?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Despite several, well-documented pro-healthy effects of regular physical training, its influence on body iron stores in elderly people remains unknown. At the same time, body iron accumulation is associated with high risk of different morbidities.
Purpose
We hypothesized that Nordic Walking training would result in pro-healthy changes in an elderly group of subjects by reducing body iron stores via shifts in iron metabolism-regulating proteins.
Methods
Thirty-seven women aged 67.7±5.3 years participated in this study. They underwent 32 weeks of training, 1-hour sessions three times a week, between October 2012 and May 2013. Fitness level, blood morphology, CRP, vitamin D, ferritin, hepcidin, and soluble Hjv were assessed before and after the training.
Results
The training program caused a significant decrease in ferritin, which serves as a good marker of body iron stores. Simultaneously, the physical cardiorespiratory fitness had improved. Furthermore, blood hepcidin was positively correlated with the ferritin concentration after the training. The concentration of blood CRP dropped, but the change was nonsignificant. The applied training resulted in a blood Hjv increase, which was inversely correlated with the vitamin D concentration.
Conclusion
Overall the Nordic Walking training applied in elderly people significantly reduced blood ferritin concentration, which explains the observed decrease in body iron stores.
Introduction
Iron is an essential element for most of the processes in our body including oxygen transport, cellular reparation, DNA synthesis, and many others. Conversely, iron may be very toxic due to its participation in the formation of reactive oxygen species (ROS). This pro-inflammatory action of iron points to the redox-regulation of the transcription factors’ activity such as NF-κB,Citation1,Citation2 which controls the expression of more than 500 different gene products, the pro-inflammatory cytokines among them.Citation3 Due to its toxicity, iron must be transported and stored in a safe way. In blood, iron is transported by transferrin and stored within cells by ferritin. Both these forms of iron are safe as neither stimulates ROS formation.
Iron induced ROS formation is called labile iron pool (LIP) or chelatable iron loosely bound to amino acids, nucleotides etc. In cells, the elevated LIP leads to an increase in ferritin biosynthesis. Thus, the high cellular ferritin concentrations usually correspond with high iron content.Citation4 In addition, ferritin is also considered to be the anti oxidant protein, which protects iron from the redox reactions.Citation5 It is debatable whether the amount of iron stored correlates with LIP and whether the iron stored is safe.Citation6 Evidence exists showing that it is not. For example, blood ferritin concentration correlated with DNA damage in humans and animals.Citation7,Citation8 Moreover, studies on cell cultures demonstrated that ferritin undergoes degradation releasing iron, when SAPK are active.Citation9,Citation10 These data indicate that any procedure which can reduce body iron stores may be beneficial for human health.
Iron metabolism is controlled by hepcidin, a hormone synthetized mainly by the liver. By blocking iron absorption from the duodenum, hepcidin limits its accumulation in the body.Citation11 The influence of exercise on iron metabolism had not been fully understood until recently, when skeletal muscles were found to produce and release myokines during exercise, exerting an anti-inflammatory effect and acting as energy sensors.Citation12 One of these myokines is IL-6, which up-regulates the anti-inflammatory cytokines such as IL-10, TNF-R, and some others.Citation13 Moreover, IL-6 induces biosynthesis and an increase in blood hepcidin concentration. In recent years, studies have demonstrated that both single and regular exercise among adults cause the rise of blood hepcidin, diminishing dietary iron absorption and body iron stores.Citation14,Citation15
A study on elderly subjects has demonstrated a relatively high prevalence (12.9%) of elevated iron stores (serum ferritin [SF] >300 ng/mL in men and SF >200 ng/mL in women).Citation16 To our knowledge, no studies so far have investigated the effect of long-lasting Nordic Walking (NW) training on hepcidin concentration in elderly individuals. Moreover, we extended our study to vitamin D status and soluble Hjv (sHjv) evaluation. Vitamin D has been demonstrated to carry an anti-inflammatory activity and its deficiency can lead to an increase in liver iron accumulation.Citation17–Citation19 On the other hand, sHjv can affect body iron metabolism by blocking hepcidin biosynthesis.Citation20 Consequently, the present study was designed to evaluate the effect of 32 weeks of a regular NW training from two perspectives. First, we evaluated the effect of this popular physical activity on the inflammation response and protein regulating iron metabolism. Second, we assessed if changes induced by applied exercise would be modified by the vitamin D concentration.
Materials and methods
Ethics statement
This study was registered with the Medical University of Gdansk Clinical Trials Registry (ID: NKBBN/523/2013) and approved by the Independent Bioethics Commission for Research of Medical University of Gdansk according to the Helsinki Declaration. Before commencing the training and testing, subjects received a verbal description of the experiment. Written informed consent was signed by all participants. Ethics approval was obtained for the referral of participants to their family physician upon detection of any abnormal pathology results and review by the study medical officer.
Subjects
Thirty-seven women originally completed this study (67.7±5.3 years of age, height of 162.5±5.1 cm, weight of 68.6±10.0 kg) (). They were recruited via an advertisement placed in a local newspaper in September 2012. All subjects underwent a medical checkup prior to the experiment. Those with uncontrolled hypertension (diastolic blood pressure over 100 mmHg), a history of cardiac arrhythmia, cardio-respiratory disorders, and orthopedic problems were excluded from the study (n=15) and six quit the study along the way. The analysis and training program were completed at the Gdansk University of Physical Education and Sport.
Experimental design
Three months before the main experiment we held a briefing with the volunteers. The aim of this meeting was to introduce the procedure of our experiment and draw attention to the participants’ dietary habits focusing on the source of iron in daily nutrition. It was recommended that the volunteers do not change their diet throughout the experiment.
One week prior to the start of the experiment, body composition and aerobic capacity were determined. Body mass and composition were estimated using bioelectrical impedance (TBF-300 Body Fat Monitor/Scale Analyzer, Tanita, Tokyo, Japan) floor scale with an accuracy of 0.1 kg. The measurements were taken 1 hour before breakfast. Participants had voided their bladders and bowels prior to the assessment. During the measurement, subjects wore only briefs and remained barefoot. The device was calibrated prior to each measurement session. Data accuracy was equal to 98%.Citation21 Additionally, the body mass index was calculated. The exercise test was preceded and followed by a cardiopulmonary function test and blood measurements.
Cardiorespiratory fitness assessment
The 2,000 m walking test was used to determine the aerobic capacity of the subjects.Citation22 Before the test verbal instruction was given to all participants. The test was performed on an athletic track where the temperature was 18°C. The test consisted of two stages: first, the reference phase, warm-up (3-minute walk and stretching exercises) and second, the main test of ten laps, each 200 m long. Time was measured using photoelectric cells (Racetime 2 SF, Microgate, Bolzano, Italy) with an accuracy of 0.001 second. The start of the movement was signaled by the instructor. To evaluate the maximal oxygen capacity a mathematic formula was applied.
NW training intervention
The physical training lasted 32 weeks between October 2012 and May 2013. Subjects met every Monday, Wednesday, and Friday at 9 am, 1 hour after eating a light breakfast. The same group of research assistants and coaches conducted and supervised all training sessions. Each training sessions lasted 1 hour (10-minute warm-up, 40-minute NW, and 10-minute cool-down) and had 60%–70% intensity of the maximal ability based on a 2,000 m walking test. During the applied training program all of the women covered 299 km 600 m (average 3 km 120 m on the training) of which 92 km 400 m were uphill (average 1 km 200 m on the training), 94 km 700 m were downhill (average 1 km 90 m on the training), and 112 km 500 m were flat (average 830 m on the training).
Blood analysis
Blood samples were collected at rest, in the morning after an overnight fasting. Next, the subjects were instructed to have a light breakfast. Blood samples were collected at two time points of the experiment: before starting the NW training and after 32 weeks of training. Serum CRP, Hjv, and hepcidin were determined by enzyme immunoassay methods using commercial kits (catalog no DCRP00, SEB979Hu, SEB995Hu). The average intra-assay coefficients of variability (CV) was <10% for all assessments. SF was measured by SYSMEX XE 2100, Architect ci 8200, and Test 1 SDL.
Vitamin D determination
The vitamin D metabolite 25-hydroxy D3 (25OHD3) was measured by high performance liquid chromatography mass spectrometry. The high performance liquid chromatography system was a Transcend TLX turboflow 2 system attached to a TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
The results were analyzed using Statistica 9.0 software. They are expressed as mean values and standard deviations. The Shapiro–Wilk test was applied to assess the homogeneity of dispersion from the normal distribution. For normally distributed results, a paired t-test analysis was performed to identify significantly different results. For not normally distributed results, a Wilcoxon signed-rank test was applied. Relationships between variables were evaluated using a Pearson correlation coefficient. Moreover, coefficient of determination was calculated. The significance level was set at P<0.05.
Results
General outcomes
Thirty-seven women completed the study with no adverse events being reported. Attendance at training units was equal to 80%±5%. Basic anthropometric and physiological characteristics of the participants are summarized in . The NW training caused significant changes in body composition. The applied 32-week procedure decreased the body fat content and increased the free fat mass (). However, the cardiorespiratory fitness capacity did not change following the training period ().
Table 1 Anthropometric and physiological characteristics of participants
Hematological parameters were within the reference ranges in all subjects at baseline as well as after the training. Still, a significant drop in hematocrit, hemoglobin (Hb), mean corpuscular Hb concentration, and mean corpuscular Hb was observed after the training period (). Importantly, however, no iron deficiency or anemia was noted.
Table 2 General characteristics of blood tests of participants
NW training induced the drop in blood ferritin
Interestingly, a strong correlation between the body iron stores and blood ferritin was recorded; thus, we aimed to estimate the effect of the NW training on the blood ferritin concentration.Citation23 The obtained data revealed that the blood ferritin concentration had significantly decreased after the 32 weeks of training (). Therefore, it might be concluded that the NW training had resulted in a significant reduction of the body iron stores.
Table 3 The effect of 32 weeks of Nordic Walking (NW) training on indicators of iron metabolism among all groups of participants
Changes in blood hepcidin concentration
Hepcidin is the main regulator of iron metabolism. Its concentration was evaluated, in order to provide insight into the mechanism of the NW training as well as check if the induced drop in the ferritin concentration had depended on the blood hepcidin. Contrary to previous observations, the applied physical training did not trigger significant changes in blood hepcidin. It is important to note that the hepcidin concentration was within reference range.Citation14,Citation24
Inflammation is one of the main factors affecting hepcidin biosynthesis; thus, the CRP was measured as a marker for this condition. The applied training procedure induced a drop in CRP; however, the changes were not statistically significant (). Interestingly, a detailed analysis showed that among 37 women, the applied training resulted in the rise of blood hepcidin in 16 subjects, whereas in the remaining 21 a decrease was noted. Still, the correlation calculated between delta CRP and delta hepcidin recorded after 32 weeks of the NW training was not significant (data not shown). It is important to note that a positive correlation was observed between hepcidin and CRP after the training. In addition to inflammation, stored iron is another factor influencing the hepcidin level.Citation25 In fact, following the training procedure blood hepcidin and ferritin were observed to be positively correlated, which is a good marker of body iron stores ().
Figure 2 Nordic Walking (NW) training induced changes in hormones regulating iron metabolism.
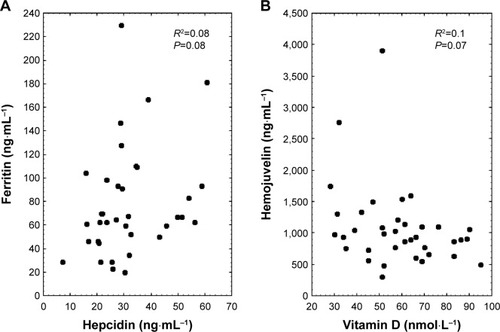
sHjv is another protein present in blood which may influence hepcidin by inhibiting its biosynthesis.Citation20 Thirty-two weeks of the NW training caused the blood sHjv concentration to rise significantly (). Contrary to previous observations, our calculations show that changes in sHjv did not correlate with changes in hepcidin or CRP (data not shown).Citation26
Vitamin D status is not associated with changes in hepcidin
Aside from the well-documented, anti-inflammatory effect of vitamin D,Citation18,Citation27 some changes in iron metabolism were noticed in animals with vitamin D deficiency.Citation17 Therefore, the next goal of our study was to evaluate whether the changes observed in the inflammation markers and iron homeostasis were related to changes in the concentration of 25OHD3 (marker of vitamin D status). As shown in , a drop in the 25OHD3 concentration was observed after the training; however, the changes did not correlate with changes in CRP (r=−0.18, P=0.27) and hepcidin (r=−0.21, P=0.22). Interestingly, a negative correlation between sHjv and 25OHD3 was observed after the training (r=−0.31, P=0.07).
Discussion
In the present study, we demonstrate that 32 weeks of NW training significantly reduced body iron stores in elderly women. To the best of our knowledge, this is the first study reporting such a phenomenon. High iron stores are regarded as a risk factor for morbidities such as cancer, heart disease, neurodegenerative disorders, arteriosclerosis, and many others.Citation28 For example, a 1% rise in blood ferritin increases the risk of heart attack by 4%.Citation29 In Finland, men with SF 200 ng·mL−1 or higher had 2.3 times as many heart attacks as men with SF 100 ng·mL−1. Notably, lower blood iron concentration was observed in nonagenarians and centenarians with respect to controls.Citation30 In another study regular phlebotomy, which is considered the best way to reduce body iron stores, was shown to reduce cancer incidence by 36.7% in elderly people.Citation31 Consequently, maintaining low body iron stores can be recommended as an effective strategy of decreasing the risk of diseases. Seventy-five percent of new cancer cases have been diagnosed among patients exhibiting mean ferritin levels during follow-up of greater than 57 ng·mL−1.Citation31 Moreover, it was reported that blood ferritin higher than 150 ng·mL−1 correlates negatively with cardiovascular fitness in young men.Citation32 Notably, 45.5% of young men are characterized by ferritin 150 ng·mL−1 or higher.Citation32 Our data demonstrate that NW training together with phlebotomy or reduction in iron consumption may constitute supplementary methods of reducing body iron stores. This observation is in agreement with an earlier study which demonstrated that people who spend more leisure time on physical activity are characterized by lower blood ferritin concentration and Hb content.Citation33 The limitation of this study is that there was no control group; however, it is worth noting that iron stores rise slowly with aging. Thus, it can be expected that effect of time could be negligible.Citation34 It is important to note that despite the drop observed in Hb, mean corpuscular Hb, and mean corpuscular Hb concentration, all of these parameters remain in normal physiological ranges. Lower Hb was previously linked with a lower risk of lung cancer in womenCitation35 and the prevalence of gestational diabetes mellitus.Citation36 In light of these arguments, the observed changes in Hb should be considered positive.
In many countries, NW is a very popular form of exercise with thousands of enthusiasts. Specially designed poles are used to push against the ground with each stride for the purpose of activating the upper as well as the lower body. NW was shown to increase gait speed and oxygen consumption more effectively than conventional walking.Citation37 NW is also commonly used in rehabilitation of patients with different kinds of morbidities.Citation38,Citation39 NW also has favorable effects on functional capacity in elderly people.Citation40–Citation42
To attain insight into the mechanism responsible for NW training induced changes in iron metabolism, hormones controlling the iron status were evaluated. It has recently been shown that single exercise leads to an increase in blood hepcidin;Citation14,Citation25,Citation43 thus, we hypothesized that a drop in body iron stores would be accompanied by an elevated level of hepcidin. Its synthesis increases in response to high iron content and inflammatory cytokines whereas its level drops during hypoxia and iron deficiency. Accordingly, our data show that post-training hepcidin concentration positively correlates with blood ferritin. Recently, Peeling et al reported that post-exercise hepcidin did not change in subjects whose blood ferritin was <30 ng/mL and significantly rose in those with blood ferritin >30 ng/mL.Citation25 Therefore, we assumed that the effects of training on hepcidin may be dependent on subjects’ body iron stores. Interestingly, such correlation was not observed at the beginning of the applied training (). Thus, it can be concluded that the NW exercise procedure somehow restored the iron dependent rise in hepcidin.
Hjv is a glycosylphosphatidyl inositol-linked protein cell membrane. It functions as a BMP co-receptor activating hepcidin expression through a BMP/SMAD signaling pathway.Citation44 In addition to residing on the cell membrane, part of Hjv can be cleaved and secreted in a soluble form present in blood. sHjv can selectively bind to BMP ligands and inhibit endogenous and BMP-induced hepcidin expression. Administration of sHjv decreases hepcidin expression in vivo.Citation20 To the best of our knowledge, this is the first report demonstrating the effect of regular training on Hjv. Our data show that sHjv significantly rises after the training program and, contrary to our expectation, positively correlates with the blood hepcidin following the exercise period (data not shown). It can be speculated that the observed rise in blood sHjv is of muscle origin although the biological meaning of these changes is not known. Recent reports showed that systemic iron homeostasis and liver hepcidin expression were not affected in knockout mice, which lack muscle Hjv;Citation45 thus, it is possible that the observed correlation is random.
Inflammation is another important factor affecting iron metabolism. Pro-inflammatory cytokines were demonstrated to induce HAMP gene expression which encodes hepcidin.Citation46 The present study reveals that the NW training-induced equivocal changes in hepcidin and delta CRP does not correlate with delta hepcidin. However, after the NW training a positive correlation is observed between hepcidin and CRP indicating a role of inflammation in changes in hepcidin. Excess in iron is considered to stimulate inflammation processes as LIP can activate the transcription factor NF-kB, which controls expression of the pro-inflammatory cytokines.Citation47 Thus, hepcidin, which impairs iron absorption and in consequence decreases body iron stores, may be considered an anti-inflammatory hormone. Since a positive correlation was noted between blood ferritin and CRP both before and after the applied training, our data support these observations. These data indicate that a decrease in iron stores is associated with a lower grade of systemic inflammation in elderly persons.
Inflammation processes can also be influenced by vitamin D. Different forms of vitamin D were shown to reduce pro-inflammatory signaling.Citation48 Our data indicate that NW-induced changes in CRP are not associated with 25OHD3, a good marker of vitamin D status. It is important to note that some subjects had inadequate vitamin D concentrations of 25OHD3 <50 nmolL−1. Moreover, a study performed on mice demonstrated that liver iron was increased in vitamin D-depleted animals.Citation17 Therefore, the effects of vitamin D status on iron metabolism were expected. Indeed, we observed that a rise in sHjv induced by the NW training negatively correlated with 25OHD3 concentration. Thus, our data support the notion that vitamin D may have an influence on iron metabolism in humans even if exact biological meaning of this data is not clear.
Conclusion
In conclusion, our data indicate that the pro-healthy effects of the NW training is manifested by a decreased inflammation and a drop in body iron stores in elderly people. Further investigation is needed to reveal the exact mechanism behind the observed changes.
Acknowledgments
This investigation was supported by the National Science Centre (Poland), project no 2014/15/B/NZ7/00976.
Disclosure
The authors report no conflict of interest in this work.
References
- JimenezLAThompsonJBrownDAActivation of NF-kappaB by PM(10) occurs via an iron-mediated mechanism in the absence of IkappaB degradationToxicol Appl Pharmacol2000166210111010896851
- XiongSSheHTakeuchiHSignaling role of intracellular iron in NF-kappaB activationJ Biol Chem200327820176461765412637578
- GhoshSHaydenMSNew regulators of NF-kappaB in inflammationNat Rev Immunol200881183784818927578
- KruszewskiMLabile iron pool: the main determinant of cellular response to oxidative stressMutat Res20035311–2819214637247
- KonijnAMGlicksteinHVaismanBMeyron-HoltzEGSlotkiINCabantchikZIThe cellular labile iron pool and intracellular ferritin in K562 cellsBlood19999462128213410477743
- SullivanJLIs stored iron safe?J Lab Clin Med2004144628028415614249
- NakanoMKawanishiYKamoharaSOxidative DNA damage (8-hydroxydeoxyguanosine) and body iron status: a study on 2507 healthy peopleFree Radic Biol Med200335782683214583347
- BarolloMD’IncaRScarpaMEffects of iron deprivation or chelation on DNA damage in experimental colitisInt J Colorectal Dis200419546146615067556
- BorkowskaASielicka-DudzinAHerman-AntosiewiczAHalonMWozniakMAntosiewiczJP66Shc mediated ferritin degradation – a novel mechanism of ROS formationFree Radic Biol Med201151365866321616139
- AntosiewiczJZiolkowskiWKaczorJJHerman-AntosiewiczATumor necrosis factor-alpha-induced reactive oxygen species formation is mediated by JNK1-dependent ferritin degradation and elevation of labile iron poolFree Radic Biol Med200743226527017603935
- GanzTNemethEHepcidin and disorders of iron metabolismAnnu Rev Med20116234736020887198
- PedersenBKMuscles and their myokinesJ Exp Biol2011214Pt 233734621177953
- PedersenBKThe anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease controlEssays Biochem20064210511717144883
- AntosiewiczJKaczorJJKasprowiczKRepeated “all out” interval exercise causes an increase in serum hepcidin concentration in both trained and untrained menCell Immunol20132831–2121723850671
- PeelingPDawsonBGoodmanCLandersGTrinderDAthletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormonesEur J Appl Physiol2008103438139118365240
- FlemingDJJacquesPFTuckerKLIron status of the free-living, elderly Framingham Heart Study cohort: an iron-replete population with a high prevalence of elevated iron storesAm J Clin Nutr200173363864611237943
- Otto-DuesselMBrewerCWoodJCInterdependence of cardiac iron and calcium in a murine model of iron overloadTransl Res20111572929921256461
- Bednarek-SkublewskaASmolenAJaroszynskiAZaluskaWKsiazekAEffects of vitamin D3 on selected biochemical parameters of nutritional status, inflammation, and cardiovascular disease in patients undergoing long-term hemodialysisPol Arch Med Wewn2010120516717420502401
- ChenYKongJSunT1,25-Dihydroxyvitamin D(3) suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-kappaB activationArch Biochem Biophys2011507224124721176770
- BabittJLHuangFWXiaYSidisYAndrewsNCLinHYModulation of bone morphogenetic protein signaling in vivo regulates systemic iron balanceJ Clin Invest200711771933193917607365
- ZiemannEGrzywaczTLuszczykMLaskowskiROlekRAGibsonALAerobic and anaerobic changes with high-intensity interval training in active college-aged menJ Strength Cond Res20112541104111220661160
- LaukkanenRMKukkonen-HarjulaTKOjaPPasanenMEVuoriIMPrediction of change in maximal aerobic power by the 2-km walk test after walking training in middle-aged adultsInt J Sports Med200021211311610727071
- SimonTLGarryPJHooperEMIron stores in blood donorsJAMA198124520203820437230400
- KanekoYMiyajimaHPipernoAMeasurement of serum hepcidin-25 levels as a potential test for diagnosing hemochromatosis and related disordersJ Gastroenterol201045111163117120533066
- PeelingPSimMBadenhorstCEIron status and the acute post-exercise hepcidin response in athletesPLoS One201493e9300224667393
- ShalevHPerez-AvrahamGKapelushnikJHigh levels of soluble serum hemojuvelin in patients with congenital dyserythropoietic anemia type IEur J Haematol2013901313623095116
- AlvarezJAZughaierSMLawJEffects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney diseaseEur J Clin Nutr2013673267269
- KellDBIron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseasesBMC Med Genomics20092219133145
- SalonenJTNyyssonenKKorpelaHTuomilehtoJSeppanenRSalonenRHigh stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish menCirculation19928638038111516192
- ForteGDeianaMPasellaSMetals in plasma of nonagenarians and centenarians living in a key area of longevityExp Gerontol20146019720625446984
- ZacharskiLRChowBKHowesPSDecreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trialJ Natl Cancer Inst200810014996100218612130
- MainousAG3rdDiazVARelation of serum ferritin level to cardiovascular fitness among young menAm J Cardiol2009103111511819101240
- LakkaTANyyssonenKSalonenJTHigher levels of conditioning leisure time physical activity are associated with reduced levels of stored iron in Finnish menAm J Epidemiol199414021481608023803
- ZacharskiLROrnsteinDLWoloshinSSchwartzLMAssociation of age, sex, and race with body iron stores in adults: analysis of NHANES III dataAm Heart J200014019810410874269
- SelbyJVFriedmanGDEpidemiologic evidence of an association between body iron stores and risk of cancerInt J Cancer19884156776823366489
- LaoTTHoLFImpact of iron deficiency anemia on prevalence of gestational diabetes mellitusDiabetes Care200427365065614988280
- ChurchTSEarnestCPMorssGMField testing of physiological responses associated with Nordic WalkingRes Q Exerc Sport200273329630012230336
- HanuszkiewiczJMalickaIBarczyk-PawelecKWozniewskiMEffects of selected forms of physical activity on body posture in the sagittal plane in women post breast cancer treatmentJ Back Musculoskelet Rehabil2015281354224968794
- LatosikEZubrzyckiIZOssowskiZPhysiological Responses Associated with Nordic-walking training in Systolic Hypertensive Postmenopausal WomenJ Hum Kinet20144318519025713659
- ParkattiTPerttunenJWackerPImprovements in functional capacity from Nordic walking: a randomized-controlled trial among elderly peopleJ Aging Phys Act20122019310521949243
- LapszoJGiovanisVPrusikKPrusikKBalance control contributors – the relationships between leg strength and balance control ability in seniorsActa Bioeng Biomech20121433823140087
- TschentscherMNiederseerDNiebauerJHealth benefits of Nordic walking: a systematic reviewAm J Prev Med2013441768423253654
- Skarpanska-StejnbornABastaPTrzeciakJSzczesniak-PilaczynskaLEffect of intense physical exercise on hepcidin levels and selected parameters of iron metabolism in rowing athletesEur J Appl Physiol2015115234535125311752
- Mleczko-SaneckaKCasanovasGRagabASMAD7 controls iron metabolism as a potent inhibitor of hepcidin expressionBlood2010115132657266520040761
- ChenWHuangFWde RenshawTBAndrewsNCSkeletal muscle hemojuvelin is dispensable for systemic iron homeostasisBlood2011117236319632521493799
- AndrewsNCAnemia of inflammation: the cytokine-hepcidin linkJ Clin Invest200411391251125315124013
- Janssen-HeiningerYMPoynterMEBaeuerlePARecent advances towards understanding redox mechanisms in the activation of nuclear factor kappaBFree Radic Biol Med20002891317132710924851
- JanjetovicZZmijewskiMATuckeyRC20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytesPLoS One200946e598819543524