Abstract
Objective
The aim of this study was to evaluate the effects of aging on metabolic, cardiovascular, autonomic, inflammatory, and oxidative stress parameters after ovarian hormone deprivation (OVX).
Methods
Female Wistar rats (3 or 22 months old) were divided into: young controls, young ovariectomized, old controls, and old ovariectomized (bilateral ovaries removal). After a 9-week follow-up, physical capacity, metabolic parameters, and morphometric and cardiac functions were assessed. Subsequently, arterial pressure was recorded and cardiac autonomic control was evaluated. Oxidative stress was measured on the cardiac tissue, while inflammatory profile was assessed in the plasma.
Results
Aging or OVX caused an increase in body and fat weight and triglyceride concentration and a decrease in both insulin sensitivity and aerobic exercise capacity. Left ventricular diastolic dysfunction and increased cardiac overload (myocardial performance index) were reported in old groups when compared with young groups. Aging and OVX led to an increased sympathetic tonus, and vagal tonus was lower only for the old groups. Tumor necrosis factor-α and interleukin-6 were increased in old groups when compared with young groups. Glutathione redox balance (GSH/GSSG) was reduced in young ovariectomized, old controls, and old ovariectomized groups when compared with young controls, indicating an increased oxidative stress. A negative correlation was found between GSH/GSSG and tumor necrosis factor-α (r=−0.6, P<0.003). Correlations were found between interleukin-6 with adipose tissue (r=0.5, P<0.009) and vagal tonus (r=−0.7, P<0.0002); and among myocardial performance index with interleukin-6 (r=0.65, P<0.0002), sympathetic tonus (r=0.55, P<0.006), and physical capacity (r=−0.55, P<0.003). The findings in this trial showed that ovariectomy aggravated the impairment of cardiac and functional effects of aging in female rats, probably associated with exacerbated autonomic dysfunction, inflammation, and oxidative stress.
Introduction
The aging process or senescence is related to morphological and functional changes in the cardiovascular system, with great emphasis on incidence of cardiovascular disease (CVD).Citation1 CVD is the leading cause of morbidity and mortality in the world. In 2012, an estimated 17.5 million people died from CVDs, representing 31% of global deaths.Citation2 In addition, CVD develops later in women than in men and is still the major cause of death in women. It is also estimated that 43 million women in the US are affected by CVDs, which strengthens the hypothesis that age and sex play a significant role in the prevalence of cardiovascular risk.Citation3,Citation4 This hypothesis postulates that climacteric women are exposed to increased cardiovascular risk, since aging and menopause have been associated with decreased exercise capacity, muscle strength, and bone mass, but increased body weight and high diabetes prevalence. In addition, the climacteric period worsens not only hemodynamic profile,Citation5,Citation6 but also the autonomic balance decreasing parasympathetic and/or increasing sympathetic activity with associated changes in cardiac function, inflammatory and oxidative stress markers.
Therefore, this experimental trial was designed to evaluate the hypothesis that early ovarian hormone deprivation (OVX) and/or aging may lead to sympathetic activation, which may interact with inflammation and oxidative stress, causing cardiac dysfunction and physical capacity loss, as observed in postmenopausal young or older women. To test this hypothesis, the effects of aging were assessed on metabolic, cardiovascular, autonomic, inflammatory, and oxidative stress parameters after OVX.
Methods
Experiments were performed on 32 female rats. 16 3-month-old and 16 22-month-old rats (Rattus norvegicus) obtained from the Federal University of São Paulo (UNIFESP) were maintained in the animal house facility of the Heart Institute of the University of Sao Paulo, receiving standard laboratory chow and water ad libitum. The animals were housed in individual cages in a temperature-controlled room (22°C–24°C) with a 12-hour dark–light cycle.
This study was approved by the Experimental Animal Use Committee of the School of Medicine, University of São Paulo (protocol 360/11), and were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
The rats were assigned to four groups: young controls (YC =8), young ovariectomized (YO =8), old controls (OC =8), and old ovariectomized (OO =8). In order to avoid any influence of the fluctuation of female sex hormones on the results observed in the YC and OC groups, the identification of the stage of the estrous cycle of the rats was performed. All experimental evaluations were performed in nonovulatory phases of the estrous cycle of rats.Citation7
Ovariectomy
After 3 months (YO) and 22 months (OO) of life, the animals were anesthetized (80 mg/kg ketamine and 12 mg/kg xylazine, intraperitoneal [IP]), and a small abdominal incision was performed. The oviduct was sectioned and the ovary removed as described in detail elsewhere.Citation8,Citation9
Estrogen concentration in the blood was measured by immunoassay to confirm OVX.Citation10
Physical capacity
All animals were adapted (1 week after ovariectomy) to the treadmill (TK-01; Ibramed, Porto Alegre, Brazil) (10 min/day; 0.3 km/h) for 1 week prior to the maximal treadmill test. A maximal treadmill testCitation11 was performed by all groups: at the beginning of the experiment and in the 4th and 8th weeks of the protocol. The purpose was to determine exercise capacity.
Determination of blood glucose and triglycerides
At the end of the protocol, all animals underwent 4 hours of fasting. A drop of blood from the tail was collected to measure plasma glucose using a glucometer, and another drop was collected for triglyceride measurement with Accutrend GTC (Hoffman-La Roche Ltd., Basel, Switzerland).
Insulin resistance test
At the end of the protocol, all animals underwent a 2-hour fasting and were anesthetized with sodium pentobarbital (40 mg/kg IP). Plasma glucose was measured from blood samples collected from the animal cannula with the use of a glucometer (Accu-Check; Hoffman-La Roche Ltd.) at times 0, 4, 8, 12, and 16 minutes after an insulin intravenous injection (0.75 U/kg body weight). Glycemia values for 4–16 minutes were used to calculate the constant decrease of plasma glucose.Citation12
Echocardiography
At the end of the protocol, a transthoracic echocardiography was performed in all the groups using double-blind observers in accordance with the guidelines of the American Society of Echocardiography. Rats were anesthetized (50 mg/kg ketamine and 12 mg/kg xylazine, IP) and the echocardiographic parameters were measured as previously described.Citation13,Citation14
Cardiovascular measurements
Ten weeks after the beginning of protocol, two catheters filled with saline solution were implanted in anesthetized rats (80 mg/kg ketamine and 12 mg/kg xylazine, IP), into the artery and femoral vein (PE-50) for direct measurements of arterial pressure (AP) and drug administration, respectively. Twenty-four hours after surgical procedures, the arterial cannula was connected to a transducer (Kent Instruments, Kent Scientific Corporation, Torrington, Connecticut, USA), and AP signals were recorded over a 30-minute period by a microcomputer equipped with an analog-to-digital converter board Windaq (DATAQ Instruments, Akron, OH, USA). Rats were conscious and allowed to move freely during the experiments. Increasing doses of phenylephrine (0.25–32 μg/kg) and sodium nitroprusside (2.5 a 100 μg/kg) were given as sequential bolus injections (0.1 mL) to produce pressure responses ranging from 5 to 40 mmHg. A 3- to 5-minute interval between doses was necessary for blood pressure to return to baseline. Peak increases or decreases in mean AP (MAP) after phenylephrine or sodium nitroprusside injection and the corresponding peak reflex changes in heart rate (HR) were recorded for each dose of the drug. Baroreceptor reflex sensitivity was evaluated by a mean index relating changes in HR to changes in MAP, allowing a separate analysis of gain for reflex bradycardia and reflex tachycardia. The mean index was expressed as beats per minute per millimeter of mercury, as described elsewhere.Citation9
Vagal and the sympathetic tonus and effects and intrinsic heart rate (IHR) were measured by determining the response to methylatropine (3 mg/kg intravenous [IV]) and propranolol (4 mg/kg IV) after basal AP recording, as described in detail elsewhere.Citation9 AP and HR were continuously recorded at a basal state and after methylatropine (3 mg/kg IV) injection (<0.2 mL). Because the HR response to this drug reaches its peak within 3–5 minutes, this time interval was allowed to elapse before HR measurement. Propranolol (4 mg/kg IV) was injected (<0.2 mL) 10 minutes after methylatropine injection, and once again, the response was evaluated after simultaneous blockade with propranolol and methylatropine. On the subsequent day, the sequence of injections was inverted (first propranolol and then methylatropine). The sympathetic effect was determined as the difference between basal HR and the lower HR after propranolol injection, vagal effect was obtained by the difference between maximum HR after methyl atropine injection and basal HR, ultimately sympathetic and vagal tonus were evaluated by intrinsic HR instead of HR.Citation6,Citation15
Body weight and visceral white adipose tissue
Animals were weighed once a week during 10 weeks of protocol. The visceral white adipose tissue (WAT) was collected and weighed at the end of protocol on an analytical balance for comparison among groups.
Inflammatory markers on plasma
Interleukin (IL) 1B, IL-4, IL-6, and tumor necrosis factor (TNF)-α levels in plasma were determined using a commercially available enzyme-linked immunosorbent assay kit (R&D Systems Inc., Minneapolis, MN, USA), in accordance with the manufacturer’s instructions. Enzyme-linked immunosorbent assay was performed in 96-well polystyrene microplates using specific monoclonal antibody coating.
Oxidative stress profile
One day after hemodynamic evaluations, the animals were euthanized and the heart (left ventricles) was immediately removed, rinsed in saline, and trimmed to remove fat tissue and visible connective tissue. The tissue was cut into small pieces, placed in ice-cold buffer, and homogenized in an Ultra80 Turrax blender (UltraStirrer, Pilatusstrasse, Muri, Switzerland) with 1 g tissue per 4 mL 120 mM KCl and 20 nM sodium phosphate buffer, pH 7.4. The homogenate was centrifuged at 600× g for 10 minutes at −26°C.
Lipoperoxidation by thiobarbituric acid reactive substances
For the thiobarbituric acid reactive substance (TBARS) assay, trichloroacetic acid (10%, w/v) was added to the homogenate to precipitate proteins and to acidify the samples. This mixture was then centrifuged (1,000× g, 3 minutes), the protein-free sample was extracted, and thiobarbituric acid (0.67%, w/v) was added to the reaction medium. The tubes were placed in a water bath (100°C) for 15 minutes. Absorbance was measured at 535 nm.Citation16
Protein oxidation by carbonyl assay
Tissue samples were incubated with 2,4-dinitrophenylhydrazine (10 mM) in a 2.5 M HCl solution for 1 hour in the dark. Samples were vortexed every 15 minutes. Subsequently, a 20% trichloroacetic acid (w/v) solution was added and the solution was incubated on ice for 10 minutes and centrifuged for 5 minutes at 1,000× g to collect protein precipitates. An additional wash was performed with 10% trichloroacetic acid (w/v). The pellet was washed three times with ethanolethyl acetate (1:1) (v/v). The final precipitates were dissolved in 6 M guanidine hydrochloride solution and incubated for 10 minutes at 37°C, and absorbance was measured at 360 nm.Citation17
Antioxidant enzyme activities
The quantification of superoxide dismutase activity was based on the inhibition of the reaction between O2·− and pyrogallol.Citation18 Catalase activity (CAT) was determined by measuring the decrease in H2O2 absorbance at 240 nm.Citation19 Glutathione peroxidase (GPx) activity was based on the consumption of NADPH at 480 nm.Citation20
Glutathione redox balance
To determine oxidized and reduced glutathione concentration, tissue was deproteinized with 2 mol/L perchloric acid, centrifuged during 10 minutes at 1,000× g, and the supernatant was neutralized with 2 mol/L potassium hydroxide. The reaction medium contained 100 mmol/L phosphate buffer (pH 7.2), 2 mmol/L nicotinamide dinucleotide phosphate acid, 0.2 U/mL glutathione reductase, and 70 mmol/L 5,5′-dithiobis(2-nitrobenzoic acid). To determine reduced glutathione, the supernatant was neutralized with 2 mol/L potassium hydroxide, to react with 70 mmol/L 5,5′-dithiobis(2-nitrobenzoic acid), and the absorbance values measured at 420 nm.Citation21
Statistical analysis
Data were reported as mean ± standard error of the mean, and (one- or two-way) analysis of variance followed by Student’s t-test and Newman–Keuls post hoc test was used to compare the groups. Associations between variables were analyzed by Pearson’s correlation. Differences were considered significant when P<0.05.
Results
Metabolic evaluations
The initial body weight was higher in OO and OC groups when compared with young groups (YC and YO). At the end of the protocol, both the YO and YC groups showed a significant increase in body weight gain when compared with the initial week, while the OC and OO groups did not. The ovariectomy has caused an additional increase in body weight in YO group when compared with the YC ().
Table 1 Metabolic evaluations and physical capacity in YC, YO, OC, and OO groups
OC and OO groups presented a significant increase of triglyceride levels when compared with the YC and YO groups (). No differences between groups have been observed regarding blood glucose and cholesterol ().
The constant decrease of plasma glucose was lower in the OC and OO groups at the end of the protocol when compared with the YC. No differences have been shown between OO and YO groups in this parameter (), which is an indicator of insulin-resistant state induced by age or OVX.
The WAT weight at the end of the protocol was increased in the YO, OC, and OO groups when compared with YC group ().
Physical capacity
Regarding the speed in the maximum physical capacity test, both ovariectomized groups (YO and OO) have shown reduced capacity when compared with both YC and OC groups at the end of the study, suggesting that ovariectomy has caused a further loss of this capacity, not only in the old animals, but also in the young ones. The OO group presented an additional impairment in the physical capacity when compared with OC group ().
Cardiac morphometry
Echocardiographic evaluation data, as shown in , have shown that left ventricular mass and left ventricular diameter during diastole increased in older groups (OC and OO) when compared with young groups (YC and YO). No differences were observed between groups regarding relative wall thickness ().
Table 2 Echocardiographic measurements in YC, YO, OC, and OO groups
Cardiac function
In young animals, there were no significant differences in systolic and diastolic function. Moreover, no differences were observed regarding ejection fraction and shortening fraction among all groups. As to diastolic function, the E/A ratio and isovolumetric relaxation time observed in the OC and OO groups were increased when compared with the YC and YO groups. E wave deceleration time (Dec E) was impaired only in the OO group when compared with YO. On the other hand, the myocardial performance index (MPI), which is the ratio of total time spent in isovolumic activity (isovolumic contraction time and isovolumic relaxation time) to the ejection time, an index of cardiac overload, was higher in OC and OO than in YC and YO groups ().
Hemodynamic and autonomic assessments
Ovariectomy induced an increase in MAP values in YO and OO groups when compared with the YC and OC groups (YC, 107±1.47; YO, 117±2.04; OC, 110±1.67; OO, 119±1.86 mmHg, P<0.001). OVX, when associated with aging process, did not show any additional increase than already observed in YO to MAP. HR was increased in OC and OO at the end of the protocol when compared with YC and YO groups (YC, 354±4; YO, 363±7; OC, 383±12; OO, 411±20 bpm, P<0.001).
The baroreceptor reflex sensitivity evaluation has shown that the baroreflex tachycardic responses were reduced in YO, OC, and OO rats in relation to controls at rest. Additionally, OO had fewer responses when compared with YO. The baroreflex bradycardic responses were significantly reduced in old animals as compared with controls at rest ().
Table 3 Cardiac autonomic control in YC, YO, OC, and OO groups
Aging has caused reduced vagal tonus. Indeed, the OC and OO groups had lower vagal tonus in relation to YC and YO groups. On the other hand, ovariectomy and/or aging seem to have caused an increase in sympathetic tonus in groups YO, OC, and OO when compared with YC group. The aging process has reduced the vagal effect on OC and OO groups when compared with the young groups (YC and YO) and OO has shown an additional reduction in relation to OC. In turn, sympathetic effect was higher in OC and OO groups when compared with YC and YO groups and increased in the YO rats when compared with the YC rats. The old groups (OC and OO) have shown reduction in IHR ().
Inflammatory profile
No difference was observed in IL-4 and IL1B among all groups. However, OC and OO groups showed a significant increase in IL-6 when compared with YC and YO. TNF-α values were increased in old controls (OC and OO) when compared with the YC group.
Oxidative stress profile
Regarding oxidative stress in cardiac tissue, protein oxidation was higher only in OO when compared with YC group. The membrane lipoperoxidation (TBARS) in the cardiac tissue was higher in OC and OO groups than in the YC group. Regarding antioxidant enzymes, no differences in superoxide dismutase activity among groups were observed. OVX increased cardiac GPx and CAT, regardless of age. Importantly, both the YO and OO rats showed a decreased glutathione redox balance (GSH/GSSG) when compared with YC and OC rats, thus indicating impairment in redox balance ().
Table 4 Inflammatory and oxidative stress evaluations in YC, YO, OC, and OO groups
Correlations
Correlations were found between increased WAT and the increase in plasma IL-6 (r=0.5, P=0.009) () and in cardiac sympathetic effect (r=0.7, P=0.0002), as well as in the reduction in insulin sensitivity (r=−0.5, P=0.004).
Figure 1 Pearson’s correlation analysis involving all studied groups: (•) represent YC and YO rats and (▪) represent OC and OO rats.
Abbreviations: GSH/GSSG, glutathione redox balance; IL, interleukin; MPI, myocardial performance index; OC, old (24 months old) control; OO, old ovariectomized; TNF, tumor necrosis factor; YC, young (5 months old) control; YO, young ovariectomized.
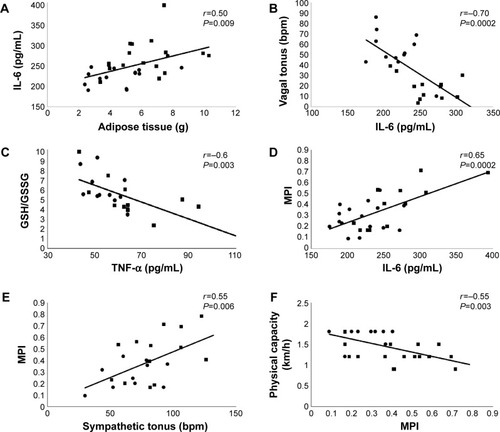
Cardiac autonomic dysfunction, represented by increased sympathetic tonus (ST) (r=0.6, P=0.002) and reduced vagal tonus (VT) (r=−0.7, P=0.0002) (), was correlated with increased levels of IL-6. The levels of IL-6 were also positively correlated with levels of TNF-α (r=0.7, P=0.0001).
The TNF-α was inversely correlated with GSH/GSSG (r=−0.6, P=0.003) (), suggesting that animals showing higher levels of inflammatory markers also presented higher levels of cardiac oxidative stress. Finally, the global cardiac function, represented by the MPI, was positively correlated with IL-6 (r=0.65, P=0.0002) (), TNF-α (r=0.5, P=0.008), and ST (r=0.55, P=0.006) () and negatively correlated with physical capacity (r=−0.55, P=0.003) ().
Discussion
The results in this trial are consistent with the hypothesis that early OVX and/or aging may lead to sympathetic activation, which interacts with inflammation and oxidative stress, promoting cardiac dysfunction and loss in physical capacity.
Metabolic changes
The finding of higher weight gain in young ovariectomized rats has been repeatedly describedCitation22 and has been attributed to the reduction in energy consumption caused by estrogen deprivation and higher food intake.Citation23 In contrast, old female ovariectomized rats did not change body weight, probably due to previous weight gain, associated with the aging process. However, the accumulation of WAT – a more important predictor of CVD than total weight – was increased in all ovariectomized rats (YO, OC, and OO) when compared with YC. Moreover, the increase in sympathetic effect associated with WAT weight (and possibly WAT function) may contribute to increased AP and central obesity.Citation24 In addition, the association between WAT and plasma IL-6 increase indicates an interaction between metabolic and inflammatory system to promote deleterious changes associated with menopause.
Insulin resistance in YO group was correlated with low levels of adiponectin,Citation25 while in old animals (OC and OO) it was associated with increased triglyceride levels. In particular, WAT has been recognized for the role that it plays in regulating energy balance, not only as a fat storage location, but also as a source of leptin and other adipokines and lipid signaling molecules, such as ceramides or diglycerides.Citation26 Regarding metabolic parameters, only triglycerides increased in OC and OO groups, while total cholesterol was similar among all groups. This finding may be associated with the fact that with the gradual decline of estrogen in the climacteric period, low-density lipoprotein receptor activity is decreased.Citation27
Autonomic and cardiac function
In the study on the influence of the autonomic nervous system in the inflammatory response, Satapathy et alCitation28 found a suppression of circulating TNF-α levels in rats treated with galantamine (cholinesterase inhibitor), together with a reduction in body weight, abdominal adiposity, and insulin resistance. In this sense, the following events could be shown in our trial: dysautonomia, increased inflammatory markers, increased body and WAT weight, and insulin resistance in all groups when compared with YC. The systolic function was similar among the four groups. However, the evaluation of diastolic function showed decreased E/A ratio, while isovolumetric relaxation time and deceleration E was increased in old (OC and OO) when compared with young groups (YC and YO). Abnormalities in these parameters suggest that the left ventricle cannot fill properly in the period between contractions. Left ventricular morphometric evaluations indicated that old rats (OC and OO) presented a higher left ventricular mass and increased left ventricular diameter during diastole when compared with young groups (YC and YO). Importantly, the global index of heart function quantified by the MPI was higher in old when compared with young animals, thus suggesting that the aging process may contribute to this impairment. Furthermore, a negative correlation has been observed between physical capacity and MPI (r=−0.55). In fact, physical capacity was decreased in aged animals (OC and OO) throughout the protocol when compared with young ones (YC and YO). Additionally, ovariectomy (YO and OO) induced lower physical capacity in young or old rats when compared with the intact controls, at the end of the protocol. The worsening performance observed in aged ovariectomized rats (OO group) suggests that deprivation of ovarian hormones in aging animals may lead to additional reduction in both lean mass (sarcopenia) and bone mass (osteopenia).Citation22
It is important to emphasize that left ventricular diastolic dysfunction and morphometric alterations can result from several factors, such as increased collagen concentration and fibrosis and decreased compliance, which is influenced by the autonomic nervous system and the renin–angiotensin system.Citation29,Citation30
Indeed, increased sympathetic activity to the heart was observed in aged and ovariectomized animals regardless of age, indicating that this could be one of the mechanisms underlying diastolic function impairment in old rats. In addition, the reduction in parasympathetic function observed only in aged animals may lend further support to the finding of a worsened heart function in these animals. These autonomic changes were accompanied by changes in baseline HR when comparing YC and YO with OC and OO groups, possibly caused by disorders observed in cardiac autonomic balance.Citation31 Indeed, it has been shown in this trial that cardiac vagal tonus decreased in old animals (OC and OO), whereas sympathetic tonus increased. However, old groups showed an IHR reduction that may be due to the age-related changes in function of the sinus node.Citation32,Citation33 The findings in this study suggest that ovariectomy aggravates autonomic dysfunction observed in older animals. This increase is probably due to a reduction in the vagal effect, which may be causing a reduction of the reflex bradycardia response mediated by baroreceptors. Although aging produces a decline in the production of ovarian hormones, the removal of the ovaries in older females abolishes the production of these hormones.Citation34,Citation35 And these changes may have led to a greater autonomic dysfunction in the OO group. Indeed, the comparison between the young groups (YC and YO) has shown that the aging process changes cardiac and autonomic profile in female rats. In addition, young female ovariectomized are similar to intact elderly rats, suggesting that many of the differences observed in young groups (YC and YO) have not been observed in the old groups (OC and OO) and these changes are probably due to the aging process. Regarding MAP, an increase in YO and OO rats was observed in comparison with their intact controls. Increased inflammatory status in ovariectomized rats associated with the increased oxidative stress in these groups might be associated with nitric oxide reduction and, consequently, with the changes in AP.Citation33
The underlying mechanisms mediating abnormal vascular function with aging and menopause remain controversial, but in vitro 17β-estradiol treatment increases the elastin and decreases collagen production in the vascular smooth muscle. Besides, 17β-estradiol also exerts protective effects in the prevention of cardiomyocyte apoptosis. Further work needs to be done for a better understanding of this kind of supplementation, since myocardial aging is associated with a myriad of changes, including left ventricular hypertrophy, heart failure, and increased myocardial stiffness that can be correlated with inflammation and oxidative stress generation.
Inflammation and oxidative stress
The decline of immune activation after aging remains unclear despite that there are several trials focusing on it. Inflammatory processes combined with cytokine release are important steps in building a response to tissue injury and they play an active role in cardiac function. Thus, both OC and OO groups were found to have increased IL-6 levels when compared with YC and YO, whereas TNF-α was higher in old groups when compared with YC. Following the hypothesis of a cholinergic anti-inflammatory pathway via vagus nerves, these results would be associated with the lower parasympathetic participation observed in old groups.Citation36,Citation37 In this sense, the correlations found between IL-6 and vagal tonus (r=−0.7) and sympathetic tonus (r=0.65) would further reinforce this hypothesis ().
Indeed, oxidative stress as expressed by TBARS measurements was enhanced in both YO and OO groups in relation to YC and OC groups, indicating that the impairment of heart function in ovariectomized animals may be related to increased oxidative stress in the tissue. It is well established that the increase in lipid membrane peroxidation may be explained by sequestration of hydrogen polyunsaturated fatty acid located in the cell membrane. This, in turn, leads to the loss of selectivity of iron exchange and release of organelles content, together with the formation of toxic substances causing cell death.Citation38 Indeed, a previous study demonstrated a positive correlation between AP and lipid peroxidation, thus reinforcing the role of oxidative stress in AP changes during hormone deprivation.Citation5 The decreased lipoperoxidation observed in both YC and OC groups when compared with ovariectomized rats seems to be related to the antioxidant properties of estrogens and their regulatory action on antioxidant enzymes.Citation30,Citation39
Moreover, protein oxidation was higher in OO when compared with YC group indicating that not only the advancing age, but also the ovariectomy increased protein damage.Citation40,Citation41
OVX has caused an increase in GPx activity in YO and OO, when compared with YC and OC. These changes could be explained as compensatory mechanisms since CAT was not different among groups and both enzymes compete for the same substrate (hydrogen peroxide).Citation42 It should be noted that the GSH/GSSG decreased in YO, OC, and OO, when compared with YC. Moreover, YO and OO had a further decrease in GSH/GSSG when compared with OC group. These results indicate that aging and/or OVX might be associated with impairment in redox balance. In this aspect, the data obtained by correlation analyses reinforce the hypothesis that cardiac remodeling is associated with increased oxidative stressCitation43 and inflammation (correlations between TNF-α with GSH/GSSG, r=−0.6; MPI with IL-6 and TNF-α, r=0.65 and r=0.5, respectively). Consequently, both mechanisms may lead to the impairment of global left ventricular function.
Study limitation
Some limitations in the current study should be pointed out, such as no data on mortality. In addition, estrogen- and progesterone-related data were not quantified in this protocol, although we have previously performed this evaluation.
Clinic implications
In women, aging coincides with menopause, during which there is a significant reduction in the production of ovarian hormones. Information in this study has shown that aging alone induces autonomic, biochemical, and functional changes that can be exacerbated by menopause, thus, giving support to the search for early interventions during this physiological process that can mitigate the deleterious effects of aging in female individuals.
Conclusion
It was shown in this study that OVX aggravates the impairment of cardiac and functional effects of aging in female rats, probably associated with exacerbated autonomic dysfunction, inflammation, and oxidative stress. In this sense, preventing and/or attenuating autonomic dysfunction triggered by aging process may result in a lower proinflammatory and oxidative stress profile, along with preserved cardiac function, possibly leading to improved quality of life and life expectancy of this female population after OVX.
Acknowledgments
This study was supported by CNPq (563961/2010- MCI, KA; 484713/2011- MCI, KA; 306 011/2010-MCI; 457200/2014-6; 309292/2014-0 KA); CAPES (PVE-074/12-JFM, KA, MM, MCI; 88881.062178/2014-01 KA), and FAPESP 2012/20141-5 KA.
Disclosure
The authors report no conflicts of interest in this work.
References
- GovindarajuDRPencinaKMRajDSMassaroJMCarnesBAD’AgostinoRBA systems analysis of age-related changes in some cardiac aging traitsBiogerontology201415213915224337960
- World Health OrganizationCardiovascular Diseases (CVDs)2015 Fact Sheet N° 317: Available from: www.who.int/mediacentre/factsheets/fs317/en/Accessed February 21, 2015
- American Heart Association’s Go Red For Women and Ad Council Launch National Public Service Advertising Campaign to Address a Leading Killer of Women in the U.S. Heart DiseaseEINNews11112014
- KochanekKDXuJMurphySLMininoAMKungHCDeaths: final data for 2009Natl Vital Stat Rep2011603111624974587
- IrigoyenMCPauliniJFloresLJExercise training improves baroreflex sensitivity associated with oxidative stress reduction in ovariectomized ratsHypertension2005464998100316157791
- FloresLJFigueroaDSanchesICEffects of exercise training on autonomic dysfunction management in an experimental model of menopause and myocardial infarctionMenopause201017471271720577132
- MarcondesFKBianchiFJTannoAPDetermination of the estrous cycle phases of rats: some helpful considerationsBraz J Biol2002624A60961412659010
- LatourMGShinodaMLavoieJMMetabolic effects of physical training in ovariectomized and hyperestrogenic ratsJ Appl Physiol (1985)200190123524111133915
- SanchesICde Oliveira BritoJCandidoGOCardiometabolic benefits of exercise training in an experimental model of metabolic syndrome and menopauseMenopause201219556256822157682
- FluesKPauliniJBritoSExercise training associated with estrogen therapy induced cardiovascular benefits after ovarian hormones deprivationMaturitas201065326727120004069
- BrooksGAWhiteTPDetermination of metabolic and heart rate responses of rats to treadmill exerciseJ Appl Physiol Respir Environ Exercise Physiol197845610091015
- BonoraEMoghettiPZancanaroCEstimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studiesJ Clin Endocrinol Metab19896823743782645308
- WichiRMalfitanoCRosaKNoninvasive and invasive evaluation of cardiac dysfunction in experimental diabetes in rodentsCardiovasc Diabetol200761417462095
- Moraes-SilvaICDe La FuenteRNMostardaCBaroreflex deficit blunts exercise training-induced cardiovascular and autonomic adaptations in hypertensive ratsClin Exp Pharmacol Physiol2010373e114e12019930428
- NegraoCEMoreiraEDBrumPCDenadaiMLKriegerEMVagal and sympathetic control of heart rate during exercise by sedentary and exercise-trained ratsBraz J Med Biol Res19922510104510521342828
- BuegeJAAustSDMicrosomal lipid peroxidationMethods Enzymol197852302310672633
- ReznickAZPackerLOxidative damage to proteins: spectrophotometric method for carbonyl assayMethods Enzymol19942333573638015470
- MarklundSLSuperoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c miceMutat Res19851481–21291343969077
- AebiHCatalase in vitroMethods Enzymol19841051211266727660
- FloheLGunzlerWAAssays of glutathione peroxidaseMethods Enzymol19841051141216727659
- AndersonMEDetermination of glutathione and glutathione disulfide in biological samplesMethods Enzymol19851135485554088074
- FerreiraJAFoleyAMBrownMSex hormones differentially influence voluntary running activity, food intake and body weight in aging female and male ratsEur J Appl Physiol201211283007301822170012
- ShimomuraKShimizuHTsuchiyaTAbeYUeharaYMoriMIs leptin a key factor which develops obesity by ovariectomy?Endocr J200249441742312402973
- LandsbergLAronneLJBeilinLJObesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of HypertensionJ Clin Hypertens (Greenwich)2013151143323282121
- GulcelikNEHalilMAriogulSUsmanAAdipocytokines and aging: adiponectin and leptinMinerva Endocrinol201338220321023732375
- WronskaALawniczakAWierzbickiPMGoykeESledzinskiTKmiecZWhite adipose tissue depot-specific activity of lipogenic enzymes in response to fasting and refeeding in young and old ratsGerontology20156144845525721559
- KullerLHMeilahnENCauleyJAGutaiJPMatthewsKAEpidemiologic studies of menopause: changes in risk factors and diseaseExp Gerontol1994293–44955097925767
- SatapathySKOchaniMDanchoMGalantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed miceMol Med2011177–859960621738953
- MostardaCMoraes-SilvaICSalemiVMExercise training prevents diastolic dysfunction induced by metabolic syndrome in ratsClinics (Sao Paulo)201267781582022892928
- ArnalJFClamensSPechetCEthinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion productionProc Natl Acad Sci U S A1996939410841138633024
- TeziniGCBecariCZanottoCZSalgadoMCPassaglia RdeCSouzaHCAgeing is the main determinant of haemodynamics and autonomic cardiac changes observed in post-menopausal female ratsAuton Neurosci20131741–2364123291358
- JoynerMJNot so fast: intrinsic heart rate vs. beta-adrenergic responsiveness in the aging human heartJ Appl Physiol (1985)200810513418483159
- Rubio-RuizMEPerez-TorresISotoMEPastelinGGuarner-LansVAging in blood vessels. Medicinal agents FOR systemic arterial hypertension in the elderlyAgeing Res Rev20141813214725311590
- LopesGSFerreiraATOshiroMEAging-related changes of intracellular Ca2+ stores and contractile response of intestinal smooth muscleExp Gerontol2006411556216343836
- LopesGSMoraOACerriPMitochondrial alterations and apoptosis in smooth muscle from aged ratsBiochim Biophys Acta20041658318719415450956
- AnderssonUTraceyKJNeural reflexes in inflammation and immunityJ Exp Med201220961057106822665702
- BorovikovaLVIvanovaSZhangMVagus nerve stimulation attenuates the systemic inflammatory response to endotoxinNature2000405678545846210839541
- HershkoCMechanism of iron toxicity and its possible role in red cell membrane damageSemin Hematol19892642772852683097
- BarbacanneMARamiJMichelJBEstradiol increases rat aorta endothelium-derived relaxing factor (EDRF) activity without changes in endothelial NO synthase gene expression: possible role of decreased endothelium-derived superoxide anion productionCardiovasc Res199941367268110435039
- CarrardGDieuMRaesMToussaintOFriguetBImpact of ageing on proteasome structure and function in human lymphocytesInt J Biochem Cell Biol200335572873912672464
- HerrmannJSoaresSMLermanLOLermanAPotential role of the ubiquitin-proteasome system in atherosclerosis: aspects of a protein quality diseaseJ Am Coll Cardiol200851212003201018498952
- De AngelisKLCestariIABarpJOxidative stress in the latissimus dorsi muscle of diabetic ratsBraz J Med Biol Res200033111363136811050669
- RosaCMXavierNPHenrique CamposDDiabetes mellitus activates fetal gene program and intensifies cardiac remodeling and oxidative stress in aged spontaneously hypertensive ratsCardiovasc Diabetol20131215224134628
