Abstract
Increased levels of oxidized proteins with aging have been considered a cardiovascular risk factor. However, it is unclear whether oxidized albumin, which is the most abundant serum protein, induces endothelial damage. The results of this study indicated that with aging processes, the levels of oxidized proteins as well as endothelial microparticles release increased, a novel marker of endothelial damage. Among these, oxidized albumin seems to play a principal role. Through in vitro studies, endothelial cells cultured with oxidized albumin exhibited an increment of endothelial damage markers such as adhesion molecules and apoptosis levels. In addition, albumin oxidation increased the amount of endothelial microparticles that were released. Moreover, endothelial cells with increased oxidative stress undergo senescence. In addition, endothelial cells cultured with oxidized albumin shown a reduction in endothelial cell migration measured by wound healing. As a result, we provide the first evidence that oxidized albumin induces endothelial injury which then contributes to the increase of cardiovascular disease in the elderly subjects.
Introduction
Aging is associated with well-known changes in protein conformation that are involved in aging-related disease. Among this modification, probably the protein oxidation is the most relevant mechanism of pathogenesis in the elderly subjects. Oxidative modifications generally cause loss of catalytic or structural function in the affected proteins; it is likely that the level of oxidatively modified proteins observed during aging will have serious deleterious effects on cellular and organ function.Citation1,Citation2 Although the oxidative damage to nucleic acids is subject to repair by highly efficient excision/insertion mechanisms, the repair of damaged to proteins appears limited to the reduction of oxidized derivatives.Citation3,Citation4 Damaged proteins are targeted for degradation to amino acid constituents by the action of various endogenous proteases, especially the 20s proteasome.Citation3,Citation4 However, the age-related accumulation of oxidized proteins may reflect age-related increases in rates of reactive oxygen species (ROS) generation, decreases in antioxidant activities, or losses in the capacity to degrade oxidized proteins.Citation2 Therefore, the importance of protein oxidation in aging is supported by the observation that levels of oxidized proteins increase with subject age.Citation5
ROS can react directly with the protein or they can react with others molecules (such a sugars and lipids). This generates products which then react with the protein. Many of reactions are mediated by free radicals.Citation6 Proteins are major targets for ROS because of their abundance in biological systems. In addition, proteins are primarily responsible for most functional processes within the cells. The major protein present in the plasma is albumin, which constitutes ~55% of the plasma proteins.Citation7 As a result, it is most susceptible to suffer an oxidative process.Citation8 In this manner, the oxidation of albumin may cause endothelial damage. Nevertheless, there are no studies analyzing the effects of oxidized albumin in aging, and as a consequence endothelial damage.
It is now recognized that the oxidative modification of proteins by reactive species, especially ROS, is implicated in the etiology or progression of an important number of diseases.Citation9 Compared to control samples, proteins are more oxidized in tissues of animals and patients suffering from Alzheimer’s disease, rheumatoid arthritis, atherosclerosis, or amyotrophic lateral sclerosis.Citation10 Oxidized proteins are also associated with aging-related diseases and diabetes,Citation5,Citation11 neurodegenerative diseases (Alzheimer’s),Citation9,Citation12 and cardiovascular diseases among others.Citation13–Citation15 In addition, cardiovascular diseases show a significantly elevated mortality in elderly patients and have been associated with endothelial cell injury. Furthermore, cardiovascular diseases have been proven to cause a decline in endothelial function.Citation16 Specifically, age-related endothelial dysfunction has been characterized in animals and humans.Citation17 In addition, oxidized proteins have been demonstrated to be a critical contributor to the development of atherosclerosis, contributing to the formation, progression, and complications of atherosclerotic plaques.Citation18 Noteworthy, in another study, oxidized proteins lead to endothelial dysfunction.Citation19 As a result, there is great interest in studying new target therapies to prevent or reverse the aging-induced oxidative stress in endothelial cells.
Furthermore, microparticles (MPs) have been used as biomarkers of cells damage and activation.Citation20,Citation21 MPs are a heterogeneous population of small membrane fragments shed from various cell types. The endothelium is one of the primary targets of circulating MPs, and MPs isolated from blood have been considered biomarkers of vascular injury and inflammation.Citation20,Citation22–Citation24 Endothelial damage and the release of membrane MPs are key steps in the pathogenesis and development of some diseases associated with damaged vasculature.Citation25 In this regard, adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), are secreted by activated endothelial cells in atherosclerotic lesions, stimulating immune cell and monocyte recruitment and migration into the intimal area of the vascular wall.Citation26,Citation27 Besides, endothelial cell apoptosis is also implicated in a number of cardiovascular conditions.Citation27
Several studies support a role of cellular senescence in aging-associated diseases.Citation28 Cellular senescence is a process in which cells cease dividing and undergo distinctive phenotypic alterations. Senescent endothelial cells are usually observed in a wide variety of diseases such as cardiovascular diseases. Senescence contributes to the overall decline in tissue regenerative potential that occurs with aging. Furthermore, an accelerated senescence in endothelial cells has been proposed to explain the premature apparition of cardiovascular diseases.
As a result, this study has determined whether the levels of oxidized proteins in sera may be involved with markers of endothelial damage associated with aging. In addition, the study also determines whether the aforementioned damage may be induced by the oxidation of the albumin.
Materials and methods
Patients and sample collection
Study population
Participants in the study were recruited from the Alcala University. All participants were volunteers who had submitted written informed consent to participate in the study and advance approval was obtained from the ethical committee of the Universidad de Alcalá. The study was performed in accordance with the World Medical Association Declaration of Helsinki. In all cases, the subjects have clinical history of age-related diseases such as type 2 diabetes mellitus, cardiovascular disease, renal disease, or cancer.
The study population included 16 subjects. The control group consisted of seven young healthy subjects aged between 28 and 31 years and who were comparable in sex and with nonsmoking habits. The elderly group consisted of eight healthy subjects aged between 75 and 82 years and who were also comparable in sex and with nonsmoking habits.
Plasma extraction
Peripheral blood samples were collected from patients in the morning using EDTA-coated tubes. Samples were centrifuged for 15 minutes at 1,200 rpm. Plasma was transferred into a new tube and stored at −20°C until use.
Culture cells
Primary human umbilical vein endothelial cells (HUVECs; ATTC Cat Number PCS-100-010) were cultured in endothelial growth media (EGM) from Lonza (Basel, Switzerland), supplemented with 10% FBS (heat inactivated). Culturing conditions were 37°C, 5% CO2, and 95% humidity. HUVECs were used for experiments between passages 2 and 7. Previously, HUVECs were serum-deprived overnight before treatments.
Determination of endothelial microparticles (EMPs) in plasma and endothelial cells
Plasma
Platelet-free plasma was obtained by centrifugation at 1,500 rpm for 10 minutes at room temperature, followed by an additional centrifugation at 15,000 rpm for 30 minutes in order to separate the MPs. MPs were resuspended in PBS to determinate the number of endothelial microparticles (EMPs) as described in the following section.
The MPs from plasma were incubated with monoclonal antibody against phycoerythrin (PE)-labeled anti-CD31 (as an endothelial marker; BD Bioscience, San Jose, CA, USA), followed by incubation with fluorescein isothiocyanate–conjugated (FITC) Annexin-V kits according to the manufacturer’s instructions (BD Bioscience). The negative control was obtained using the anti-isotype antibodies. An equal volume of flow count calibrator beads (Beckman Coulter, Marseilles, France) were added. Fluorescence-activated cell sorter analysis was performed in an Accuri C6 flow cytometer (BD Bioscience).
Endothelial cells (HUVEC)
Culture supernatants from endothelial cells treated with native and oxidized albumin for 24 hours were collected and cleared from detached cells and cell fragments by centrifugation at 1,500 rpm for 10 minutes at room temperature. The supernatants were then subjected to centrifugation at 15,000 rpm for 30 minutes. Pelleted EMPs were resuspended in PBS and then quantified. An equal volume of flow count calibrator beads (Beckman Coulter) was added. The EMPs in HUVEC were determined and quantified in an Accuri C6 flow cytometer (BD Bioscience) using forward scatter intensity value (size) and side scatter intensity value (granularity).
Preparation of oxidized albumin
Albumin (Albutein 20%; GRIFOLS, Barcelona, Spain) was diluted to 0.1 mg protein/mL with EDTA-free PBS and incubated with CuSO4 (10 µmol/L) for 18 hours at 37°C. At the end of incubation, 0.1 mmol/L EDTA was added to prevent further oxidation.Citation29 Oxidized albumin was diluted to 20 mg/mL in PBS. Either native albumin or oxidized albumin was added at a final concentration of 2 mg/mL.Citation30
Determination of plasma AOPPs
Plasma concentrations of advanced oxidation protein products (AOPPs) were measured in duplicate using a commercially available ELISA kit (CUSABIO, Wuhan, Hubei, People’s Republic of China, Cat Number CSB-E09925h) following the manufacturer’s protocol.
Protein carbonyl assay (OxyBlot)
OxyBlot analysis was performed, according to manufacturer’s specifications (Millipore, Billerica, MA, USA), to identify carbonyl groups that are introduced into the amino acid side chain after oxidative modification of proteins. The level of protein oxidation was determined by an Oxidized Protein Detection Kit (OxyBlot, Chemicon, Billerica, MA, USA, Cat# S7150-Kit). The OxyBlot kit derivatizes carbonyl groups to a 2,4-dinitrophenylhydrazone (DNP) moiety. The DNP moiety can then be detected using anti-DNP antibodies and is a method to assay for one form of oxidative damage to a protein. The proteins were derivatized as per the protocol given in the kit. These proteins were separated on 10% SDS-PAGE gels and transferred membranes were washed in washing buffer (PBS with 0.2% Tween-20). Protein bands were visualized with Luminata Crescendo Western HRP Substrate (Millipore).
Sample preparation and bidimensional electrophoresis (2D PAGE)
The samples used (native and oxidized albumin) for the identification of carbonylated proteins were prepared for analysis by two-dimensional gel electrophoresis.
First, both native and oxidized albumin were precipitated with acetone overnight at −20°C. The following day, aliquots of solubilized samples containing 500 µg of proteins were mixed with sample buffer containing 30 mM DTT, 0.5% v/v ampholytes (Biolytes pH 3–10; Bio-Rad, Hercules, CA, USA), and 0.001% w/v bromophenol blue to a total volume of 300 µL and loaded on IPG strips (17 cm, pH range: 4–7; Bio-Rad) for protein electrofocusing in a Protean IEF cell system (Bio-Rad). Strips were passively rehydrated for 2 hours at 20°C, and then the voltage was gradually increased (30–8,000 V) till it reached 60,000 Vh.
Prior to the separation of proteins according to their molecular weight, strips were equilibrated for 20 minutes in equilibration buffer (6 M urea, 1.5 M Tris–HCl pH 8.8, 2% SDS, and 20% glycerol) containing 2% DTT, followed by 20 minutes in equilibration buffer containing 135 mM iodoacetamide. Second-dimension separation was performed in polyacrylamide Mini-Protean TGX precast gel (BioRad) using a Mini-Protean tetra cell (Bio-Rad). Gels were run at a constant current intensity of 60 mA during 3 hours. A total of two gels were performed for each condition from two different biological samples.
Gel staining, image acquisition, and analysis
Two-dimensional gels were stained with Coomassie brilliant blue G-250 (Bio-Rad) for 24 hours (according to the study by Herbert et al),Citation31 and then destained by washing with 0.1 M Tris–H3PO4 (pH 6.5) for 5 minutes, followed by 1 minute with 25% v/v methanol and finally 5 minutes with 20% w/v ammonium sulfate. Gels were digitized using a GS-800 calibrated densitometer (Bio-Rad) and the images analyzed with the PDQUEST software 8.0.1 (Bio-Rad), using spot-by-spot manual matching between the studied conditions.
Endothelial adhesion molecules expression
Expression of VCAM-1 and ICAM-1 were measured from endothelial cells treated with vehicle and different doses of native and oxidized albumin for 24 hours. After treatment, HUVECs were obtained by mechanical disruption and washed once with PBS 1×. Next, 10 µL VCAM-1 (CD106-PE conjugate, BD Pharmingen [BD Pharmingen, San Diego, CA, USA]) and 10 µL ICAM-1 (MHCD5401-FITC conjugate, Invitrogen, Waltham, MA, USA) antibodies were used to assess the expression in the different experimental conditions. HUVECs were incubated with the antibodies for 20 minutes in darkness. Then, cells were washed with PBS 1× and fixed with BD CellFIX™ (Becton Dickinson, Cat Number 340181 [Becton Dickinson Bioscience, San José, CA, USA]). Finally, we proceeded to the data acquisition in the cytometer, with HUVECs without antibody labeling used as a reference (as a negative control). We performed the experiment in duplicates (n=3). For the analysis of data acquired in the cytometer, we used the mean fluorescence intensity (MFI) of different antibodies (VCAM-1-PE and ICAM-1-FITC).
Apoptosis quantification
The percentage of apoptosis was measured by annexin-V and propidium iodide (PI) staining of endothelial cells treated with vehicle and different doses of native and oxidized albumin for 24 hours. HUVECs were obtained by mechanical disruption and washed once with annexin-V binding buffer (FITC Annexin-V Apoptosis Detection Kit I, Becton Dickinson). Cells were then suspended in 195 µL of annexin-V binding buffer and 5 µL of annexin-V (556419, Becton Dickinson) and PI (51-66211E, Becton Dickinson) were added following the manufacture’s protocol. The negative tube controls that did not contain either annexin-V or PI were placed with 195 µL of annexin-V binding buffer. Cells were incubated for 15 minutes in darkness. Next, the cells were washed once with buffer and finally suspended in 490 µL of buffer. The samples were acquired within 1 hour in flow cytometer (FACSCalibur, Becton Dickinson). We performed this experiment in duplicates (n=3). For the analysis of the data acquired, cell populations that were positive for both annexin-V and PI were utilized. These cells were considered to be in late apoptosis state. Finally, we analyzed the differences, in percentages, obtained in the experimental conditions.
Detection of ROS
Hydroethidine (Invitrogen), a substance that is oxidized by ROS to become ethidium, which emits red color, was used to measure superoxide anion. The HUVEC monolayer was incubated in EGM medium, with vehicle, native albumin, and oxidized albumin at different doses (mg/mL), and without FBS for 4 hours at 37°C with CO2. At the end of the treatments, the cells were exposed for 15 minutes at 37°C to 2 µM hydroethidine (HE). Analyses were performed in a flow cytometer (FACSCalibur, Becton Dickinson). Intracellular ROS production was measured as a percentage of positive cells marked with HE.
Senescence assay
HUVECs were cultured in their own medium with the different treatments. Cells were fixed at 70% confluence and then incubated at 37°C overnight with the staining solution containing the X-gal substrate (Catalog #JM-K320-250; Senescence Detection kit, MBL International Corp, Woburn, MA, USA). Cells were then observed under a microscope for development of blue color.
Wound healing assay
HUVECs were seeded in six-well plates (1×105 cells/well), incubated in medium with 10% FBS for 24 hours, and subsequently washed twice with PBS and incubated in medium with 0% FBS. After the cells grew to confluence, a straight line was scratched across the culture with a 10–200 µL micropipettor tip. The cells were treated with the different treatments and were incubated at 37°C and 5% CO2. Images of the cells were obtained with an inverted phase contrast microscope at different times. The wound width was determined with ImageJ software (National Institutes of Health, New York, NY, USA).
Statistical analysis
Data are presented as mean ± SD. Significance of differences from control values was determined with the Student’s t-test, with two-tailed distribution between groups as indicated in (AOPPs ELISA). Mean separations were performed with one-way analysis of variance, with Bonferroni correction used for the in vitro experiments. A value of P<0.05 was considered to indicate statistical significance. All statistical tests were performed with GraphPad Prism software version 5.0 (Graph Pad Software, Inc., La Jolla, CA, USA).
Table 1 AOPPs and levels of endothelial microparticles in the plasma of healthy young subjects compared with healthy elderly subjects
Results
Increase of AOPPs plasma levels in aging subjects compared with young subjects (controls)
Plasma protein oxidation and its correlation to human aging were analyzed in this study. Oxidized plasma proteins were evaluated as AOPPs by ELISA. Plasma concentration levels of AOPPs from aging subjects were significantly increased compared with young subjects, who comprised the control group (). Therefore, we observed that the levels of oxidized proteins increase with subject age.
EMPs rise in elderly subjects
There are studies that show the MPs ratio as a marker of the endothelial damage.Citation20,Citation21 To study the role of MPs ratio in the aging process, we measured the number of EMPs in the plasma of different subjects by flow cytometry. As a result, we observed that there was an incremental increase in the amount of EMPs in the elderly subjects compared with young subjects (). This finding implicates that the increase of EMPs is associated with aging.
Determination of albumin oxidation in vitro
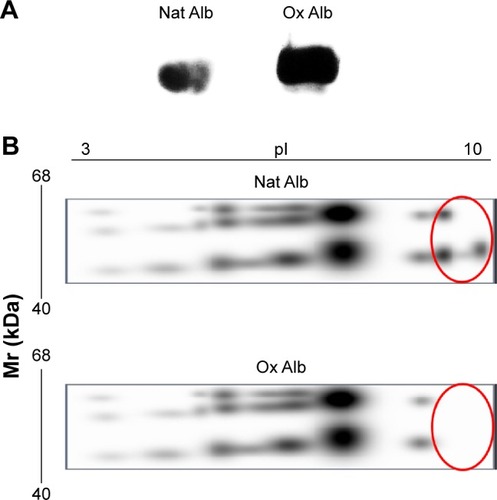
To analyze the biological effect of oxidized albumin on the endothelial cells, we utilized the albumin oxidation. The albumin oxidation with CuSO4 procedure was confirmed by different techniques. First, the albumin oxidation was confirmed by OxyBlot analysis. The amount of oxidation was proportional to the signal intensity and we found a higher OxyBlot band in the oxidized albumin versus native albumin (). We also probed the albumin oxidation using 2D PAGE technique. The gel with oxidized albumin presented a clear difference in the position of albumin spots (). In addition, the 2D PAGE analysis revealed (as shown in ) a change in the pI of oxidized albumin (6.8) of −1.5 units with respect to native albumin pI (8.3).
Figure 1 Confirmation of the albumin oxidation.
Abbreviations: DNPH, 2,4-dinitrophenylhydrazine; DNP, 2,4-dinitrophenyl; TCA, Trichloroacetic acid; Mr, relative molecular mass; IPG, immobilized pH gradient; Ox Alb, oxidized albumin; Nat Alb, native albumin.
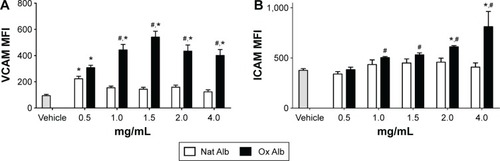
Oxidized albumin increases adhesion markers in a dose–response effect in endothelial cells
To quantify the endothelial damage in vitro, we measured some typical markers of adhesion,Citation32,Citation33 such as VCAM-1 and ICAM-1, in endothelial cells (HUVECs). We performed a dose–response treatment with native and oxidized albumin for 24 hours in serum-deprived HUVEC. We observed an increase in VCAM-1 expression () when treating with different doses of oxidized albumin for 24 hours. Furthermore, we observed increased levels of ICAM-1 () in a dose–response pattern after a 24-hour exposure period with oxidized albumin treatment versus treatment with native albumin and vehicle. These observations suggest that treatment with oxidized albumin activates the endothelial cells.
Figure 2 Flow cytometer analysis of endothelial adhesion molecules, (A) VCAM-1 and (B) ICAM-1, expression in HUVEC incubated with vehicle CuSO4 (10 µmol/L) + 0.1 mmol/L EDTA (gray bar), Nat Alb (white bars), or Ox Alb (black bars).
Abbreviations: Ox Alb, oxidized albumin; Nat Alb, native albumin; MFI, median fluorescence intensity; HUVECs, human umbilical vein endothelial cells; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; EDTA, ethylenediaminetetraacetic acid; SD, standard deviation.
Oxidized albumin enhances the apoptosis levels in endothelial cells
It has been described that apoptosis plays an important role in the endothelial damage.Citation34,Citation35 To check the possibility that oxidized albumin produces endothelial damage via apoptosis, we measured the apoptosis levels with different concentrations of native and oxidized albumin () in resting HUVECs. After a 24-hour exposure period, we observed upregulated apoptosis levels in a dose–response effect with the oxidized albumin treatment compared with different native albumin concentrations and vehicle. This confirms that another marker of endothelial damage is modified with oxidized albumin treatment.
Figure 3 Flow cytometer determination of (A) cellular apoptosis and (B) endothelial microparticles expression in the supernatants of albumin-treated cells.
Abbreviations: Ox Alb, oxidized albumin; Nat Alb, native albumin; HUVEC, human umbilical vein endothelial cells; EMPs, endothelial microparticles; PI, propidium iodide; SD, standard deviation.
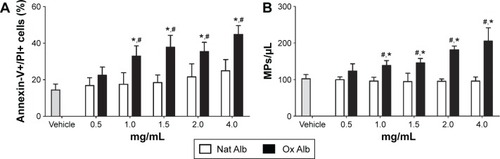
Oxidized albumin treatment elevates the EMPs production in supernatants of endothelial cells
As mentioned before, there exists a relationship between the number of EMPs in plasma and the aging process, which is exhibited by the elevated levels of oxidized protein. We also performed studies in vitro to measure the numbers of EMPs generated by oxidized albumin. The results showed an increase in the production of EMPs with different doses of oxidized albumin treatment, whereas there was no change in the EMPs production with different concentrations of native albumin compared with vehicle supernatants cells (). Thereupon, as in the case of plasma samples, there is also a link between the number of EMPs and the oxidized protein amount.
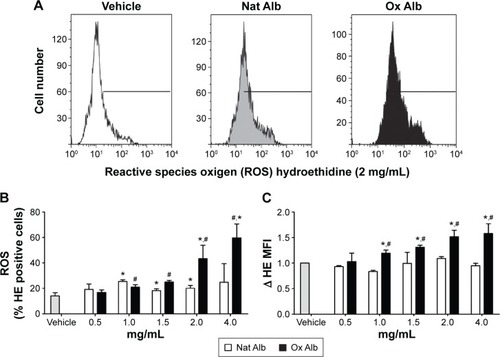
Oxidized albumin generates oxidative stress
To test the possibility of oxidized albumin increasing ROS production, we have used a HE as a probe for measurement of intracellular ROS. Resting HUVECs were treated with and without different doses of native and oxidized albumin for 4 hours and vehicle. Flow cytometry intracellular ROS production () and the total ROS production () were significantly higher after oxidized albumin treatments compared with the native albumin and vehicle cells. Surprisingly, we also observed that the native albumin treatments upregulated the percentage of ROS () production versus vehicle-treated cells, whereas there were no differences in the total ROS production between native albumin-treated cells and vehicle-treated cells (). Thereby, albumin oxidation is associated with oxidative stress due to endogenous production of ROS by mitochondria.
Figure 4 (A) Flow cytometer analysis of intracellular ROS production in HUVEC incubated with vehicle (white), Nat Alb (gray), or Ox Alb (black). HUVEC were treated with native and oxidized albumin (2 mg/mL) for 4 hours, (B) the histogram shows ROS as the percentage of HE-positive cells, and (C) MFI with vehicle (gray bar) and different doses of Nat Alb (white bars) and Ox Alb (black bars) for 4 hours.
Abbreviations: Ox Alb, oxidized albumin; Nat Alb, native albumin; HUVEC, human umbilical vein endothelial cells; ROS, reactive oxygen species; HE, hidroethidine; MFI, median fluorescence intensity; SD, standard deviation.
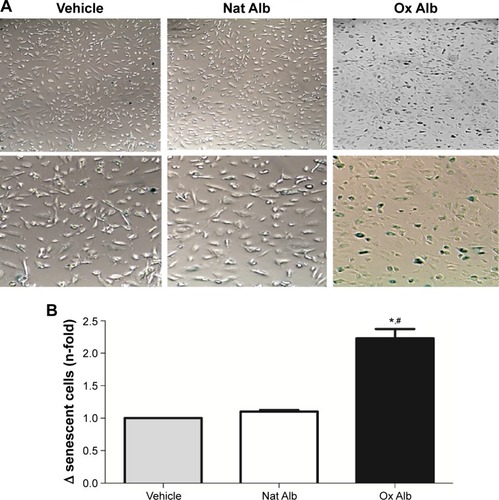
Increment of senescent endothelial cells by albumin oxidation treatment
To investigate the role of oxidized albumin in the senescence of endothelial cells, staining for senescence-associated β-galactosidase was carried out (). Staining revealed that there was a 2.3-fold increase in the number of β-galactosidase-positive cells in oxidized albumin-treated HUVECs (), whereas the value with native albumin was significantly lower and similar to vehicle cells. By morphological inspection, we found that senescent cells increase in size and display a more flat morphology than native albumin-treated HUVECs (). Importantly, the senescence effect observed in the oxidized albumin-treated endothelial cells was reproducible with the replicative senescence (population doublings undergone by human endothelial cells) of HUVEC.Citation36
Figure 5 Senescent-associated β-galactosidase activity staining of native and oxidized albumin treatments.
Abbreviations: Ox Alb, oxidized albumin; Nat Alb, native albumin; HUVEC, human umbilical vein endothelial cells; SD, standard deviation.
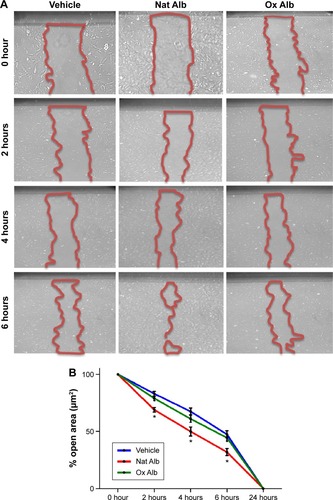
Oxidized albumin inhibits endothelial cell wound healing migration
Finally, we studied migration associated with the albumin oxidation in endothelial cells. The wound healing migration assay illustrated a reduction in migration in oxidized albumin-treated HUVEC, whereas no significant changes were observed between native albumin-treated cells and vehicle-treated cells (). Hence, the treatment with the oxidized albumin showed a reduction of migration activity in endothelial cells.
Figure 6 HUVEC with Nat Alb and Ox Alb (2 mg/mL) were scratched and wound margins were imaged from 0 up to 24 hours later.
Abbreviations: Ox Alb, oxidized albumin; Nat Alb, native albumin; HUVEC, human umbilical vein endothelial cells.
Discussion
Endothelial dysfunction associated with cardiovascular diseases is a property related to aging. The mechanism by which endothelial cells undergo senescence is still largely unclear and yet to be discovered. Although this mechanism probably involves a multifactorial response, oxidative stress has been proposed as a mediator to explain the process of cellular senescence. Oxidative stress is characterized by excess free radical activity and plays an important role in the oxidation of proteins. Several studies have implicated the oxidation of low-density lipoprotein (LDL) in atherosclerosis.Citation5,Citation37,Citation38 However, there is no evidence that relates the oxidized albumin, which is the most abundant protein in serum, with endothelial injury. Therefore, in this study, we investigated whether aging induced an increase in oxidized protein and whether oxidized albumin may be involved in aging-related endothelial damage.
The results of this study demonstrated that elderly subjects exhibit an increase of AOPPs and EMPs, reflecting endothelial damage and cellular senescence. To date, all the studies have analyzed the effect of oxidized LDL on endothelial damage. In plasma, the major protein presented is the albumin and LDL is present in lower concentrations, consequently, the albumin is more susceptible to suffer oxidation. In particular, there is no evidence showing that the oxidized albumin produced by oxidative stress generates cellular senescence. For this reason, our study is focused on oxidized albumin. Notwithstanding, plenty of data exist regarding the association between the presence of oxidized LDL presence and some senescence markers.Citation39–Citation41 It has also been described that AOPPs are carried by oxidized plasma proteins, especially albumin, and accumulate in subjects with renal disease and coronary artery disease.Citation42 In this sense, these reported data indicate that oxidized proteins are increased in the plasma of elderly subjects.
In accordance, there are no findings demonstrating the presence of oxidized albumin in the plasma when there is endothelial dysfunction. In contrast, accumulating evidence suggest that the oxidation of LDL results in severe vascular damage.Citation40,Citation43 Here, we have shown an increase of the EMPs in the elderly subjects, suggesting that EMPs measurement is identified as a parameter in the endothelial damage. So, the in vitro model was used in this study to determine whether the oxidized albumin induces senescence and endothelial damage.
In this study, oxidized albumin-treated HUVECs cause the release of EMPs in the media and an increment of apoptosis levels. These findings support the idea that the endothelial cells are suffering from an endothelial activation, which is an apoptosis phenomenon not observed with native albumin treatment. Recent evidence also suggests that the endothelial cell is damaged as a consequence of cardiovascular disease. Furthermore, released EMPs are considered a marker of endothelial damage in patients.Citation20,Citation44 Several studies have demonstrated that adhesion molecules are secreted by activated endothelial cells and contributed to endothelial cell injury.Citation26,Citation27 Supporting this, our results demonstrate an increase of VCAM-1 and ICAM-1.
In addition, other studies have indicated the increase of modification proteins may be associated with oxidative stress development in aging. In this regard, there is a wealth of data evidencing the fact that protein modifications cause ROS production.Citation37,Citation45 As the results showed, oxidized albumin results in ROS production increment in endothelial cells as well as in the amount of ROS per cell (MFI increment). The enhancement of oxidative stress is considered a key mechanism in the cellular senescence development (replicative and premature induced by cardiovascular factors).Citation40,Citation46 In this study, the upregulation of ROS induced by oxidized albumin is correlated with an increase in the number of senescent cells. These data support the idea that the oxidized albumin may be considered a cardiovascular risk factor to induce oxidative stress. As a consequence, the cell may suffer senescence processes to prevent a possible damage due to oxidative stress. Additionally, we observed a slight ROS increment when the endothelial cells were treated with native albumin, which is not comparable with the elevated ROS levels observed with the oxidized albumin treatment; moreover, it is not associated with changes in other markers of cellular damage, such as adhesion molecules and apoptosis. In this sense, it seems that the ROS modification by native albumin is linked to normal cell proliferation and physiological signaling processes. Additional experiments are needed to consider the oxidized albumin quantification as a biological marker associated with endothelial damage and aging. Consequently, research is needed to explore the possibility of utilizing oxidized albumin as a potential therapeutic target.
Finally, we have shown that the migratory capacity of endothelial cells was reduced with oxidized albumin treatment. To date, experimental studies have found that oxidized LDL inhibits endothelial cell migration and may impair healing of arterial injuries.Citation47 In addition, this study reported that stimulation of ROS production by oxidized LDL inhibited endothelial cell migration. Likewise, oxidized albumin promotes intracellular ROS production and therefore is implicated in many cellular processes, including migration. As mentioned earlier, the endothelial senescence induced by oxidized albumin is a cellular mechanism to avoid ROS damage. Furthermore, this effect is associated with reduction in the endothelial migration activity.
Conclusion
Our data have shown that the plasma of elderly subjects contain oxidized proteins, especially oxidized albumin. The oxidation of this protein indicates an endothelial activation by adhesion molecule releases and the consequent endothelial damage through an oxidative stress and increase in apoptosis levels. Although further experiments are needed to better understand the role of oxidized albumin in endothelial injury, the identification of oxidized albumin as a possible marker for use in the clinic may be helpful in the diagnosis of the endothelial damage associated with aging. As a result, oxidized albumin may be utilized in prognosis and as a therapeutic target in preventing cardiovascular disease development.
Author contributions
Rafael Ramírez, Carlos Luna, Matilde Alique, and Estefania Navalmoral conceived and designed the experiments. Estefanía Navalmoral, Maria-Victoria Noci, Lourdes Bohorquez-Magro, and Julia Carracedo performed the experiments. Carlos Luna, Matilde Alique, and Julia Carracedo analyzed the data. Rafael Ramírez and Julia Carracedo contributed reagents/materials/analysis tools. Rafael Ramírez, Julia Carracedo, and Matilde Alique wrote the paper. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We are grateful to María José Jimenez and Rosa Moyano for technical assistance. We also thank Miss Annie Bailey for English and scientific revisions of the manuscript. This work was supported by Plan Nacional Proyectos de Investigación en Salud of Instituto de Salud Carlos III (ISCIII) Fondos Feder European Grants (PI11/01536, PI12/01489, PI014/0041 and PI14/00806); Red de Investigación Renal (REDinREN; RD12/0021/002); Comunidad de Madrid (Fibroteam; S2010/BMD-2321); Junta de Andalucía Grants JA0797-2010, P010-CTS-6337, P11-CTS-7352.
Disclosure
Julia Carracedo is a fellow from Fundación de Investigaciones Biomédicas de Córdoba (Programa Nicolás Monardes). Carlos Luna is a fellow from Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía (CTS-6337). Matilde Alique is a fellow of the program “Ayuda Postdoctoral Programa Propio” from Universidad de Alcala, Madrid, Spain. The authors report no other conflicts of interest in this work.
References
- LevineRLStadtmanEROxidative modification of proteins during agingExp Gerontol20013691495150211525872
- StadtmanERProtein oxidation and agingFree Radic Res200640121250125817090414
- GruneTReinheckelTDaviesKJDegradation of oxidized proteins in K562 human hematopoietic cells by proteasomeJ Biol Chem19962712615504155098663134
- RivettAJRegulation of intracellular protein turnover: covalent modification as a mechanism of marking proteins for degradationCurr Top Cell Regul1986282913372878793
- GradinaruDBorsaCIonescuCMarginaDAdvanced oxidative and glycoxidative protein damage markers in the elderly with type 2 diabetesJ Proteomics20139231332223587667
- StadtmanERLevineRLProtein oxidationAnn N Y Acad Sci200089919120810863540
- AndersonNLAndersonNGThe human plasma proteome: history, character, and diagnostic prospectsMol Cell Proteomics200211184586712488461
- BorgesCRRehderDSJensenSElevated plasma albumin and apolipoprotein A-I oxidation under suboptimal specimen storage conditionsMol Cell Proteomics20141371890189924736286
- BerlettBSStadtmanERProtein oxidation in aging, disease, and oxidative stressJ Biol Chem19972723320313203169252331
- Dalle-DonneIGiustariniDColomboRRossiRMilzaniAProtein carbonylation in human diseasesTrends Mol Med20039416917612727143
- FanXZhangJThevesMMechanism of lysine oxidation in human lens crystallins during aging and in diabetesJ Biol Chem200928450346183462719854833
- ChoiJForsterMJMcDonaldSRWeintraubSTCarrollCAGracyRWProteomic identification of specific oxidized proteins in ApoE-knockout mice: relevance to Alzheimer’s diseaseFree Radic Biol Med20043691155116215082069
- BrennanMLHazenSLAmino acid and protein oxidation in cardiovascular diseaseAmino Acids2003253–436537414661097
- De MarchiEBaldassariFBononiAWieckowskiMRPintonPOxidative stress in cardiovascular diseases and obesity: role of p66Shc and protein kinase COxid Med Cell Longev2013201356496123606925
- StrobelNAFassettRGMarshSACoombesJSOxidative stress biomarkers as predictors of cardiovascular diseaseInt J Cardiol2011147219120120864194
- BrandesRPFlemingIBusseREndothelial agingCardiovasc Res200566228629415820197
- MatzRLAndriantsitohainaRAge-related endothelial dysfunction: potential implications for pharmacotherapyDrugs Aging200320752755012749750
- DavignonJGanzPRole of endothelial dysfunction in atherosclerosisCirculation200410923 Suppl 1III27III3215198963
- BanfiCBrioschiMBarcellaSOxidized proteins in plasma of patients with heart failure: role in endothelial damageEur J Heart Fail200810324425118331966
- BuendíaPMontes de OcaAMadueñoJAEndothelial microparticles mediate inflammation-induced vascular calcificationFASEB J201529117318125342130
- BartoloniEAlunnoABistoniOCharacterization of circulating endothelial microparticles and endothelial progenitor cells in primary Sjögren’s syndrome: new markers of chronic endothelial damage?Rheumatology (Oxford)201554353654425190637
- LovrenFVermaSEvolving role of microparticles in the pathophysiology of endothelial dysfunctionClin Chem20135981166117423529703
- FinkKMoebesMVetterCSelenium prevents microparticle-induced endothelial inflammation in patients after cardiopulmonary resuscitationCrit Care2015195825886988
- LeeSKYangSHKwonILeeOHHeoJHRole of tumour necrosis factor receptor-1 and nuclear factor-κB in production of TNF-α-induced pro-inflammatory microparticles in endothelial cellsThromb Haemost2014112358058825008247
- Dignat-GeorgeFBoulangerCMThe many faces of endothelial microparticlesArterioscler Thromb Vasc Biol2011311273321160065
- ZhuYPShenTLinYJAstragalus polysaccharides suppress ICAM-1 and VCAM-1 expression in TNF-α-treated human vascular endothelial cells by blocking NF-κB activationActa Pharmacol Sin20133481036104223728723
- Al-MutairiMAl-HarthiSCadalbertLPlevinROver-expression of mitogen-activated protein kinase phosphatase-2 enhances adhesion molecule expression and protects against apoptosis in human endothelial cellsBr J Pharmacol2010161478279820860659
- van DeursenJMThe role of senescent cells in ageingNature2014509750143944624848057
- IbanezBGiannarelliCCimminoGRecombinant HDL(Milano) exerts greater anti-inflammatory and plaque stabilizing properties than HDL(wild-type)Atherosclerosis20122201727722030095
- RubensteinDAMariaZYinWGlycated albumin modulates endothelial cell thrombogenic and inflammatory responsesJ Diabetes Sci Technol20115370371321722586
- HerbertBGalvaniMHamdanMReduction and alkylation of proteins in preparation of two-dimensional map analysis: why, when, and how?Electrophoresis200122102046205711465505
- QuagliaroLPiconiLAssaloniRIntermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide productionAtherosclerosis2005183225926716285992
- YuHJiangWDuHInvolvement of the Akt/NF-κB pathways in the HTNV-mediated increase of IL-6, CCL5, ICAM-1, and VCAM-1 in HUVECsPLoS One201494e9381024714064
- WinnRKHarlanJMThe role of endothelial cell apoptosis in inflammatory and immune diseasesJ Thromb Haemost2005381815182416102048
- CarracedoJBuendíaPMerinoACellular senescence determines endothelial cell damage induced by uremiaExp Gerontol201348876677323624226
- WagnerMHampelBBernhardDHalaMZwerschkeWJansen-DürrPReplicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosisExp Gerontol20013681327134711602208
- ChuangCYDegendorferGHammerAWhitelockJMMalleEDaviesMJOxidation modifies the structure and function of the extracellular matrix generated by human coronary artery endothelial cellsBiochem J2014459231332224517414
- SchaerCADeuelJWBittermannAGMechanisms of haptoglobin protection against hemoglobin peroxidation triggered endothelial damageCell Death Differ201320111569157923995229
- UchidaKKanematsuMSakaiKProtein-bound acrolein: potential markers for oxidative stressProc Natl Acad Sci U S A1998959488248879560197
- ShiYLüscherTFCamiciGGDual role of endothelial nitric oxide synthase in oxidized LDL-induced, p66Shc-mediated oxidative stress in cultured human endothelial cellsPLoS One201499e10778725247687
- CarracedoJMerinoABriceñoCCarbamylated low-density lipoprotein induces oxidative stress and accelerated senescence in human endothelial progenitor cellsFASEB J20112541314132221228221
- MarscheGFrankSHrzenjakAPlasma-advanced oxidation protein products are potent high-density lipoprotein receptor antagonists in vivoCirc Res2009104675075719179658
- HuangCSLinAHLiuCTIsothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NFκB activationMol Nutr Food Res201357111918193023836589
- SchiroAWilkinsonFLWestonRSmythJVSerracino-InglottFAlexanderMYEndothelial microparticles as conveyors of information in atherosclerotic diseaseAtherosclerosis2014234229530224721189
- WuJHeZGaoXOxidized high-density lipoprotein impairs endothelial progenitor cells’ function by activation of CD36-MAPK-TSP-1 pathwaysAntioxid Redox Signal201522430832425313537
- BuendíaPCarracedoJSorianoSKlotho prevents NFκB translocation and protects endothelial cell from senescence induced by uremiaJ Gerontol A Biol Sci Med Sci201570101198120925246106
- van AalstJAZhangDMMiyazakiKCollesSMFoxPLGrahamLMRole of reactive oxygen species in inhibition of endothelial cell migration by oxidized low-density lipoproteinJ Vasc Surg20044061208121515622376
