111759176312433263
- EzquerraMLleóACastellvíMA novel mutation in the PSEN2 gene (T430M) associated with variable expression in a family with early-onset Alzheimer diseaseArch Neurol20036081149115112925374
- LleoABlesaRGendreJA novel presenilin 2 gene mutation (D439A) in a patient with early-onset Alzheimer’s diseaseNeurology200157101926192811723295
- KauweJSKJacquartSChakravertySExtreme cerebrospinal fluid amyloid β levels identify family with late-onset Alzheimer’s disease presenilin 1 mutationAnn Neurol200761544645317366635
- RacitiLNicolettiALe PiraFPresenilin-2 gene mutation presenting as Lewy body dementia?Neurol Sci201132353353421409510
- DengGPikeCJCotmanCWAlzheimer-associated presenilin-2 confers increased sensitivity to poptosis in PC12 cellsFEBS Lett1996397150548941712
- PeltonenHMHaapasaloAHiltunenMKatajaVKosmaVMMannermaaAγ-Secretase components as predictors of breast cancer outcomePLoS One2013811e7924924223915
- NearyDSnowdenJSGustafsonLFrontotemporal lobar degeneration: a consensus on clinical diagnostic criteriaNeurology1998516154615549855500
- HodgesJRFrontotemporal dementia (Pick’s disease): clinical features and assessmentNeurology200156Suppl 4S6S1011402143
- RatnavalliEBrayneCDawsonKThe prevalence of frontotemporal dementiaNeurology200258111615162112058088
- CairnsNJNeuronal intermediate filament inclusion diseaseHandb Clin Neurol20088944344818631766
- SnowdenJSFrontotemporal dementiaBr J Psychiatry200218014014311823324
- RabinoviciGDMillerBLFrontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and managementCNS Drugs201024537539820369906
- BorroniBAlbericiAArchettiSNew insights into biological markers of frontotemporal lobar degeneration spectrumCurr Med Chem201017101002100920156164
- RossoSMRoksGStevensMComplex compulsive behaviour in the temporal variant of frontotemporal dementiaJ Neurol20012481196597011757960
- HodgesJRMillerBThe neuropsychology of frontal variant frontotemporal dementia and semantic dementia. Introduction to the special topic papers: part IINeurocase20017211312111320159
- MackenzieIRANeumannMBigioEHNomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an updateActa Neuropathol201011911419924424
- LindquistSGBrændgaardHSvenstrupKFrontotemporal dementia linked to chromosome 3 (FTD-3) – current concepts and the detection of a previously unknown branch of the Danish FTD-3 familyEur J Neurol200815766767018484988
- BernardiLMalettaRGTomainoCThe effects of APOE and tau gene variability on risk of frontotemporal dementiaNeurobiol Aging200627570270915904995
- Levy-LahadEPoorkajPWangKGenomic structure and expression of STM2, the chromosome 1 familial Alzheimer disease geneGenomics19963421982048661049
- HébertSSSerneelsLDejaegereTCoordinated and widespread expression of γ-secretase in vivo: evidence for size and molecular heterogeneityNeurobiol Dis200417226027215474363
- NakajimaMMoriizumiEKosekiHPresenilin 1 is essential for cardiac morphogenesisDev Dyn2004230479579915254914
- DonovielDBHadjantonakisAKIkedaMMice lacking both presenilin genes exhibitearlyembryonic patterning defectsGenes Dev199913212801281010557208
- HanssonEMLendahlUChapmanGNotch signaling in development and diseaseSemin Cancer Biol200414532032815288257
- NosedaMMcLeanGNiessenKNotch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformationCirc Res200494791091714988227
- TakedaTAsahiMYamaguchiOPresenilin 2 regulates the systolic function of heart by modulating Ca2+ signalingFASEB J200519142069207116204356
- KowalskaAGenetic counseling and testing for families with Alzheimer’s diseaseNeurol Neurochir Pol200438649550115654674