Abstract
Cerebral small vessel disease (CSVD) is a group of pathological processes with multifarious etiology and pathogenesis that are involved into the small arteries, arterioles, venules, and capillaries of the brain. CSVD mainly contains lacunar infarct or lacunar stroke, leukoaraiosis, Binswanger’s disease, and cerebral microbleeds. CSVD is an important cerebral microvascular pathogenesis as it is the cause of 20% of strokes worldwide and the most common cause of cognitive impairment and dementia, including vascular dementia and Alzheimer’s disease (AD). It has been well identified that CSVD contributes to the occurrence of AD. It seems that the treatment and prevention for cerebrovascular diseases with statins have such a role in the same function for AD. So far, there is no strong evidence-based medicine to support the idea, although increasing basic studies supported the fact that the treatment and prevention for cerebrovascular diseases will benefit AD. Furthermore, there is still lack of evidence in clinical application involved in specific drugs to benefit both AD and CSVD.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease of the brain. AD is clinically characterized by cognitive impairment at first and dementia eventually, which gradually worsen over a number of years.Citation1 Increasing research indicates that AD results from the destruction and death of nerve cells triggered by amyloid plaques and neuro-fibrillary tangles,Citation1–Citation3 both of which are thought to contribute to the neurodegenerative death of the neurons in the brain and the subsequent symptoms of AD. The main risk factor for AD is advanced age, while the incidence of AD increases with the progress of aging.Citation4 Substantial clinical research implicates the involvement of vascular risk factors in AD, such as hypertension,Citation5 coronary artery disease,Citation6 diabetes,Citation7,Citation8 and hyperlipidemia.Citation9,Citation10 These vascular factors increase the risk of AD occurrence.Citation5,Citation11
Cerebral small vessel disease (CSVD) is considered as an important pathological process of subcortical structures such as lacunar infarcts, white matter lesions, and microbleeds.Citation12 CSVD is responsible for the pathogenesis of ischemic strokes, cerebral hemorrhages, and encephalopathy, which are associated with advanced age, and deteriorated by hypertension and diabetes mellitus.Citation13 CSVD mainly contains lacunar infarct or lacunar stroke, leukoaraiosis, Binswanger’s disease, and cerebral microbleeds.Citation11 With the development of imaging examination and its clinical usage, it has been well identified that cerebral vascular diseases, especially CSVD, contribute to the occurrence of AD ().Citation9,Citation14
Figure 1 CSVD and Alzheimer’s disease.
Abbreviation: CSVD, cerebral small vessel disease.
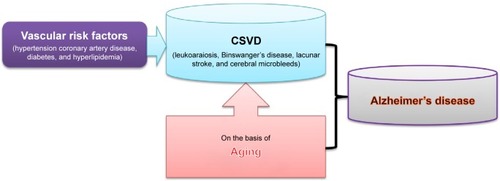
This work focused on the relationship between AD and CSVD. An overview was provided for the association between AD and lacunar infarct, leukoaraiosis, Binswanger’s disease, and cerebral microbleeds, respectively. It was reviewed that CSVD plays an important role in the development of cognitive impairment and dementia. It was also discussed that it is possibly an effective measure to prevent cerebrovascular disorders as an attractive target for AD.
Cerebral small vessel disease
CSVD, microangiopathy of the cerebral white and gray matter, indicates a group of pathological process with multifarious etiology and pathogenesis that involve the small vessels, including arterioles, venules, and capillaries of the brain.Citation15,Citation16 CSVD is responsible for 20%–30% cases of ischemic strokes, as well as for a considerable proportion of cerebral hemorrhages and encephalopathy.Citation17 CSVD is regarded as an important pathological process of subcortical structures such as lacunar infarcts, white matter lesions, and microbleeds.Citation12 Despite atherosclerotic plaque biology has not been considered into being suitable to small cerebral vessels, a lesion by fibrinoid necrosis causing lacunar infarcts and primary intracerebral bleeds has been identified as an important pathology in the CSVD.Citation18 CSVD is a common aging phenomenon that is exacerbated by hypertension and diabetes mellitus,Citation13 including leukoaraiosis, Binswanger’s disease, lacunar stroke, and cerebral microbleeds.Citation19 Leukoaraiosis refers to white matter changes (WMC) in the brain, often occurring after 65 years of age. Leukoaraiosis pathogenesis includes breakdown of axons, an unnatural paleness of myelin, gliosis, loss of ependymal cells, and enlarged perivascular spaces. Binswanger’s disease is also known as subcortical leukoencephalopathy caused by arteriosclerosis and thromboembolism influencing the blood vessels on the blood supply of white matter. Lacunar stroke or lacunar infarct is a little stroke that is caused from occlusion of the penetrating small arteries that supply blood into the brain’s deep distributions. Cerebral microbleeds result from impaired small vessel integrity, mainly being attributed to either hypertensive vasculopathy or cerebral amyloid angiopathy. Microbleeds are commonly combined with Alzheimer’s dementia and stroke.Citation20 Research findings demonstrate that vascular factors play a pathogenic role in the early stages of AD because these cerebrovascular alterations impair the delicate balance between the brain’s energy requirements and cerebral blood supply,Citation21–Citation23 resulting in the upregulation of beta-amyloid (Aβ) production and impaired Aβ drainage.Citation24,Citation25
Age-related and hypertension-related small vessel diseases are the most common forms of CSVD ().Citation15 CSVD is often recognized as the most common etiology of vascular cognitive impairment and dementia, acting as the same role of large vessel disease and other forms of cerebrovascular disease (CVD) in vascular cognitive impairment and dementia. CSVD gradually develops into decline of cognition, vascular dementia (VaD), impairment of gait and balance, mood depression, and urinary incontinence and often results in great social and economic burdens.Citation17 Increasing evidences suggest that there are decreased vascular density and cerebral microvascular pathology in the process of aging and neurodegeneration and cerebrovascular dysfunction, and CSVD precedes or accompanies cognitive dysfunction and neurodegeneration.Citation26 Several investigations indicate that cerebrovascular atherosclerosis, especially atherosclerosis of the Willis circle, is more severe in AD and correlates with the severity of AD pathology,Citation27,Citation28 implicating that atherosclerosis-induced cerebrovascular hypoperfusion correlates with AD pathology and the clinical manifestations of AD. CSVD on the basis of atherosclerosis will accelerate the development of AD since cerebral hypoperfusion and reduced glucose uptake have been found very early in AD,Citation28,Citation29 in consideration that the regulation of cerebral blood flow plays an important role in the coordinated interaction of neurons, glia, and vascular cells. Many studies show that CSVD increases the risk of AD occurrence, while signs of AD pathology can often coexist with both VaD and CSVD.Citation30
Lacunar infarct and AD
Lacunar infarct or lacunar stroke is a small (0.2–15 mm in diameter) noncortical infarct, which is generally believed to be the cause of occlusion of the penetrating arteries. Lacunar infarct onsets when one of the penetrating arteries that provide blood to the brain’s deep distributions is obstructed. It is assessed that lacunar infarcts make up 25% of all ischemic strokes, with an annual incidence of approximately 15 per 100,000 people.Citation31,Citation32 The clinical manifestations of lacunar stroke vary, such as the disorders of sensation, movement, sight, speech, balance, and coordination, because different functions are determined by different areas of the brain involved in lacunar stroke.Citation8 Diagnosis of lacunar stroke is mainly according to the setting of clinical representations and neuroimaging results.Citation33 In many cases, a lacunar infarct manifests a silent stroke, which does not have any outside symptoms. Patients are not aware of it at all when they suffer a lacunar stroke. Lacunar infarct induces a significant impairment to the cells in small parts of the brain and leads to death of corresponding parts of the brain tissue. In spite of no symptoms, lacunar stroke still gradually destroys the brain function and acts as an existing risk of major stroke attacks therein.Citation34,Citation35 The onset of multiple lacunar strokes occur and get accumulated with the progress of aging and role of risk factors. This ultimately results in a state of cognitive impairment and development of dementia.Citation36–Citation38
It is well accepted that lacunar infarct is an age-related cerebral ischemia disorder,Citation39,Citation40 and the different location of lacunar infarct within subcortical gray matter is a determinant of different cognitive impairment.Citation40,Citation41 The greatest known risk factor of AD is the advancing age, and the majority of patients with AD are >60 years old.Citation42 Accordingly, aging is a common contributor to the occurrence of lacunar infarct and the development of AD. Furthermore, AD and lacunar infarct have a considerable degree of overlap in risk factors,Citation43–Citation45 such as hypertension, diabetes, hyperlipidemia, adiposity, smoking, and drinking, as well as unhealthy lifestyle.
A number of clinical and basic findings indicate that CVD is closely associated with both the presence and the severity of the clinical symptoms of AD.Citation46 As the progress of cerebrovascular lesions was inextricably linked with vascular risk factors and age, the involvement of vascular risk factors will fully aid in clinical presentation of elderly patients with AD.Citation42,Citation47 It has been found that AD is highly prevalent in the elderly and often coexists with multimicroinfarcts; the number of microinfarcts is roughly in proportion to the degree of cognitive impairment, and the presence of micro-infarcts was intimately linked with AD rather than cerebral amyloid angiopathy.Citation48 Lacunar infarct, one of the cerebrovascular disorders, is also known to play important roles in the pathogenesis underlying AD, especially in the elderly patients with AD.Citation42 Additionally, several studies implicate the associations between lacunar infarct and Alzheimer’s pathology, including Aβ and tau pathology.Citation49–Citation51 A cross-sectional study showed that the presence of lacunar infarct was associated with higher Aβ42 in VaD and lower tau in AD in cerebrospinal fluid (CSF).Citation49 Another cross-sectional study demonstrated that the presence of lacunar infarctions could be tightly coupled to increased plasma Aβ40 and plasma Aβ40 concentration in the patients with AD.Citation51
However, the relationship between AD and lacunar infarct remains largely unclear. Although it appears that there are several shares in risk factors and related pathogenesis in AD and lacunar infarct, how these neglected silent infarcts develop multiple domain cognitive deficits and AD? It seems that cerebral atherosclerosis plays a strong role in the occurrence of cystic infarcts and microinfarcts but not Alzheimer’s pathogenesis.Citation9
Cerebral microbleeds and AD
Currently, cerebral microbleeds have been well identified as an important imaging marker of CSVD. Cerebral microbleeds are small hypointense imaging appearances according to T2* gradient-recall echo and susceptibility-weighted magnetic resonance imaging (MRI) sequences.Citation20,Citation52,Citation53 With the development of MRI techniques, cerebral microbleeds have been found in the patients of stroke and cognitive impairment, as well as in healthy people and in people with other disorders.Citation54,Citation55 A confirmed diagnosis of cerebral microbleeds indicates the acceptance of the underlying small vessel disease, the safety of antithrombotic use, the risk of symptomatic intracerebral hemorrhage, and the cause of cognitive impairment and dementia.Citation56,Citation57 Substantial literature to date has indicated that cerebral microbleeds contribute to the clinical manifestation and are linked with the occurrence of biochemical hallmarks of AD, suggesting the involvement of cerebral microbleeds in the pathogenesis of AD.Citation58
Multiple cerebral microbleeds have been shown to occur in AD and are linked with cerebral network breakdowns in the patients with AD,Citation59 affecting approximately one-third of subjects who are present with the clinical manifestations of AD dementia.Citation60 Furthermore, cerebral microbleeds in AD are more closely linked with cerebral amyloid angiopathy than CVD.Citation61 Atypical AD subjects seem to be at a particular risk for developing large numbers of microbleeds and for developing microbleeds in the frontal lobe under T2*-weighted MRI.Citation60,Citation62 In a review literature, it has been proved that the neuropathology of AD results in the breakdown of the silent microbleeds, caused by pulse-induced damage to the cerebral vessels underlying the age-related stiffening of the aorta and great arteries, which increases the intensity of the pressure pulse.Citation63 It has been reported that the incidence of multiple lobar microbleeds that have occurred in the preclinical AD and microbleeds in AD are particularly linked with additional Aβ burden.Citation64 Cerebral microbleeds were also related to the alterations of Aβ metabolism in AD.Citation65 Concomitantly, older age, higher Aβ burden, and CVD may all facilitate the occurrence of lobar microbleeds.Citation66 A cross-sectional study, investigating the prevalence, locations, and risk factors for cerebral microbleeds in neurodegenerative disorders identified that cerebral microbleeds are linked with vascular burden and AD diagnosis.Citation67 It has been reported that there was a high prevalence of cerebral microbleeds in patients with early AD according to 7 T MRI tests.Citation68
A growing body of research studies has proved that sortilin-related receptor 1 gene (SORL1) is genetically linked to AD pathological changes.Citation69–Citation73 The SORL1 was downregulated in the AD brain and positively contributed to Aβ accumulation. Emerging data suggest that SORL1 acts as a central regulating role of the trafficking and processing of amyloid precursor protein and interacts with ApoE and tau protein.Citation71 A recent study found that SORL1 single nucleotide polymorphism (SNP) rs2070045-G allele was linked to CSF-tau and hip-pocampal atrophy, two endophenotype markers of AD.Citation74 In addition, haplotype-based analyses discovered an association between haplotype rs11218340-A/rs3824966-G/rs3824968-A and higher CSF-tau and CSF-tau phosphorylated at threonine 181, implicating that SORL1 may be the tau pathology in AD.Citation74 The association of SORL1 with cerebral microbleeds indicates that the amyloid cascade sweeps into microbleeds into the etiology of AD.Citation75
Cerebral microbleeds have been highlighted as a potential key risk factor in the pathogenesis of AD, linking the main pathological contributors of Aβ accumulation with cerebrovascular damage.Citation76 However, it seems that cerebral microbleeds are likely to unfavorably affect cognitive functioning.Citation77 There is a debate concerning the relationship between microangiopathy and the clinical course of AD or the conversion of mild cognitive impairment to AD.Citation78,Citation79 Cerebral microbleeds did not affect disease course in terms of progression to AD.Citation77,Citation80 Hence, the role of cerebral microbleeds in AD remains to be identified.
Leukoaraiosis and AD
Leukoaraiosis describes a diffuse, aberrant appearance of white matter on imaging, often presented in the normal elderly and in the patients with vascular risk factors or those suffering with cognitive impairment.Citation81,Citation82 The most common imaging to find leukoaraiosis is hyperintensity on T2-weighted MR in cerebral white matter. Age, hypertension, diabetes mellitus, and cardiovascular diseases are the major risk factors for leukoaraiosis.Citation83 Leukoaraiosis was characterized by reduced cerebral blood flow and cerebrovascular reactivity and a leakage in the blood–brain barrier.Citation84 It is gradually becoming clearer that leukoaraiosis is associated with CVD,Citation85 AD, and other diseases.Citation83
It has been reported that in compelling neuroimaging data, similar white matter abnormalities of leukoaraiosis appear in the patients with AD and in elderly healthy people.Citation85–Citation88 Coffman et al confirmed that the presence of leukoaraiosis might be of important significance in understanding changes in the white matter among populations at increased risk for AD.Citation89 A longitudinal study indicated that leukoaraiosis is associated with a greater degree of cognitive impairment in patients with AD.Citation90 A cross-sectional, descriptive, multicenter study involving 109 patients with AD and 59 with mild cognitive impairment identified that AD had a higher prevalence of leukoaraiosis and apathy.Citation91 A recent pilot study suggests that jugular venous reflux is associated with cerebral WMC in individuals with AD, implying that cerebral venous outflow impairment might play a role in the dynamics of WMC in patients with AD, particularly in the periventricular regions.Citation92 It is well accepted that white matter hyperintensities (WMH) in imaging are linked with aging and AD.Citation93 Makedonov et al reported that WMH perfusion was lower than normal-appearing white matter perfusion in both age-matched elderly controls and AD groups. WMH tended to have a lower perfusion in AD compared with age-matched elderly.Citation94 This imaging study suggests a common WMH etiology in AD and healthy aging. This finding is also suggestive of susceptible WMH hypoperfusion in AD compared with healthy aging, implicating that AD pathology decreases the perfusion at anatomic locations susceptible to the formation of WMH through either the neurodegenerative process or AD-related vasculopathy or both.Citation94 The leukoaraiosis and disability prospective multinational European study evaluated the influence of WMC on the transition of independent elderly subjects, disclosing that self-perceived memory complaints predicted AD.Citation95
Binswanger’s disease and AD
In 1894, Dr Otto Binswanger, professor of psychiatry in Switzerland, first described a new clinical and neuropathological phenomenon that he termed “encephalitis subcorticalis chronica progressive” and Binswanger’s disease is named after him.Citation96,Citation97 Other names for Binswanger’s disease are also known as subcortical leukoencephalopathy, subcortical arteriosclerotic encephalopathy, ischemic periventricular leukoencephalopathy, or subcortical dementia.Citation98–Citation101 Binswanger’s disease is featured by damage to small penetrating blood vessels in the subcortical regions of the brain. Binswanger’s disease is often associated with advanced age, chronic hypertension, stroke, disease of the large blood vessels in the neck, alcohol and smoking, and cardiovascular diseases. Signs and symptoms of Binswanger’s disease usually start after the age of 60 years. The onset of this disease is often between 54 years and 66 years of age, and the first symptoms are usually mental disorders or little stroke. A slow progressive dementia is identified as an important clinical feature of the disease. Binswanger’s disease sometimes presents a rare form of dementia and often referred to as subcortical dementia.
Like other CSVD, Binswanger’s disease shares some backgrounds with AD,Citation102,Citation103 including aging and vascular risk factors, such as hypertension, diabetes, cardiovascular diseases, metabolic syndrome, obesity, hyperlipidemia, smoking, and drinking. Binswanger’s disease seems to be a risk factor for AD.Citation104 However, there are no biomolecular reports concerning the relationship between Binswanger’s disease and AD until now.
Cerebral atrophy: a common morphological feature in both AD and CSVD
Cerebral atrophy, a common feature of brain disorders, is a condition in which brain cells are lost, or the connections between them are damaged, resulting in the decrease of normal brain volume.Citation105,Citation106 A number of conditions can lead to brain atrophy, including epilepsy,Citation107 traumatic brain injuries,Citation108,Citation109 strokes,Citation110 AD,Citation105 multiple sclerosis,Citation111 cerebral palsy,Citation112 and Huntington’s disease.Citation113 Like other atrophies, cerebral atrophy involves loss of tissue. Loss of brain tissue can lead to sinister consequences, including a variety of neurological and cognitive problems. Focal cerebral atrophy, the corresponding damage concerned with a particular area of the brain, manifests the corresponding function impairment of concerned area of brain. Generalized cerebral atrophy will be linked with a range of clinical problems because of the involvement of the whole brain.
Cerebral atrophy can usually be identified in medical imaging such as Computed Tomography (CT) and/or MRI, which can demonstrate structural changes in the brain. Increasing imaging results have evidenced that CSVD has a close association with cerebral atrophy.Citation114,Citation115 A prospective follow-up research conducted by Nitkunan et al showed that brain volume is decreased in the patients with CSVD with respect to normal aging subjects, and this decrease was correlated with cognition decline during 1-year prospective follow-up.Citation116 The leukoaraiosis and disability study further proved that brain atrophy facilitates cognitive decline, and brain atrophy is independently related to longitudinal cognitive decline in CSVD.Citation117 According to MRI measurement, the severity of brain atrophy was correlated with the number of lacunar infarcts and the size of subcortical and periventricular white matter lesions, while shorter telomere length is associated with brain atrophy and WMH.Citation114,Citation118–Citation120
A large number of imaging studies have confirmed that cerebral atrophy is the most significant morphological characteristic of AD,Citation121–Citation125 which has the widening grooves and fissures of the cerebral cortex, indicating progressively severe brain atrophy and loss of brain mass. It has been found that the acceleration of hippocampal atrophy in mild cognitive impairment subjects enhances the progress to clinical AD within 3 years of baseline,Citation105 and regional measures of hippocampal atrophy are the strongest predictors of progression to AD.Citation126
On the basis of the above information, it can be suggested that cerebral atrophy is a common morphologic feature in both AD and CSVD. On the background of cerebral atrophy, what is the relationship between AD and CSVD? The problem is still pending. Thus, it is important to reveal the exact role of CSVD in AD underlying cerebral atrophy.
Dementia: a common clinical result of both AD and CSVD
CSVD is common in the elderly and enhances the process of cognitive impairment and dementia.Citation127,Citation128 Clinical studies implicate the link between cognitive disorders with white matter lesions (a marker of small vessel disease in the brain) caused by aging, hypertension, systemic circulatory disturbances, or other processes (cerebral amyloid angiopathy, cerebral autosomal dominant arteriopathy with subcortical infarcts, and leukoencephalopathy).Citation129–Citation131 Genetic predispositions and environmental exposures may promote the development of CSVD and interact with normal aging to impact cognitive function.Citation132,Citation133 It has been demonstrated that the increasing severity of white matter lesions was associated with a steeper decline in cognitive function, accompanying with generalized brain atrophy and the presence of brain infarcts.Citation127,Citation134 White matter diffuse lesion could directly influence the recall processes controlled by the frontal lobe.Citation135 The cognitive impairment is specifically focused on information processing speed and executive function in older people, implicating that CSVD may lead to cognitive impairment by blemishing information processing speed and executive function.Citation127 The epidemiology of dementia in Singapore study showed that cerebral microbleeds were, independent of other concomitant markers of CSVD, helpful in poorer cognitive function.Citation136
Available evidence demonstrates that cognitive impairment and dementia are the clinical manifestation for both AD and CSVD.Citation137,Citation138 Increasing evidence of a complex relationship between AD and cognitive impairment and VaD has been reported while CVD and stroke are related to high risk of both cognitive impairment and AD.Citation139,Citation140 The manifestation of CVD in imaging is extremely similar to AD in the elderly, whereas imaging markers of subcortical vascular diseases (leukoaraiosis, lacunar infarcts, microbleeds, ventricular enlargement, cortical, and hippocampal atrophy).Citation141–Citation143 Leukoaraiosis is linked with functional impairment in older patients with AD but not VaD, while leukoaraiosis might play a synergistic effect with cognitive and behavioral disturbances to the onset and progression of cognitive disability of AD.Citation144 The Sunnybrook Dementia Study demonstrated that Visible Virchow–Robin spaces in the white matter, markers for small vessel diseases, may be more related to AD-related vascular pathology since AD had significantly greater volumes of WMH, lacunes, and Virchow–Robin spaces in the white matter,Citation145 and WMH may be related to regional neurodegeneration.Citation146,Citation147 It has been described and illustrated that WMH volume, particularly in parietal regions, is elevated among individuals with risk for AD, predicts future diagnosis of AD, predicts the rate of progression of cognitive symptoms among individuals with AD, and increases over time among individuals destined to develop AD.Citation148 The vascular pathology of CSVD in AD may interact with neurodegenerative process and deteriorate cognitive decline. Thus, the progress of disorders from cognitive impairment to dementia can be attributed to cerebrovascular causes.Citation149–Citation151
Conclusion and perspective
CSVD has been readily identified on CT and/or MRI scans. CSVD, a leading cause of cognitive impairment, ultimately leads to dementia and contributes to neurodegenerative progress, including Alzheimer’s onset. Up to now, a number of studies have confirmed that CSVD can co-occur with AD, and signs of AD pathology have been found in CSVD, indicating that the pathology of CSVD and AD are interconnected. It seems that CSVD could stimulate amyloid pathology, while AD-associated cerebral amyloid pathology may enhance auxiliary vascular damage. The association between CSVD and incident dementia and Alzheimer’s onset has never been well clarified. CSVD has been highlighted as a potential key risk factor for AD, linking the main pathological contributors of Aβ accumulation with cerebrovascular damage. However, there are no biomolecular reports concerning the relationship Binswanger’s disease and AD until now. The role of cerebral microbleeds in AD remains to be identified. Furthermore, it remains unclear if and how associations between CSVD and Alzheimer’s pathology result in cognitive impairment and dementia.
According to the above information, it can be suggested that CSVD may play a crucial role in AD. Based on the theory of CVD, the treatment and prevention for CVDs will benefit AD. It seems that statins have such a role in the treatment and prevention of Alzheimer’s neurodegeneration since they can benefit CVDs. In spite of this, there is no strong evidence-based medicine to support the idea. So far, increasing basic molecular biology findings report that the treatment and prevention for CVDs will benefit AD. However, there is still lack of evidence in clinical application involved in specific drugs to benefit both AD and CSVD.
Acknowledgments
This work was supported by the Natural Science Foundation of Hubei Province (2015CFB260), the Hubei Province Health and Family Planning Scientific Research Project (WJ2015MB219), the Shiyan Natural Science Foundation (15K70), and the Natural Science Foundation of Renmin Hospital, Hubei University of Medicine to Dr Zhiyou Cai.
Disclosure
The authors report no conflicts of interest in this work.
References
- VintersHVEmerging concepts in Alzheimer’s diseaseAnnu Rev Pathol20151029131925387055
- WurtmanRBiomarkers in the diagnosis and management of Alzheimer’s diseaseMetabolism2015643 suppl 1S47S5025468144
- BloomGSAmyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesisJAMA Neurol201471450550824493463
- CaiZMonoamine oxidase inhibitors: promising therapeutic agents for Alzheimer’s disease (Review)Mol Med Rep2014951533154124626484
- TomimotoHVascular cognitive impairment: the relationship between hypertensive small vessel disease and cerebral amyloid angiopathyBrain Nerve2012641213771386 Japanese23209064
- de la TorreJCCardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementiaCardiovasc Psychiatry Neurol2012201236751623243502
- PasquierFBoulogneALeysDFontainePDiabetes mellitus and dementiaDiabetes Metab2006325 pt 140341417110895
- PalacioSMcClureLABenaventeORBazanC3rdPergolaPHartRGLacunar strokes in patients with diabetes mellitus: risk factors, infarct location, and prognosis: the secondary prevention of small subcortical strokes studyStroke20144592689269425034716
- ZhengLVintersHVMackWJZarowCEllisWGChuiHCCerebral atherosclerosis is associated with cystic infarcts and microinfarcts but not Alzheimer pathologic changesStroke201344102835284123887837
- SiegelGJChauhanNBFeinsteinDLStatin therapy is associated with reduced neuropathologic changes of Alzheimer diseaseNeurology2008715383 Author reply 38318663190
- ZhangMChenMWangQRelationship between cerebral microb-leeds and cognitive function in lacunar infarctJ Int Med Res201341234735523569023
- LammieGABrannanFSlatteryJWarlowCNonhypertensive cerebral small-vessel disease. An autopsy studyStroke19972811222222299368569
- SchmidtHZeginiggMWiltgenMGenetic variants of the NOTCH3 gene in the elderly and magnetic resonance imaging correlates of age-related cerebral small vessel diseaseBrain2011134pt 113384339722006983
- PaquetCJouventEMineMA cortical form of CADASIL with cerebral Abeta amyloidosisActa Neuropathol2010120681382020957378
- PantoniLCerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challengesLancet Neurol20109768970120610345
- SchmidtkeKHullMCerebral small vessel disease: how does it progress?J Neurol Sci2005229–230132015760614
- ZhangAJYuXJWangMThe clinical manifestations and pathophysiology of cerebral small vessel diseaseNeurosci Bull201026325726420502505
- LammieGAHypertensive cerebral small vessel disease and strokeBrain Pathol200212335837012146804
- OnoderaOWhat is cerebral small vessel disease?Rinsho Shinkeigaku2011516399405 Japanese21735731
- YatesPAVillemagneVLEllisKADesmondPMMastersCLRoweCCCerebral microbleeds: a review of clinical, genetic, and neuroimaging associationsFront Neurol2014420524432010
- IadecolaCRescuing troubled vessels in Alzheimer diseaseNat Med200511992392416145570
- IadecolaCThe overlap between neurodegenerative and vascular factors in the pathogenesis of dementiaActa Neuropathol2010120328729620623294
- LockhartALambJROsredkarTPIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosisBrain2007130pt 102607261517698496
- IadecolaCCerebrovascular effects of amyloid-beta peptides: mechanisms and implications for Alzheimer’s dementiaCell Mol Neurobiol2003234–568168914514024
- RoherAEKuoYMEshCCortical and leptomeningeal cerebro-vascular amyloid and white matter pathology in Alzheimer’s diseaseMol Med200393–411212212865947
- BrownWRThoreCRReview: cerebral microvascular pathology in ageing and neurodegenerationNeuropathol Appl Neurobiol2011371567420946471
- RoherAEEshCKokjohnTACircle of Willis atherosclerosis is a risk factor for sporadic Alzheimer’s diseaseArterioscler Thromb Vasc Biol200323112055206214512367
- YarchoanMXieSXKlingMACerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementiasBrain2012135pt 123749375623204143
- GirouardHIadecolaCNeurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer diseaseJ Appl Physiol (1985)2006100132833516357086
- Henry-FeugeasMCAlzheimer’s disease in late-life dementia: a minor toxic consequence of devastating cerebrovascular dysfunctionMed Hypotheses200870486687517825499
- SaccoSMariniCTotaroRRussoTCeroneDCaroleiAA population-based study of the incidence and prognosis of lacunar strokeNeurology20066691335133816682663
- LastillaMLacunar infarctClin Exp Hypertens2006283–420521516833026
- DasTSettecaseFBoulosMMultimodal CT provides improved performance for lacunar infarct detectionAJNR Am J Neuroradiol20153661069107525721075
- LabovitzDLBoden-AlbalaBHauserWASaccoRLLacunar infarct or deep intracerebral hemorrhage: who gets which? The Northern Manhattan studyNeurology200768860660817310033
- MasanaYIwamotoFYamadaMMotozakiTYoshimineTPrevalence and risk factors for silent lacunar infarct in white matter lesion – brain multiphasic screeningNo To Shinkei2003551210271032 Japanese14870572
- ArboixALacunar infarct and cognitive declineExpert Rev Neurother20111191251125421864071
- van ZandvoortMJvan der GrondJKappelleLJde HaanEHCognitive deficits and changes in neurometabolites after a lacunar infarctJ Neurol2005252218319015729524
- ChenCFLanSHKhorGTLaiCLTaiCTCognitive dysfunction after acute lacunar infarctKaohsiung J Med Sci200521626727116035569
- GrinbergLTThalDRVascular pathology in the aged human brainActa Neuropathol2010119327729020155424
- BenistySGouwAAPorcherRLocation of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS studyJ Neurol Neurosurg Psychiatry200980547848319211595
- WenHMMokVCFanYHEffect of white matter changes on cognitive impairment in patients with lacunar infarctsStroke20043581826183015205490
- NagataKTakanoDYamazakiTCerebrovascular lesions in elderly Japanese patients with Alzheimer’s diseaseJ Neurol Sci20123221–2879122868090
- WhiteheadSNMassoniEChengGTriflusal reduces cerebral ischemia induced inflammation in a combined mouse model of Alzheimer’s disease and strokeBrain Res2010136624625620934412
- RichardEGouwAAScheltensPvan GoolWAVascular care in patients with Alzheimer disease with cerebrovascular lesions slows progression of white matter lesions on MRI: the evaluation of vascular care in Alzheimer’s disease (EVA) studyStroke201041355455620056923
- GoldGGiannakopoulosPHerrmannFRBourasCKovariEIdentification of Alzheimer and vascular lesion thresholds for mixed dementiaBrain2007130pt 112830283617878206
- SnowdonDAGreinerLHMortimerJARileyKPGreinerPAMarkesberyWRBrain infarction and the clinical expression of Alzheimer disease. The Nun studyJAMA1997277108138179052711
- HeymanAFillenbaumGGWelsh-BohmerKACerebral infarcts in patients with autopsy-proven Alzheimer’s disease: CERAD, part XVIII. Consortium to establish a registry for Alzheimer’s diseaseNeurology19985111591629674796
- van RoodenSGoosJDvan OpstalAMIncreased number of microinfarcts in Alzheimer disease at 7-T MR imagingRadiology2014270120521124029643
- KesterMIGoosJDTeunissenCEAssociations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkersJAMA Neurol201471785586224818585
- Erten-LyonsDWoltjerRKayeJNeuropathologic basis of white matter hyperintensity accumulation with advanced ageNeurology2013811197798323935177
- GurolMEIrizarryMCSmithEEPlasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathyNeurology2006661232916401840
- De ReuckJLCordonnierCDeramecourtVMicrobleeds in postmortem brains of patients with Alzheimer disease: a T2*-weighted gradient-echo 7.0 T magnetic resonance imaging studyAlzheimer Dis Assoc Disord201327216216722546781
- YakushijiYHaraHCerebral microbleeds: clinical features and managementRinsho Shinkeigaku2012521111061109 Japanese23196531
- ParkJHSeoSWKimCPathogenesis of cerebral microbleeds: in vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairmentAnn Neurol201373558459323495089
- GhelmezDSorin TutaSPopaCCerebral microbleeds (CMBs) – relevance for mechanisms of cerebral hemorrhage – analysis of 24 MRI evaluated patientsJ Med Life20136443743924868257
- ShamsSMartolaJGranbergTCerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis – the Karolinska Imaging Dementia StudyAJNR Am J Neuroradiol201536466166625523590
- NagasawaJKiyozakaTIkedaKPrevalence and clinicoradiological analyses of patients with Alzheimer disease coexisting multiple microbleedsJ Stroke Cerebrovasc Dis20142392444244925174565
- GoosJDKesterMIBarkhofFPatients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognitionStroke200940113455346019762705
- HeringaSMReijmerYDLeemansAKoekHLKappelleLJBiesselsGJMultiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s diseaseJ Alzheimers Dis201438121122123948936
- WhitwellJLKantarciKWeigandSDMicrobleeds in atypical presentations of Alzheimer’s disease: a comparison to dementia of the Alzheimer’s typeJ Alzheimers Dis20154541109111725649655
- Nakata-KudoYMizunoTYamadaKMicrobleeds in Alzheimer disease are more related to cerebral amyloid angiopathy than cerebrovascular diseaseDement Geriatr Cogn Disord200622181416645275
- Martinez-RamirezSGreenbergSMViswanathanACerebral microbleeds: overview and implications in cognitive impairmentAlzheimers Res Ther2014633324987468
- StoneJJohnstoneDMMitrofanisJO’RourkeMThe mechanical cause of age-related dementia (Alzheimer’s disease): the brain is destroyed by the pulseJ Alzheimers Dis201544235537325318547
- BenedictusMRGoosJDBinnewijzendMASpecific risk factors for microbleeds and white matter hyperintensities in Alzheimer’s diseaseNeurobiol Aging201334112488249423731952
- GoosJDTeunissenCEVeerhuisRMicrobleeds relate to altered amyloid-beta metabolism in Alzheimer’s diseaseNeurobiol Aging20123351011.e1011e101922118945
- YatesPADesmondPMPhalPMIncidence of cerebral microbleeds in preclinical Alzheimer diseaseNeurology201482141266127324623839
- OlazaranJRamosABoyanoIPattern of and risk factors for brain microbleeds in neurodegenerative dementiaAm J Alzheimers Dis Other Demen201429326326924408753
- BrundelMHeringaSMde BresserJHigh prevalence of cerebral microbleeds at 7Tesla MRI in patients with early Alzheimer’s diseaseJ Alzheimers Dis201231225926322531417
- WenYMiyashitaAKitamuraNSORL1 is genetically associated with neuropathologically characterized late-onset Alzheimer’s diseaseJ Alzheimers Dis201335238739423455993
- FengXHouDDengYLiWTianMYuZSORL1 gene polymorphism association with late-onset Alzheimer’s diseaseNeurosci Lett201558438238925450149
- YinRHYuJTTanLThe role of SORL1 in Alzheimer’s diseaseMol Neurobiol201551390991824833601
- XueXZhangMLinYXuEJiaJAssociation between the SORL1 rs2070045 polymorphism and late-onset Alzheimer’s disease: interaction with the ApoE genotype in the Chinese Han populationNeurosci Lett2014559949824309291
- FelskyDSzeszkoPYuLThe SORL1 gene and convergent neural risk for Alzheimer’s disease across the human lifespanMol Psychiatry201419101125113224166411
- LouwersheimerERamirezACruchagaCThe influence of genetic variants in SORL1 gene on the manifestation of Alzheimer’s diseaseNeurobiol Aging20153631605.e1613e162025659857
- SchuurMvan SwietenJCSchol-GelokSGenetic risk factors for cerebral small-vessel disease in hypertensive patients from a genetically isolated populationJ Neurol Neurosurg Psychiatry2011821414420667857
- CordonnierCBrain microbleedsPract Neurol20101029410020308236
- LoitfelderMSeilerSSchwingenschuhPSchmidtRCerebral microbleeds: a reviewPanminerva Med201254314916022801432
- HommetCMondonKConstansTReview of cerebral microangiopathy and Alzheimer’s disease: relation between white matter hyperintensities and microbleedsDement Geriatr Cogn Disord201132636737822301385
- CordonnierCvan der FlierWMBrain microbleeds and Alzheimer’s disease: innocent observation or key player?Brain2011134pt 233534421257651
- van der FlierWMClinical aspects of microbleeds in Alzheimer’s diseaseJ Neurol Sci20123221–2565822836015
- O’SullivanMLeukoaraiosisPract Neurol200881263818230707
- AurielEBornsteinNMBerenyiEClinical, radiological and pathological correlates of leukoaraiosisActa Neurol Scand20111231414720219022
- HuangYHZhangWWLinLCould changes in arterioles impede the perivascular drainage of interstitial fluid from the cerebral white matter in leukoaraiosis?Neuropathol Appl Neurobiol201036323724719889176
- UhJYezhuvathUChengYLuHIn vivo vascular hallmarks of diffuse leukoaraiosisJ Magn Reson Imaging201032118419020578025
- BogousslavskyJLeukoencephalopathy, leukoaraiosis and cerebral infarctionRev Neurol (Paris)198814411117 Japanese3279483
- DuronEVidalJSBounatiroSRelationships between personality traits, medial temporal lobe atrophy, and white matter lesion in subjects suffering from mild cognitive impairmentFront Aging Neurosci2014619525120483
- PlutaRJanuszewskiSUlamekMIschemic blood-brain barrier and amyloid in white matter as etiological factors in leukoaraiosisActa Neurochir Suppl200810235335619388344
- MoodyDMBrownWRChallaVRGhazi-BirryHSReboussinDMCerebral microvascular alterations in aging, leukoaraiosis, and Alzheimer’s diseaseAnn N Y Acad Sci19978261031169329684
- CoffmanJATorelloMWBornsteinRAChakeresDBurnsENasrallahHALeukoaraiosis in asymptomatic adult offspring of individuals with Alzheimer’s diseaseBiol Psychiatry19902711124412482354229
- DiazJFMerskeyHHachinskiVCImproved recognition of leukoaraiosis and cognitive impairment in Alzheimer’s diseaseArch Neurol19914810102210251929892
- Sarabia-CoboCMPerezVHermosillaCNunezMJde LorenaPApathy and leukoaraiosis in mild cognitive impairment and Alzheimer’s disease: multicenter diagnostic criteria according to the latest studiesDement Geriatr Cogn Dis Extra20144222823525177331
- ChungCPBeggsCWangPNJugular venous reflux and white matter abnormalities in Alzheimer’s disease: a pilot studyJ Alzheimers Dis201439360160924217278
- ChenWSongXZhangYAssessment of the Virchow-Robin spaces in Alzheimer disease, mild cognitive impairment, and normal aging, using high-field MR imagingAJNR Am J Neuroradiol20113281490149521757525
- MakedonovIBlackSEMacIntoshBJCerebral small vessel disease in aging and Alzheimer’s disease: a comparative study using MRI and SPECTEur J Neurol201320224325022742818
- VerdelhoAMadureiraSMoleiroCSelf-perceived memory complaints predict progression to Alzheimer disease. The LADIS studyJ Alzheimers Dis201127349149821841255
- van SwietenJCCaplanLRBinswanger’s diseaseAdv Neurol1993621932118517210
- SchorerCEAlzheimer and Kraepelin describe Binswanger’s diseaseJ Neuropsychiatry Clin Neurosci19924155581627963
- FerrerIBellaRSerranoMTMartiEGuionnetNArteriolosclerotic leucoencephalopathy in the elderly and its relation to white matter lesions in Binswanger’s disease, multi-infarct encephalopathy and Alzheimer’s diseaseJ Neurol Sci199098137502230830
- PetersonBSummergradPBinswanger’s disease (Part II): pathogenesis of subcortical arteriosclerotic encephalopathy and its relation to other dementing processesJ Geriatr Psychiatry Neurol1989241711812699555
- FisherCMBinswanger’s encephalopathy: a reviewJ Neurol1989236265792651569
- SandykRSubcortical arteriosclerotic encephalopathy (Binswanger’s disease)S Afr Med J19836362042056823631
- TomimotoHAkiguchiIAkiyamaHVascular changes in white matter lesions of Alzheimer’s diseaseActa Neuropathol199997662963410378382
- KosakaKIkedaKMatsushitaMIizukaRA combination of Alzheimer’s and Binswanger’s diseases – a clinicopathological study of four casesJpn J Psychiatry Neurol19864046856923599567
- WatanabeTShiinoAAkiguchiIAbsolute quantification in proton magnetic resonance spectroscopy is superior to relative ratio to discriminate Alzheimer’s disease from Binswanger’s diseaseDement Geriatr Cogn Disord20082618910018617735
- LeungKKBartlettJWBarnesJManningENOurselinSFoxNCCerebral atrophy in mild cognitive impairment and Alzheimer disease: rates and accelerationNeurology201380764865423303849
- BeckCKruetzelmannAForkertNDA simple brain atrophy measure improves the prediction of malignant middle cerebral artery infarction by acute DWI lesion volumeJ Neurol201426161097110324687898
- HennyCDesplandPARegliFInitial epileptic crisis after the age of 60: etiology, clinical aspects and EEGSchweiz Med Wochenschr199012021787792 Japanese2349461
- BiglerEDThe clinical significance of cerebral atrophy in traumatic brain injuryArch Clin Neuropsychol19872329330414589621
- TateDFKhedrakiRNeeleyESRyserDKBiglerEDCerebral volume loss, cognitive deficit, and neuropsychological performance: comparative measures of brain atrophy: II. Traumatic brain injuryJ Int Neuropsychol Soc201117230831621352625
- AoiMCHuKLoMTSelimMOlufsenMSNovakVImpaired cerebral autoregulation is associated with brain atrophy and worse functional status in chronic ischemic strokePLoS One2012710e4679423071639
- Dell’OglioECeccarelliAGlanzBIQuantification of global cerebral atrophy in multiple sclerosis from 3T MRI using SPM: the role of misclassification errorsJ Neuroimaging201525219119925523616
- CordatoNJDugginsAJHallidayGMMorrisJGPantelisCClinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsyBrain2005128pt 61259126615843423
- KassubekJBernhard LandwehrmeyerGEckerDGlobal cerebral atrophy in early stages of Huntington’s disease: quantitative MRI studyNeuroreport200415236336515076769
- AribisalaBSValdes HernandezMCRoyleNABrain atrophy associations with white matter lesions in the ageing brain: the Lothian Birth Cohort 1936Eur Radiol20132341084109223114884
- SalaSAgostaFPaganiECopettiMComiGFilippiMMicrostructural changes and atrophy in brain white matter tracts with agingNeurobiol Aging2012333488498.e48220594616
- NitkunanALanfranconiSCharltonRABarrickTRMarkusHSBrain atrophy and cerebral small vessel disease: a prospective follow-up studyStroke201142113313821148440
- JokinenHLipsanenJSchmidtRBrain atrophy accelerates cognitive decline in cerebral small vessel disease: the LADIS studyNeurology201278221785179222592361
- ThongJYHilalSWangYAssociation of silent lacunar infarct with brain atrophy and cognitive impairmentJ Neurol Neurosurg Psychiatry201384111219122523933740
- WikgrenMKarlssonTSoderlundHShorter telomere length is linked to brain atrophy and white matter hyperintensitiesAge Ageing201443221221724231584
- KanhaiDAde KleijnDPKappelleLJExtracellular vesicle protein levels are related to brain atrophy and cerebral white matter lesions in patients with manifest vascular disease: the SMART-MR studyBMJ Open201441e003824
- GuoHSongXVandorpeREvaluation of common structural brain changes in aging and Alzheimer disease with the use of an MRI-based brain atrophy and lesion index: a comparison between T1WI and T2WI at 1.5T and 3TAJNR Am J Neuroradiol201435350451223988753
- AnandhKRSujathaCMRamakrishnanSAtrophy analysis of corpus callosum in Alzheimer brain MR images using anisotropic diffusion filtering and level setsConf Proc IEEE Eng Med Biol Soc201420141945194825570361
- WhitwellJLJackCRJrPankratzVSRates of brain atrophy over time in autopsy-proven frontotemporal dementia and Alzheimer diseaseNeuroimage20083931034104017988893
- SluimerJDVrenkenHBlankensteinMAWhole-brain atrophy rate in Alzheimer disease: identifying fast progressorsNeurology20087019 pt 21836184118458218
- BokdeALPietriniPIbanezVThe effect of brain atrophy on cerebral hypometabolism in the visual variant of Alzheimer diseaseArch Neurol200158348048611255453
- HennemanWJSluimerJDBarnesJHippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measuresNeurology20097211999100719289740
- PrinsNDvan DijkEJden HeijerTCerebral small-vessel disease and decline in information processing speed, executive function and memoryBrain2005128pt 92034204115947059
- SierraCCerebral small vessel disease, cognitive impairment and vascular dementiaPanminerva Med201254317918822801435
- PantoniLGarciaJHCognitive impairment and cellular/vascular changes in the cerebral white matterAnn N Y Acad Sci1997826921029329683
- RichardsonKStephanBCIncePGBrayneCMatthewsFEEsiriMMThe neuropathology of vascular disease in the medical research council cognitive function and ageing study (MRC CFAS)Curr Alzheimer Res20129668769622471870
- CostanzaAXekardakiAKovariEGoldGBourasCGiannakopoulosPMicrovascular burden and Alzheimer-type lesions across the age spectrumJ Alzheimers Dis201232364365222842869
- RinconFWrightCBCurrent pathophysiological concepts in cerebral small vessel diseaseFront Aging Neurosci201462424715862
- MeierIBManlyJJProvenzanoFAWhite matter predictors of cognitive functioning in older adultsJ Int Neuropsychol Soc201218341442722390883
- PasquierFLeysDWhy are stroke patients prone to develop dementia?J Neurol199724431351429050953
- Suades-GonzalezEJodar-VicenteMPerdrix-SolasDMemory deficit in patients with subcortical vascular cognitive impairment versus Alzheimer-type dementia: the sensitivity of the ‘word list’ subtest on the Wechsler Memory Scale-IIIRev Neurol20094912623629 Japanese20013713
- HilalSSainiMTanCSCerebral microbleeds and cognition: the epidemiology of dementia in Singapore studyAlzheimer Dis Assoc Disord201428210611224322485
- De DeynPPGoemanJEngelborghsSFrom neuronal and vascular impairment to dementiaPharmacopsychiatry199932suppl 1172410338104
- KovacicJCFusterVAtherosclerotic risk factors, vascular cognitive impairment, and Alzheimer diseaseMt Sinai J Med201279666467323239205
- ErkinjunttiTDiagnosis and management of vascular cognitive impairment and dementiaJ Neural Transm Suppl2002639110912597611
- AttemsJJellingerKAThe overlap between vascular disease and Alzheimer’s disease – lessons from pathologyBMC Med20141220625385447
- Henry-FeugeasMCMRI of the ‘Alzheimer syndrome’J Neuroradiol200734422022717719631
- CechettoDFHachinskiVWhiteheadSNVascular risk factors and Alzheimer’s diseaseExpert Rev Neurother20088574375018457531
- ChalmersKWilcockGLoveSContributors to white matter damage in the frontal lobe in Alzheimer’s diseaseNeuropathol Appl Neurobiol200531662363116281911
- BleARanziniMZurloALeukoaraiosis is associated with functional impairment in older patients with Alzheimer’s disease but not vascular dementiaJ Nutr Health Aging2006101313516453055
- RamirezJBerezukCMcNeelyAAScottCJGaoFBlackSEVisible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer’s disease patients and normal elderly from the Sunnybrook Dementia StudyJ Alzheimers Dis201543241542425096616
- GuzmanVACarmichaelOTSchwarzCTostoGZimmermanMEBrickmanAMWhite matter hyperintensities and amyloid are independently associated with entorhinal cortex volume among individuals with mild cognitive impairmentAlzheimers Dement201395 supplS124S13123375566
- UmegakiHPathophysiology of cognitive dysfunction in older people with type 2 diabetes: vascular changes or neurodegeneration?Age Ageing201039181019917634
- BrickmanAMContemplating Alzheimer’s disease and the contribution of white matter hyperintensitiesCurr Neurol Neurosci Rep2013131241524190781
- KlohsJRudinMShimshekDRBeckmannNImaging of cerebrovascular pathology in animal models of Alzheimer’s diseaseFront Aging Neurosci201463224659966
- JellingerKAPathogenesis and treatment of vascular cognitive impairmentNeurodegener Dis Manag20144647149025531689
- Targosz-GajniakMSiudaJOchudloSOpalaGCerebral white matter lesions in patients with dementia – from MCI to severe Alzheimer’s diseaseJ Neurol Sci20092831–2798219268974
