Abstract
The use of multi drug regimens among the elderly population has increased tremendously over the last decade although the benefits of medications are always accompanied by potential harm, even when prescribed at recommended doses. The elderly populations are particularly at an increased risk of adverse drug reactions considering comorbidity, poly-therapy, physiological changes affecting the pharmacokinetics and pharmacodynamics of many drugs and, in some cases, poor compliance due to cognitive impairment and/or depression. In this setting, drug–drug interaction may represent a serious and even life-threatening clinical condition. Moreover, the inability to distinguish drug-induced symptoms from a definitive medical diagnosis often results in addition of yet another drug to treat the symptoms, which in turn increases drug–drug interactions. Cognitive enhancers, including acetylcholinesterase inhibitors and memantine, are the most widely prescribed agents for Alzheimer’s disease (AD) patients. Behavioral and psychological symptoms of dementia, including psychotic symptoms and behavioral disorders, represent noncognitive disturbances frequently observed in AD patients. Antipsychotic drugs are at high risk of adverse events, even at modest doses, and may interfere with the progression of cognitive impairment and interact with several drugs including anti-arrhythmics and acetylcholinesterase inhibitors. Other medications often used in AD patients are represented by anxiolytic, like benzodiazepine, or antidepressant agents. These agents also might interfere with other concomitant drugs through both pharmacokinetic and pharmacodynamic mechanisms. In this review we focus on the most frequent drug–drug interactions, potentially harmful, in AD patients with behavioral symptoms considering both physiological and pathological changes in AD patients, and potential pharmacodynamic/pharmacokinetic drug interaction mechanisms.
Introduction
A potential drug interaction is defined as an event in which two drugs known to interact were concurrently prescribed, regardless of whether adverse events occurred.Citation1 Drug interactions may have potentially life-threatening consequences, especially in frail elderly subjects.Citation2 Indeed, the elderly are particularly at an increased risk of adverse drug reactions (ADRs) considering comorbidity and the consequent poly-therapy as well as the age related changes of pharmacokinetics and pharmacodynamics of many drugs and, in some cases, the poor compliance due to cognitive impairment or behavior alteration.Citation3,Citation4 The use of multi drug regimens among the elderly population has increased tremendously over the last decade although the benefits of medications are always accompanied by potential harm (eg, adverse reaction due to drug–drug interaction), even when prescribed at recommended doses.Citation2,Citation3 An ADR is not always easy to recognize, especially in the elderly, in whom many clinical conditions coexist. Indeed, an ADR may be much more easily ascribed to “frailty” itself, an already existing diagnosis or the onset of a new clinical problem rather than to a pharmacological adverse effect. For example, falls, delirium, drowsiness, lethargy, light-headedness, apathy, urinary incontinence, chronic constipation, and dyspepsia are frequently accepted as a primary diagnosis rather than a potential ADR.Citation5 The inability to distinguish drug-induced symptoms from a definitive medical diagnosis often results in the addition of another drug to treat the symptoms increasing the risk of drug–drug interactions.Citation5
Alzheimer’s disease (AD) is the most common neurodegenerative disorder with a huge prevalence in the elderly population. This clinical condition is characterized by a slow progressive impairment of cognitive function.Citation6 Psychiatric and behavioral symptoms are common in patients with AD and contribute substantially to the morbidity of the illness.Citation7–Citation9 Delusions or hallucinations appear in 30%–50% of AD patients and, as many as 70% of them exhibit agitated or aggressive behaviour.Citation8 Considering the late onset of the syndrome, AD patients are often co-affected by other age-related diseases such as systemic hypertension, heart disease, dyslipidemia, diabetes, arthritis, renal failure, endocrine alteration, neoplasm etc, and, consequently, receive several drugs.Citation10,Citation11 For a variety of reasons (eg, increased sensitivity to certain adverse effects, potential difficulty with adhering to a regimen, reduced ability to recognize and report adverse events) the risk of ADR may be less favorable in AD patients as compared to those without dementia.Citation12,Citation13
Generally, Alzheimer patients with mild-to-severe disease are treated by cognitive enhancers like acetylcholinesterase inhibitors (AChEIs) and memantine with the intent to decrease the rate of disease progression.Citation14 Moreover, AD patients with behavioral symptoms need specific treatments such as psychotherapy and, when symptoms are not controlled, pharmacotherapy. As recommended by several authors, non-pharmacological interventions (eg, psychosocial/psychological counseling, interpersonal management, and environmental management) should be the first strategy and, when ineffective, it should be combined with specific drug classes for the shortest time possible. In particular, the most represented medications are first- and second-generation antipsychotic drugs.Citation13,Citation15–Citation19 These medications present a high risk of adverse events, even at modest doses, and may favor the progression of cognitive impairment.Citation20–Citation22 Moreover, antipsychotics may interact with several drugs including antiarrhythmics and AChEIs.Citation23,Citation24 Long-term studies of efficacy and safety of antipsychotics in elderly patients have been limited in number, and some evidences suggest that antipsychotic drugs could be related with cardiovascular events (strokes and heart arrhythmia).Citation25–Citation30 In this review we focus on the most frequent drug–drug interactions, potentially harmful, in AD patients with behavioral symptoms. The potential pharmacodynamic/pharmacokinetic drug interaction mechanisms are also analyzed.
Alzheimer patient-associated alterations affecting drug pharmacokinetics and pharmacodynamics
Pharmacokinetics is the study of drug absorption, distribution, metabolism, and excretion. Drug pharmacokinetics is affected by several conditions related to the patients, such as genetic determinants and age related alterations.Citation1 Considering the late onset of AD, the major pharmacokinetic alteration of drugs, observed in Alzheimer patients, is similar to those described in the elderly population. The most important age-related processes, observed also in healthy old people, are related to muscle structure, liver and kidney function.Citation31,Citation32 In elderly patients, muscle mass and total body water are reducedCitation33 affecting pharmacokinetics of hydrophilic drugs with a smaller volume of distribution. Conversely, body fat increases from 20% to 40% with age,Citation34,Citation35 resulting in a larger volume of distribution of lipophilic drugs. The distribution of drugs into the central nervous system (CNS) tissue might be affected in the elderly considering that the blood–brain barrier becomes more porous in the elderly, resulting in drug availability to the CNS being increased.Citation36 Moreover, in the elderly, an age-related decrease in the apparent liver blood flow has been reported.Citation37,Citation38 As a consequence, both total drug clearance and free drug clearance can decrease. However, the most significant organ changes in the elderly occur in the aging kidneys where a glomerulus loss is accompanied by a decrease in renal plasma flow.Citation39
Pharmacodynamics is the study of the time course and intensity of drugs’ pharmacologic effects. Pharmacodynamic interactions involve changes in a drug action on a receptor or a biologically active site.Citation1 At pharmacodynamics level, although the biologic mechanisms in many cases are not well explained, elderly patients are particularly vulnerable to adverse effects related to CNS drugs, such as antipsychotics, including delirium, extrapyramidal symptoms, arrhythmias, and postural hypotension.Citation40 Moreover, several age-related diseases increase the sensitivity to drug effects and lower the susceptibility threshold for drug-related adverse effects, independently of their mechanisms. For example, ageing is associated with increased sensitivity to the CNS effects of benzodiazepines:Citation41,Citation42 sedation is induced by diazepam at lower doses and lower plasma concentrations in elderly subjects.Citation43,Citation44 Ageing is also associated with increased sensitivity to the effects of nitrazepam, flurazepam, and loprazolam,Citation45,Citation46 but the exact mechanisms responsible for the increased sensitivity to benzodiazepines are unknown. Moreover, the elderly present a response to postural changes different from young subjects; indeed, cardiac output is maintained by increasing heart rate in the young, while in elderly subjects it is maintained by an increased stroke volume.Citation41 These age related changes make the elderly more susceptible to the effects of drugs affecting cardiac rhythm or vasculature, as well as vagomimetic or antihypertensive drugs, resulting in an increased risk of hypotension episodes.Citation41
However, if the pharmacokinetic changes in Alzheimer patients generally resemble those observed in the elderly population and several predisposing factors for ADR and drug–drug interactions are present among the normal elderly population, the pharmacodynamics of some CNS drugs may differ between normal elderly and Alzheimer patients. Indeed, AD selectively damages brain regions and neurotransmission pathways and is characterized by the presence of amyloid beta plaques and neurofibrillary tangles.Citation47 Recent radiologic studies have shown that neuronal death is limited in normal aging, while in AD there is considerable neuronal loss.Citation47,Citation48 The latter is a constant feature and eventually the direct cause of dementia. Interestingly, the distribution of the pathological features of AD seems to follow a region-specific pattern: the amyloid plaques are more prevalent in the neocortex and the neuronal/synaptic loss is more represented in the hippocampus, posterior cingulate, and corpus callosum areas of the brain closely involved with memory formation and higher cortical activities.Citation49–Citation52 These pathological anatomical alterations underline a more complex modification in neurotransmission resulting in a reduction of the cholinergic transmission. The cholinergic system plays a fundamental role not only in the cognitive processes of dementia, but also in the behavioral symptoms associated with AD. Alterations in the cholinergic system can cause symptoms such as apathy, affective disorders, psychomotor impairment, agitation, and psychosis.Citation53,Citation54 Although the impaired neurotransmission in AD patients represents the major pharmacological target (especially cholinergic system) for disease treatment, it might also represent a point of weakness for AD patients, especially for those with behavioral symptoms, increasing CNS drug activity or sensitivity and thus favoring ADR and/or negative pharmacodynamic drug–drug interaction.
Pharmacokinetic drug interaction
Cognitive enhancers, including AChEIs and memantine, are the most widely prescribed agents for AD patients. The US Food and Drug Administration (FDA) has approved galantamine and rivastigmine for mild-to-moderate dementia, memantine for moderate-to-severe dementia, and donepezil for mild-to-severe dementia.Citation55,Citation56 Although these drugs did not present frequent pharmacokinetic drug interactions, in some cases they may occur. In the following section, we describe the major drug–drug interactions of AD pharmacological treatments. By definition, pharmacokinetic interactions involve alterations in the plasma concentration of a drug by a second agent that affects absorption, distribution, metabolism, and elimination phases.Citation1
AChEIs
As pharmacological class, AChEIs have relatively few pharmacokinetic interactions. However, considering that donepezil and galantamine are metabolized in the liver through CYP2D6 and CYP3A4, their hepatic metabolism may be affected by specific substrates, inhibitors, or enhancers of the same enzymes.Citation57 Several drugs, reported in , may potentially interact with donepezil and galantamine at this level with different mechanisms: 1) by direct enzyme inhibition (eg, ketoconazole strongly inhibits CYP3A4 by non-competitive mechanism) and 2) competing for the catalytic site of the enzyme CYP3A4 (eg, benzodiazepines are metabolized by CYP3A4 cytochrome thus reducing the rate of transformation of the concurrent drugs eliminated by the same enzyme such as donepezil).Citation58 Tiseo et al documented that in healthy humans the concurrent administration of ketoconazole and donepezil produces no change in ketoconazole plasma concentrations, but a statistically significant change in donepezil plasma concentrations.Citation59 However, given that donepezil may be metabolized by two cytochromes (CYP3A4 and CYP2D6), the competitive inhibition with other CYP2D6 or CYP3A4 drug substrates, such as antidepressant or benzodiazepine, may not be clinically relevant. Interestingly, the oral coadministration of once-daily donepezil 5 mg for 15 days and once-daily oral sertraline (50 mg for 5 days increased to 100 mg for 10 days) did not result in any clinically significant pharmacokinetic interactions, and no unexpected ADRs were observed in healthy humans.Citation60 Instead, the coadministration of galantamine with ketoconazole (CYP3A4 strong inhibitor) or paroxetine (CYP2D6 strong inhibitor) leads to a 30% and 40% increase, respectively, in galantamine exposure, compared to galantamine alone.Citation61 The exposure to galantamine in patients with moderate and severe renal impairment is significantly higher than in healthy subjects, and is further approximately 30% higher in patients with moderate hepatic impairment.Citation61,Citation62 Therefore, attention should be paid when galantamine is coadministered with other drugs in patients with renal or hepatic impairment.
Table 1 List of important drugs that are substrates or inhibitors of cytochromes CYP2D6 and CYP3A4
The competitive drug–drug interaction for the CYP may become clinically significant also in the elderly where an age-related reduced hepatic and renal clearance (see “AChEIs” section) has been observed. It is noteworthy that rivastigmine, another AChEI, is least likely among the cognitive enhancers to have pharmacokinetic interactions with other medications since it does not undergo hepatic metabolism. Grossberg et al reported that rivastigmine did not lead to increased adverse events when administered concomitantly with 22 different classes of medications, including antidiabetic, cardiovascular, gastrointestinal, and nonsteroidal anti-inflammatory drugs in Alzheimer patients enrolled in a prospective study.Citation63
Memantine
Memantine is a weak base with a pKa of 10.27 and it is predominantly excreted unchanged by the kidneys.Citation64 Urine pH has been shown to be a major determining factor for the excretion of alkaline drugs like memantine, reducing the excreted amount.Citation65 In this setting, Freudenthaler et al demonstrated a considerable effect of urine pH of healthy volunteers on memantine excretion.Citation66 Since the renal excretion of memantine may have a relevant impact on the pharmacokinetic profile, changing of dietary habits that may alter urine pH should be avoided during treatment with such agent. Marked changes of urine pH might lead to tissue memantine overexposure resulting in toxic effects, especially in the elderly, where a reduced renal function has been described. Moreover, increased plasma levels of memantine may arise if coadministered with agents using the same renal cationic transport system as amantadine, such as cimetidine, ranitidine, procainamide, quinidine, quinine, and nicotine.Citation67 Serum hydrochlorothiazide levels may be reduced by coadministration with memantine, and patients treated with warfarin should be monitored for possible increases in international normalized ratio or prothrombin time.Citation67
Miscellaneous
Other pharmacokinetic drug–drug interactions that may occur in Alzheimer patients are related to psychiatric drugs such as benzodiazepine, antidepressant, and antipsychotic agents that are largely used in such patients, especially for behavioral alterations. In this setting, the major part of pharmacokinetic drug–drug interactions is related to hepatic metabolism (). For example, hepatic CYP enzymes of the phase 1 metabolism are most susceptible, regarding the pharmacokinetic interactions of antipsychotic drugs.Citation24 Among the multiple isoenzymes in the liver, CYP1A2, CYP2C19, CYP2D6, and CYP3A4 are practically relevant for the degradation of antipsychotic drugs.Citation68 Coadministration of an antipsychotic drug with paroxetine, a potent inhibitor of CYP2D6, may lead to elevated plasma concentrations of the antipsychotic drug, and thus enhance concentration-dependent side effects such as extrapyramidal symptoms.Citation57 Other pharmacokinetic drug–drug interactions that may occur in patients are represented by hepatic enzyme induction, thus resulting in reduced tissue exposure and/or drug failure. In the clinical setting, the most important inducers of CYP1A2 are polycyclic aromatic hydrocarbons that are present in cigarette smoke. For CYP3A4, carbamazepine is the most important inducer.Citation69,Citation70 As example, a susceptible substrate of CYP3A4 is represented by quetiapine; it was described that quetiapine plasma concentration decreases by approximately 90% when coadministered with carbamazepine.Citation69,Citation70 Inducer enzyme drugs that may affect the pharmacokinetics of other drugs are listed in .
Table 2 Principal CYP3A4 inducer drugs
Pharmacodynamic drug interaction
Pharmacodynamic drug–drug interactions may result from an antagonistic or synergistic mechanism. Several CNS drugs may interact directly at the receptor level or indirectly affecting one or more neurotransmission system. In particular, the risk to develop an ADR related to a negative drug–drug interaction is increased in the elderly due to CNS ageing alterations and may be more relevant in Alzheimer patients (see the previous section). The cholinergic theory of ADCitation71 has led to development of the cholinesterase inhibitors. When used appropriately, these medications may slow the decline in cognition and functional impairment associated with AD. However, based on their opposing mechanisms of action, concomitant use of cholinesterase inhibitors and anticholinergics may result in pharmacological antagonism ().Citation72
Figure 1 Pharmacodynamic drug–drug interaction in a cholinergic synapse: acetylcholinesterase inhibitor (AChEI) and anti-muscarinic drug.
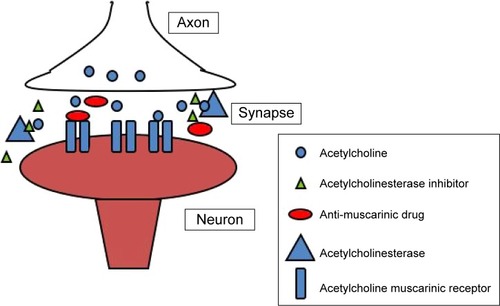
Cholinergic antagonism
The anticholinergic effect is a feature of several drugs such as antipsychotics, antidepressants, antihistamines, bronchodilators, drugs for urinary incontinence etc. There is evidence that the use of these classes of drugs, even in non-Alzheimer elderly individuals, is considered potentially inappropriateCitation73 and the use of anticholinergics by those with dementia is especially troubling because these individuals are more prone to develop medication-induced cognitive impairment.Citation73 It is noteworthy to mention that tertiary anticholinergics such as atropine have been successfully used as an antidote for donepezil over-dosage,Citation74 and concomitant use of anticholinergics was not allowed in randomized placebo-controlled trials of AChEIs.Citation75–Citation77 As shown by Roe et al the concomitant use of AChEIs and anticholinergic drugs is quite frequent in the Alzheimer population.Citation72 Indeed, they found that community-based elderly individuals with probable dementia are more likely to take anticholinergics than controls. Patients taking donepezil frequently use an anticholinergic medication concomitantly. In particular, they found that 33% of those taking donepezil also were receiving anticholinergics, compared with 23% of controls and 26% of all patients in the study used multiple anticholinergic medications. Similar results were obtained by Carnahan et al in a cross-sectional study to measure the prevalence of anticholinergic drug use in patients receiving cholinesterase inhibitors and to describe change in use of anticholinergics upon inception of AChEI treatment.Citation78 They showed that the concurrent use of anticholinergics and AChIs in this cohort reaches 35%. Also Robinson et al, in an Australian naturalistic study, highlights that co-prescribing of cholinesterase inhibitors and drugs with anticholinergic activity is a common practice and is similar to that seen in other developed countries.Citation79 An interesting survey on AChEIs’ negative drug–drug interaction was performed by Tavassoli et al.Citation80 Analyzing the spontaneous reported ADRs related to AChEIs in France up to 2006, they found that the most frequently reported ADRs, due to drug–drug interaction, in patients treated with AChEIs were associated with bradycardia (54.5%) and anticholinergic (31.4%) drugs, in most cases via pharmacodynamic mechanisms. ADRs due to drug–drug interactions were mainly represented by cardiovascular (bradycardia, atrioventricular block [AVB] and arterial hypotension) and neurological (mainly mental confusion) events.Citation80
In order to evaluate the impact of the atypical antipsychotics, olanzapine, quetiapine, and risperidone on cognition in patients with AD, Vigen et al carried out a clinical study in 421 AD outpatients with psychosis or agitated/aggressive behavior, randomized to masked, flexible-dose olanzapine, quetiapine, risperidone or placebo.Citation21 Patients were followed for 36 weeks and cognitive assessments were obtained at baseline, 12 weeks, 24 weeks, and 36 weeks. Approximately 60% of those patients were treated concomitantly with AChEIs. The authors found that, in this cohort, atypical antipsychotics were associated with worsening cognitive function compared with placebo.Citation21 Anticholinergic related drug effects, as well as those described for antipsychotic agents, can counteract AChEIs’ beneficial effect. Considering that anticholinergic drugs can worsen cognitive impairment, they should be administered with caution in elderly patients, especially in AD. The development of an adverse outcome when a demented patient is exposed to anticholinergic drugs, may depend mainly on two factors: total anticholinergic load (ie, use of multiple drugs with anticholinergic activity or use of high doses of these products), and individual pharmacokinetic and pharmacodynamic variability.Citation72 Overall, the concurrent use of anticholinergics and AChEIs is quite common in a clinical setting but is rarely appropriate considering the pharmacologic antagonism.Citation72
Potential ADR at cardiovascular level
Most of the cardiovascular ADRs to AChEIs might be related to stimulation of the parasympathetic nervous system.Citation81 The parasympathetic nervous system can affect heart as well as brain function, and its effect on the heart is more complicated than is generally thought.Citation81 Arrhythmia and syncope have been reported with the use of AChEIs. The heart is naturally rich with cholinesterase, and its inhibition may affect cardiac function, especially in elderly patients, many of whom have concomitant cardiovascular disease. The inhibition of cholinesterase by AChEIs retards acetylcholine degradation and potentiates the cardio-inhibitory effect.Citation81 Moreover, AChEIs increase arterial blood pressure through central M1 and M2 subtypes of muscarinic receptors.Citation80 However, the effect of AChEIs is only slight in patients who receive a typical dose. Morganroth et al pooled data from four large placebo-controlled trials in order to assess possible electrocardiogram (ECG) changes associated with rivastigmine.Citation82 The authors determined that the incidence of first-degree AVB and bradycardia were higher in the rivastigmine higher dose group when compared with the lower dose and placebo groups, although this difference did not reach statistical significance. A higher incidence of first-degree AVB, increased PR intervals, or decreased heart rates was also found in two trials assessing the safety of donepezil in Alzheimer patients with or without the use of cardiac-related drugs, also in this case, the results were not statistically significant.Citation82,Citation83 Although the relationship of bradycardia and syncope with AChEIs remains unclear, there is an increasing number of case reports suggesting a causal relationship.Citation84–Citation86 The cardiovascular effects reported for AChEIs and the underlying molecular mechanisms may explain most of the negative drug–drug interactions with other drugs with cardiac activity.
In last few years, there have also been concerns regarding the mortality risk of antipsychotics in people with dementia. The FDA conducted a meta-analysis based on 17 of the short-term atypical antipsychotics in people with AD, highlighting a significant increase in mortality risk for individuals treated with atypical antipsychotics compared to individuals receiving placebo, leading to a “black box” warning regarding mortality risk.Citation87 Five meta-analyses on antipsychotic drugs reported a significantly increased risk of cerebrovascular events with atypical antipsychotics compared to placebo.Citation88–Citation93 These findings should be interpreted considering that antipsychotic drugs might present a direct effect on the development of cardiovascular events such as QT prolongation,Citation24 that could be worsened by drug–drug interactions. In particular, QTc interval prolongation is reported for a number of old and new antipsychotic drugs, such as, haloperidol, levomepromazine, melperone, pimozide, quetiapine, sertindole, thioridazine, or ziprasidone.Citation94–Citation96 When an antipsychotic drug affecting QTc prolongation is combined with another QTc-lengthening drug or drugs slowing cardiac frequency (AChEIs), the concomitant use may have additive or even potentiating effects.Citation67 Moreover, as suggested by Pariente et al,Citation88 we can hypothesize that antipsychotic effects, both on body weight and metabolic syndrome development, seem unlikely in the context of Alzheimer patients. In this setting, if such a pathway is involved, one would expect an over-time progressive increasing risk rather than, as they found, a transient acute risk that decreases thereafter only.
Memantine
With respect to drug–drug interactions related to memantine it is important to consider that memantine presents a weak dopaminergic agonist with atropinic effects. Memantine should not be administered alongside compounds acting upon the same receptor (NMDA) system (amantadine, ketamine, dextromethorphan) due to the risk of pharmacotoxic psychosis.Citation66 There is one published case report on a possible risk for the combination of memantine and phenytoin.Citation67
Conclusion
Alzheimer patients present an increased risk of ADRs and drug–drug interaction ADRs due to several factors such as age, age of disease onset, the presence of multi-pharmacotherapy as well as disease-related CNS alterations that cause sensitization to the effects of psychotropic drugs. Although there are few studies of pharmacokinetic interaction related to Alzheimer patients, considering the potential hepatic effects, it is important to pay attention when prescribing drugs for patients who:Citation97,Citation98 1) take cytochrome inhibitors of CYP3A4 and CYP2D6; 2) take several cytochrome substrates that compete for the metabolic pathway; 3) present reduced glomerular filtration rate; and 4) are affected by hepatic disease affecting metabolic functions. Another pharmacokinetic interaction that could affect memantine plasma concentration profile in AD patients is represented by drugs that may alter the urinary pH.
One of the most important pharmacodynamic interactions, based on their opposing mechanisms of action, is represented by the concomitant use of AChEIs and anticholinergic drugs that results in pharmacological antagonism.Citation72 The anticholinergic effect is a feature of several drugs such as antipsychotics, antidepressants, antihistamines, bronchodilators, and drugs for urinary incontinence that are frequently prescribed to Alzheimer patients, especially to those with behavioral and psychotic symptoms. Finally, in this setting, we have to consider the cardiovascular adverse reaction related to the drugs, keeping in mind the possible alteration in the rhythm and the QT.
Disclosure
The authors have no conflicts of interest to disclose.
References
- BuxtonILBenetLZPharmacokinetics: The Dynamics of Drug Absorption, Distribution, Metabolism, and EliminationBruntonLLChabnerBAKnollmannBCGoodman & Gilman’s The Pharmacological Basis of Therapeutic12th edNew YorkMcGraw-Hill2011
- MalletLSpinewineAHuangAThe challenge of managing drug interactions in elderly peopleLancet2007370958218519117630042
- HanlonJTLindbladCIHajjarERMcCarthyTCUpdate on drug-related problems in the elderlyAm J Geriatr Pharmacother200311384315555464
- GurwitzJHFieldTSHarroldLRIncidence and preventability of adverse events among older persons in the ambulatory settingJAMA200328991107111612622580
- TangiisuranBWrightJVan der CammenTRajkumarCAdverse drug reactions in elderly: challenges in identification and improving preventative strategiesAge Ageing200938435835919420141
- SeeleyWWMillerBLDementiaLongoDLFauciASKasperDLHauserSHJamesonJLLoscalzoJHarrison’s Principles of Internal Medicine18th edNew YorkMcGraw-Hill2012
- LyketsosCGSteinbergMTschanzJTMental and behavioral disturbances in dementia: indings from the Cache County Study on Memory in AgingAm J Psychiatry2000157570871410784462
- RopackiSAJesteDVEpidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003Am J Psychiatry2005162112022203016263838
- LópezOLZivkovicGSmithGBeckerJTMeltzerCCDeKoskySTPsychiatric symptoms associated with cortical-subcortical dysfunction in Alzheimer’s diseaseJ Neuropsychiatry Clin Neurosci2001131566011207330
- AndersenFViitanenMHalvorsenDSStraumeBEngstadTACo-morbidity and drug treatment in Alzheimer’s disease. A cross sectional study of participants in the dementia study in northern NorwayBMC Geriatr2011115821970467
- HeunRSchoepfDPotluriRNatalwalaAAlzheimer’s disease and co-morbidity: increased prevalence and possible risk factors of excess mortality in a naturalistic 7-year follow-upEur Psychiatry2013281404821924588
- SmallGWRabinsPVBarryPPDiagnosis and treatment of Alzheimer’s disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics SocietyJAMA199727816136313719343469
- BraunerDJMuirJCSachsGATreating nondementia illnesses in patients with dementiaJAMA2000283243230323510866871
- O’BrienJTBurnsABAP Dementia Consensus GroupClinical practice with anti-dementia drugs: a revised (second) consensus statement from the British Association for PsychopharmacologyJ Psychopharmacol2011258997101921088041
- GironMSForsellYBernstenCThorslundMWinbladBFastbomJPsychotropic drug use in elderly people with and without dementiaInt J Geriatr Psychiatry200116990090611571771
- OlinJTKatzIRMeyersBSSchneiderLSLebowitzBDProvisional diagnostic criteria for depression of Alzheimer disease: rationale and backgroundAm J Geriatr Psychiatry200210212914111925274
- SinkKMHoldenKFYaffeKPharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidenceJAMA2005293559660815687315
- GauthierSCummingsJBallardCManagement of behavioral problems in Alzheimer’s diseaseInt Psychogeriatr201022334637220096151
- SchneiderLSDagermanKInselPSEfficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trialsAm J Geriatr Psychiatry200614319121016505124
- GardetteVLapeyre-MestreMColeyNAntipsychotic use and mortality risk in community-dwelling Alzheimer’s disease patients: evidence for a role of dementia severityCurr Alzheimer Res2012991106111622950915
- VigenCLMackWJKeefeRSCognitive effects of atypical antipsychotic medications in patients with Alzheimer’s disease: outcomes from CATIE-ADAm J Psychiatry2011168883183921572163
- LeePEGillSSFreedmanMBronskillSEHillmerMPRochonPAAtypical antipsychotic drugs in the treatment of behavioural and psychological symptoms of dementia: systematic reviewBMJ200432974577515194601
- ParienteASanctussyDJMiremont-SalameGFactors associated with serious adverse reactions to cholinesterase inhibitors: a study of spontaneous reportingCNS Drugs2010241556320030419
- PereraGKhondokerMBroadbentMBreenGStewartRFactors associated with response to acetylcholinesterase inhibition in dementia: a cohort study from a secondary mental health care case register in LondonPLoS One2014911e10948425411838
- WangPSSchneeweissSAvornJRisk of death in elderly users of conventional vs atypical antipsychotic medicationsN Engl J Med2005353222335234116319382
- GillSSRochonPAHerrmannNAtypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort studyBMJ20053307489e445
- DouglasIJSmeethLExposure to antipsychotics and risk of stroke: self-controlled case series studyBMJ2008337a122718755769
- MaherARMaglioneMBagleySEfficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysisJAMA2011306121359136921954480
- LiuMETsaiSJChangWCPopulation-based 5-year follow-up study in Taiwan of dementia and risk of strokePLoS One201384e6177123626726
- TrifiròGVerhammeKMZiereGCaputiAPCh StrickerBHSturkenboomMCAll-cause mortality associated with atypical and typical antipsychotics in demented outpatientsPharmacoepidemiol Drug Saf200716553854417036366
- YoungAAgeing and physiological functionsPhilos Trans R Soc Lond B Biol Sci19973521363183718439460068
- MuhlbergWPlattDAge-dependent changes of the kidneys: Pharmacological implicationsGerontology199945524325310460985
- FulopTJrWorumICsongorJFórisGLeoveyABody composition in elderly people: I. Determination of body composition by multiisotope method and the elimination kinetics of these isotopes in healthy elderly subjectsGerontology19853116143972257
- McLeanAJLe CouteurDGAging biology and geriatric clinical pharmacologyPharmacol Rev200456216318415169926
- GinsbergGHattisDRussASonawaneBPharmacokinetic and pharmacodynamic factors that can affect sensitivity to neurotoxic sequelae in elderly individualsEnviron Health Perspect200511391243124916140636
- Bentue-FerrerDTributOPolardEAllainHClinically significant drug interactions with cholinesterase inhibitors: a guide for neurologistsCNS Drugs2003171394796314533945
- ZoliMMagalottiDBianchiGTotal and functional hepatic blood flow decrease in parallel with ageingAge Ageing1999281293310203201
- WynneHACopeLHMutchERawlinsMDWoodhouseKWJamesOFThe effect of age upon liver volume and apparent liver blood flow in healthy manHepatology1989922973012643548
- FliserDZeierMNowackRRitzERenal functional reserve in healthy elderly subjectsJ Am Soc Nephrol199337137113778439649
- TrifiròGSpinaEAge-related changes in pharmacodynamics: focus on drugs acting on central nervous and cardiovascular systemsCurr Drug Metab201112761162021495972
- MangoniAAJacksonSHAge-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applicationsBr J Clin Pharmacol200457161414678335
- KruseWHProblems and pitfalls in the use of benzodiazepines in the elderlyDrug Saf1990553283342222867
- SwiftCGEwenJMClarkePStevensonIHResponsiveness to oral diazepam in the elderly: relationship to total and free plasma concentrationsBr J Clin Pharmacol19852021111183929807
- ReidenbergMMLevyMWarnerHRelationship between diazepam dose, plasma level, age, and central nervous system depressionClin Pharmacol Ther1978234371374630787
- CastledenCMGeorgeCFMarcerDHallettCIncreased sensitivity to nitrazepam in old ageBr Med J1977160521012318894
- GreenblattDJDivollMHarmatzJSMacLaughlinDSShaderRIKinetics and clinical effects of flurazepam in young and elderly non-insomniacsClin Pharmacol Ther19813044754867285482
- CummingsJLAlzheimer’s diseaseN Engl J Med20043511566715229308
- ArcherHAEdisonPBrooksDJAmyloid load and cerebral atrophy in Alzheimer’s disease: an 11C-PIB positron emission tomography studyAnn Neurol200660114514716802294
- WestMJKawasCHStewartWFRudowGLTroncosoJCHippocampal neurons in pre-clinical Alzheimer’s diseaseNeurobiol Aging20042591205121215312966
- MorrisonJHHofPRLife and death of neurons in the aging brainScience199727853374124199334292
- MorrisonJHHofPRSelective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s diseaseProg Brain Res200213646748612143403
- HofPRBussièreTGoldGStereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 field in Alzheimer diseaseJ Neuropathol Exp Neurol2003621556712528818
- CummingsJLBackCThe cholinergic hypothesis of neuropsychiatric symptoms in Alzheimer’s diseaseAm J Geriatr Psychiatry199862 Suppl 16478
- MufsonEJCountsSEPerezSEGinsbergSDCholinergic system during the progression of Alzheimer’s disease: therapeutic implicationsExpert Rev Neurother20088111703171818986241
- RainaPSantaguidaPIsmailaAEffectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guidelineAnn Intern Med2008148537939718316756
- HowardRMcShaneRLindesayJDonepezil and memantine for moderate-to-severe Alzheimer’s diseaseN Engl J Med20123661089390322397651
- SpinaEScordoMGD’ArrigoCMetabolic drug interactions with new psychotropic agentsFundam Clin Pharmacol200317551753814703714
- SweeneyBPBromilowJLiver enzyme induction and inhibition: implications for anaesthesiaAnaesthesia200661215917716430569
- TiseoPJPerdomoCAFriedhoffLTConcurrent administration of donepezil HCl and ketoconazole: assessment of pharmacokinetic changes following single and multiple dosesBr J Clin Pharmacol199846Suppl 130349839763
- NagyCFKumarDPerdomoCAWasonSCullenEIPrattRDConcurrent administration of donepezil HCl and sertraline HCl in healthy volunteers: assessment of pharmacokinetic changes and safety following single and multiple oral dosesBr J Clin Pharmacol200458Suppl 1253315496220
- HuangFFuYA review of clinical pharmacokinetics and pharmacodynamics of galantamine, a reversible acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease, in healthy subjects and patientsCurr Clin Pharmacol20105211512420156150
- PiotrovskyVVan PeerAVan OsselaerNArmstrongMAerssensJGalantamine population pharmacokinetics in patients with Alzheimer’s disease: modeling and simulationsJ Clin Pharmacol200343551452312751272
- GrossbergGTStahelinHBMessinaJCAnandRVeachJLack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medicationsInt J Geriatr Psychiatry200015324224710713582
- WesemannWSonntagK-HMajJOn the pharmacodynamics and pharmacokinetics of memantineDrug Res198333811221134
- RoySDHawesEMMidhaKKInfluence of urinary pH on the disposition of methoxyphenamine and three metabolites in humansJ Pharm Sci19877664274323625484
- FreudenthalerSMeinekeISchreebKHBoakyeEGundert-RemyUGleiterCHInfluence of urine pH and urinary flow on the renal excretion of memantineBr J Clin Pharmacol19984665415469862242
- European Medicine AgencyEbixa Tablets SPC, Summary of Product CharacteristicsEuropean Medicine Agency (EMA) Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000463/WC500058763.pdfAccessed August 15, 2015
- HiemkeCPfuhlmannBInteractions and monitoring of antipsychotic drugsHandb Exp Pharmacol201221224126523129335
- CastbergISkogvollESpigsetOQuetiapine and drug interactions: evidence from a routine therapeutic drug monitoring serviceJ Clin Psychiatry200768101540154517960969
- Nickl-JockschatTPaulzenMSchneiderFGrozingerMDrug interaction can lead to undetectable serum concentrations of quetiapine in the presence of carbamazepineClin Neuropharmacol20093215519471186
- WhitehousePJPriceDLStrubleRGClarkAWCoyleJTDelonMRAlzheimer’s disease and senile dementia: loss of neurons in the basal forebrainScience19822154537123712397058341
- RoeCMAndersonMJSpivackBUse of anticholinergic medications by older adults with dementiaJ Am Geriatr Soc200250583684212028169
- American Geriatrics Society 2012 Beers Criteria Update Expert PanelAmerican Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adultsJ Am Geriatr Soc201260461663122376048
- ShepherdGKlein-SchwartzWEdwardsRDonepezil overdose: A tenfold dosing errorAnn Pharmacother199933781281510466911
- RöslerMAnandRCicin-SainAEfficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomised controlled trialBMJ1999318718463363810066203
- Corey-BloomJAnandRVeachJA randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s diseaseInt J Ger Psychopharmacol199815565
- TariotPNSolomonPRMorrisJCA 5-month, randomized, placebocontrolled trial of galantamine in ADNeurology200054122269227610881251
- CarnahanRMLundBCPerryPJChriscillesEAThe concurrent use of anticholinergics and cholinesterase inhibitors: rare event or common practice?J Am Geriatr Soc200452122082208715571547
- RobinsonMRowettDLevertonAMabbottVChanges in utilisation of anticholinergic drugs after initiation of cholinesterase inhibitorsPharmacoepidemiol Drug Saf200918865966419548222
- TavassoliNSommetALapeyre-MestreMBagheriHMontrastrucJLDrug interactions with cholinesterase inhibitors: an analysis of the French pharmacovigilance database and a comparison of two national drug formularies (Vidal, British National Formulary)Drug Saf200730111063107117973542
- MasudaYCardiac effect of cholinesterase inhibitors used in Alzheimer’s disease – from basic research to bedsideCurr Alzheimer Res20041431532115975060
- MorganrothJGrahamSHartmanRAnandRElectrocardiographic effects of rivastigmineJ Clin Pharmacol200242555856812017350
- BordierPGarrigueSBaroldSSBressollesNLanusseSClémentyJSignificance of syncope in patients with Alzheimer’s disease treated with cholinesterase inhibitorsEuropace20035542943114753643
- BordierPGarrigueSLanusseSCardiovascular effects and risk of syncope related to donepezil in patients with Alzheimer’s diseaseCNS Drugs200620541141716696580
- KayrakMYaziciMAyhanSSKocFUlgenMSComplete atrioventricular block associated with rivastigmine therapyAm J Health Syst Pharm200865111051105318499878
- PaulisonBLéosCLPotential cardiotoxic reaction involving rivastigmine and beta-blockers: a case report and review of the literatureCardiovasc Toxicol201010430631020865460
- US Food Drug AdministrationDeaths with Antipsychotics in Elderly Patients with Behavioral DisturbancesUS Food and Drug Administration2005 Available from: http://www.cchrint.org/pdfs/US_Food_and_Drug_Administration_Warnings_on_Antipsychotic_Drugs.pdfAccessed August 15, 2015
- ParienteAFourrier-RéglatADucruetTAntipsychotic use and myocardial infarction in older patients with treated dementiaArch Intern Med2012172864865322450214
- PrattNRougheadEESalterARyanPChoice of observational study design impacts on measurement of antipsychotic risks in the elderly: a systematic reviewBMC Med Res Methodol2012127222682666
- BallardCWaiteJThe effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s diseaseCochrane Database Syst Rev20061CD00347616437455
- De DeynPPKatzIRBrodatyHLyonsBGreenspanABurnsAManagement of agitation, aggression, and psychosis associated with dementia: a pooled analysis including three randomized, placebo-controlled double-blind trials in nursing home residents treated with risperidoneClin Neurol Neurosurg2005107649750815922506
- SchneiderLSTariotPNDagermanKSEffectiveness of atypical antipsychotic drugs in patients with Alzheimer’s diseaseN Engl J Med2006355151525153817035647
- HerrmannNLanctotKLDo atypical antipsychotics cause stroke?CNS Drugs20051929110315697324
- Van NoordCEijgelsheimMStrickerBHDrug- and non-drug-associated QT interval prolongationBr J Clin Pharmacol2010701162320642543
- Wenzel-SeifertKWittmannMHaenEQTc prolongation by psychotropic drugs and the risk of Torsade de PointesDtsch Arztebl In201110841687693
- BresslerRGrapefruit juice and drug interactions. Exploring mechanisms of this interaction and potential toxicity for certain drugsGeriatrics20066111121817112309
- Ingelman-SundbergMGenetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversityPharmacogenomics J20055161315492763
- ZhouSFXueCCYuXQLiCWangGClinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoringTher Drug Monit200729668771018043468
