Abstract
The purpose of this study was to evaluate the systemic effects of intravitreal ranibizumab (Lucentis) treatment in patients with neovascular age-related macular degeneration (AMD). The impact of intravitreal ranibizumab injections on central retinal thickness (CRT) of treated and contralateral untreated eyes, and differences in gene expression patterns in the peripheral blood mononuclear cells were analyzed. The study included 29 patients aged 50 years old and over with diagnosed neovascular AMD. The treatment was defined as 0.5 mg of ranibizumab injected intravitreally in the form of one injection every month during the period of 3 months. CRT was measured by optical coherence tomography. The gene expression profile was assigned using oligonucleotide microarrays of Affymetrix HG-U133A. Studies have shown that there was a change of CRT between treated and untreated eyes, and there were differences in CRT at baseline and after 1, 2, and 3 months of ranibizumab treatment. Three months after intravitreal injection, mean CRT was reduced in the treated eyes from 331.97±123.62 to 254.31±58.75 μm, while mean CRT in the untreated fellow eyes reduced from 251.07±40.29 to 235.45±36.21 μm at the same time. Furthermore, the research has shown that among all transcripts, 3,097 expresses change after the ranibizumab treatment in relation to controls. Among these transcripts, 1,339 were up-regulated, whereas 1,758 were down-regulated. Our results show the potential systemic effects of anti-VEGF therapy for AMD. Moreover, our study indicated different gene expression in peripheral blood mononuclear cells before and after intravitreal ranibizumab treatment.
Introduction
Age-related macular degeneration (AMD) is the most common cause of severe, irreversible vision loss in the elderly.Citation1 In the last decades, therapies for neovascular AMD treatments were: photodynamic therapy, thermal laser photocoagulation, or transpupillary thermotherapy. Currently, the most widely used treatments for the neovascular exudative form of AMD include intravitreal injections of anti-VEGF agents, such as ranibizumab (Lucentis), aflibercept, or bevacizumab.Citation2
Anti-VEGF therapies have been used with increasing frequency to treat other ocular diseases, including diabetic macular edema,Citation3 branch retinal vein occlusion, central retinal vein occlusion,Citation4 and uveitis.Citation5,Citation6
The use of anti-VEGF drugs improved visual prognosis of AMD patients.Citation1 It is also suggested that anti-VEGF agents may reach the contralateral eye via the systemic circulation.Citation7,Citation8 However, the effects of intravitreal injections of anti-VEGFs on untreated contralateral eyes are not well documented. Moreover, systemic side effects of intraocular anti-VEGF therapy for AMD are also not well-known.Citation9 Previous studies suggested that this therapy may lead to serious cardiovascular complications.Citation1,Citation10,Citation11 On the contrary, Campbell et al observed that anti-VEGF therapy is not associated with increased risk of stroke.Citation12 Moreover, there are only few published data regarding gene expression/protein level differences in blood samples of patients with AMD after intravitreal anti-VEGF treatment.Citation13–Citation15 Nassar et al suggested that selected cytokine serum levels may be used as biomarkers for AMD or to predict patient responses to anti-VEGF therapies.Citation13
The purpose of this study was to evaluate the systemic effects of intravitreal ranibizumab treatment in patients with neovascular AMD. The influence of intravitreal ranibizumab injections on central retinal thickness (CRT) of treated and contralateral untreated eyes, as well as the differences in gene expression patterns in the peripheral blood mononuclear cells (PBMCs) of these patients were analyzed.
Materials and methods
Subjects
The study included 29 patients (15 women and 14 men, mean age 73 years, range: 54–86 years) who received ranibizumab because of neovascular AMD diagnosed based on routine ophthalmologic diagnostic procedures (optical coherence tomography [OCT] and fluorescein angiography). All the patients were treated at the Department of Ophthalmology, University Hospital No 5, Medical University of Silesia, Katowice, Poland.
All subjects underwent a complete ophthalmic examination: best-corrected visual acuity using Snellen charts, Goldmann applanation tonometry, indirect biomicroscopy in mydriasis (+78 D lens; Volk Optical, Mentor, OH, USA), OCT (Cirrus HD-OCT 4000, Carl Zeiss Meditec AG, Jena, Germany) and fluorescein angiography (Fundus Camera FF 450 plus IR; Carl Zeiss Meditec AG).
The inclusion criteria for the study group were as follows: choroidal neovascularization (CNV) resulting from AMD, aged ≥50 years, best-corrected visual acuity 0.1 to 0.5 with Snellen chart and no previous CNV therapies, such as anti-VEGF injections, photodynamic, or laser therapies. Patients with uncontrolled, elevated intraocular pressure, glaucomatous optic neuropathy, chronic uveitis, retinal vein occlusion, or other ocular neovascular diseases were excluded from the study. Moreover, patients with a previous history of myocardial infarction or stroke were also excluded. Intravitreal injections were performed by an ophthalmologist, who applied an aseptic technique of the procedure, using infiltration anesthesia in the injection site. The treatment was defined as 0.5 mg (0.05 mL) of ranibizumab (Lucentis, Novartis International AG, Basel, Switzerland) injected intravitreally in the form of one injection every month during the period of 3 months. The reinjections were dependent on CNV activity. The study was approved by the Bioethics Committee of the Medical University in Katowice (KNW) in accordance with the Declaration of Helsinki regarding medical research involving human subjects. All patients were informed about the research and signed an informed consent form.
Tissues
For molecular analysis venous blood samples were collected into ethylenediaminetetraacetic acid-containing tubes before ranibizumab injections and 30 days after the application of three injections of Lucentis at around the same time of day. PMBCs were isolated from 5 mL specimens derived from each patient by using Ficoll-Conray density gradient centrifugation for 30 minutes at 1,500 rpm at room temperature immediately after blood collection (specific gravity 1.077; Immunobiological Co., Gunma, Japan).
RNA extraction
Total RNA was extracted using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. RNA extracts were treated with DNase I (RNeasy Mini Kit; Qiagen NV, Venlo, the Netherlands) according to the manufacturer’s instructions. The quality of extracts was checked electrophoretically using 0.9% agarose gel stained with ethidium bromide (Sigma-Aldrich Co., St Louis, MO, USA). The results were analyzed and recorded using the 1D Bas-Sys gel documentation system (Biotech-Fisher, Perth, Australia). Nucleic acid concentration was determined using a GeneQuant II RNA/DNA spectrophotometer (Pharmacia Biotech, Cambridge, UK).
Oligonucleotide microarray analysis
Eight samples of patients with AMD were collected for microarray analysis (control – three samples; after ranibizumab treatment – five samples) before and after intravitreal ranibizumab injections. The injections were conducted every month during a 3-month period. The analysis of the expression profile of genes was performed using commercially available oligonucleotide microarrays of HG-U133A (Affymetrix, Santa Clara, CA, USA) in accordance with the manufacturer’s recommendations, as described previously.Citation16 Each gene chip contains 22,238 probe sets that correspond to more than 18,400 transcripts and 14,500 well-characterized human genes. Here, approximately 8 μg of total RNA were used for the complementary DNA (cDNA) synthesis using SuperScript Choice System (Thermo Fisher Scientific). During the next step, cDNA was used as a template to produce biotin-labeled complementary RNA (cRNA) using BioArray HighYield RNA transcript labeling kit (Enzo Life Sciences, Farmingdale, NY, USA). cRNA was purified on Rneasy Mini Kit columns (Qiagen NV). Next, the biotin-labeled cRNA was fragmented using Sample Cleanup Module (Qiagen NV) and hybridized with the HG-U133A microarray (Affymetrix). The cRNA probes hybridized to oligonucleotide arrays were stained with streptavidin phycoerythrin conjugate and were scanned using GeneArray Scanner G2500A (Agilent Technologies, Santa Clara, CA, USA). The scanned data were processed for signal values using Microarray Suite 5.0 software (Affymetrix). The obtained results were normalized using RMAExpress software (Robust Multichip Average).
Statistical analyses
Statistical analyses were performed using Statistica 9.0 software (StatSoft, Tulsa, OK, USA), and the level of significance was set at P<0.05. Values were expressed as mean and standard deviation. The differences of repeated measures of continuous variables were analyzed with the two-factor analysis of variance (ANOVA) for repeated measures. Paired t-test was used to assess the significant differences between CRT at baseline and after ranibizumab treatment in treated and untreated contralateral eyes.
The data from all arrays were analyzed using Gene-Spring 12.0 platform (Agilent Technologies) to identify transcriptome differences between the control and ranibizumab treatment groups. The oligonucleotide microarrays of Affymetrix HG-U133A enabled an analysis of 22,283 mRNA transcripts. The normalized data were used to compile a list of genes, the expression of which appeared to be up- or down-regulated by an arbitrary, at least 2-fold, cutoff. A significant differential gene expression was identified by 2.0-fold change at P<0.05 (t-test). The Benjamin–Hochberg false discovery rate multiple test correction was applied whenever applicable. Gene ontology analysis was carried out with the PANTHER 8.0 (Protein Analysis Through Evolutionary Relationships; http://www.pantherdb.org) Classification System database to classify genes based on their biological processes, molecular functions, cellular components, and pathways.
Results
The differences in CRT between ranibizumab treated and untreated contralateral eyes
In all patients, CRT was measured by OCT in both eyes: before the first dose of treatment was administered (baseline) and again at 1, 2, and 3 months after the initiation of treatment in treated eye and untreated contralateral eyes. There was a statistically significant change of CRT between treated and untreated eyes (ANOVA for repeated measures, P=0.0028). Moreover, there were statistically significant differences in CRT at baseline and after 1, 2, and 3 months of ranibizumab treatment (ANOVA for repeated measures, P<0.0001) (). The mean CRT decreased over time in both groups, with a sharper decrease in the treatment group. The differences in CRT between treated and untreated contralateral eyes means have been narrowing over time (ANOVA for repeated measures, P=0.0004) ().
Figure 1 The differences in central retinal thickness (CRT) over time in treated and untreated contralateral eyes.
Abbreviations: CI, confidence interval; ANOVA, analysis of variance.
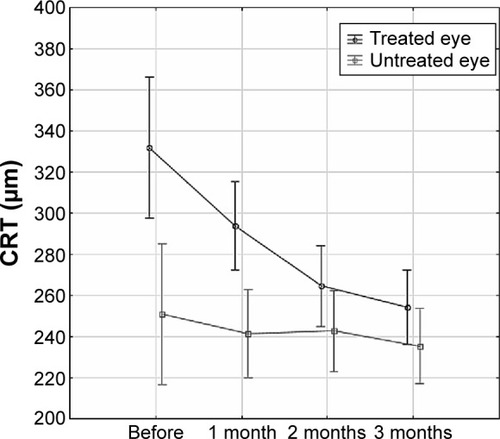
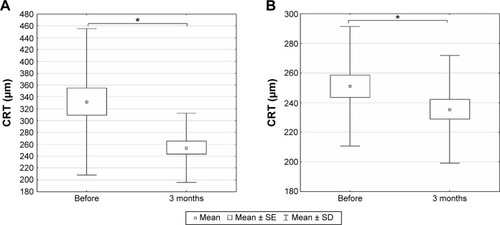
While mean CRT was significantly reduced in the treated eyes 3 months after intravitreal injection from 331.97±123.62 to 254.31±58.75 μm (paired t-test, P<0.0001) (), mean CRT in the untreated fellow eyes reduced significantly from 251.07±40.29 to 235.45±36.21 μm 3 months later (paired t-test, P=0.0405) ().
Figure 2 The central retinal thickness (CRT) measured by optical coherence tomography before the first dose of treatment and 3 months after the initiation of treatment in treated and untreated contralateral eyes.
Abbreviations: SE, standard error; SD, standard deviation.
The differences in gene expression profile before and after ranibizumab treatment
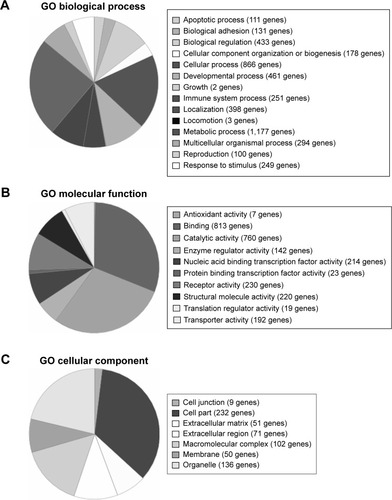
During the next step of the research, the gene expression profile was assigned using oligonucleotide microarrays of Affymetrix HG-U133A, enabling analysis of 22,283 mRNA transcripts. The expression of genes was compared in PBMCs of AMD patients before and after ranibizumab treatment. Among all transcripts, 3,097 for 2,806 genes expressed more than 2-fold statistically significant change after ranibizumab treatment in relation to controls (). Among these transcripts, 1,339 for 1,137 genes were up-regulated, whereas 1,758 for 1,669 genes were down-regulated. Gene ontology analysis identified 14 subgroups for differentially expressed genes at P<0.05 based on biological processes (), ten subgroups based on molecular functions (), and seven subgroups based on cellular components (). In the last part of the study, PANTHER analysis revealed several functional categories that were significantly enriched in statistically differentially expressed gene set compared to the entire National Center for Biotechnology Information reference list of human genome based on biological processes, molecular functions, and cellular components of these genes (). Moreover, PANTHER pathway analysis revealed that differentially up-regulated genes were enriched for several molecular pathways, including CCKR signaling map (21 genes), T-cell activation (13 genes), ubiquitin proteasome pathways (eleven genes), p53 pathways (12 genes), glycolysis (six genes), integrin signaling pathway (19 genes), apoptosis signaling pathway (14 genes), inflammation mediated by chemokine and cytokine signaling pathway (23 genes), gonadotropin releasing hormone receptor pathway (21 genes), cell cycle (five genes), cytoskeletal regulation by Rho GTPase (eleven genes), pentose phosphate pathway (three genes), oxidative stress response (five genes), p38 MAPK pathway (six genes), angiotensin II-stimulated signaling through G proteins and beta-arrestin (six genes), fructose galactose metabolism (three genes), Ras pathways (nine genes), cadherin signaling pathway (two genes), cholesterol biosynthesis (three genes), axon guidance mediated by netrin (five genes), isoleucine biosynthesis (two genes), and B-cell activation (seven genes).
Table 1 Changes in gene expression in PBMCs of AMD patients before and after ranibizumab treatment
Table 2 PANTHER classification of biological processes, molecular functions, and cellular components significantly enriched in the set of differentially expressed genes
Figure 3 PANTHER classification of differentially expressed genes.
Abbreviations: PANTHER, Protein Analysis Through Evolutionary Relationships; GO, gene ontology.
In turn, molecular pathways that were significantly enriched in the list of down-regulated genes included cadherin signaling pathways (26 genes), TGFβ signaling pathway (17 genes), 5HT2 type receptor mediated signaling pathway (eleven genes), gonadotropin releasing hormone receptor pathway (29 genes), 5HT3 type receptor mediated signaling pathway (six genes), 5HT1 type receptor mediated signaling pathway (eight genes), heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway (19 genes), CCKR signaling map (22 genes), endothelin signaling pathway (13 genes), 5HT4 type receptor mediated signaling pathway (five genes), 5-hydroxytryptamine degradation (three genes), integrin signaling pathway (21 genes), angiogenesis (18 genes), Wnt signaling pathway (31 genes), de novo purine biosynthesis (seven genes), heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway (14 genes), EGF receptor signaling pathway (15 genes), and oxidative stress response (five genes).
Discussion
The determination of systemic effects of intravitreal ranibizumab injections seems to be important.Citation17 Therefore, this report focused on effects of intravitreal ranibizumab injections in patients with neovascular AMD on CRT both in treated and contralateral untreated eyes. Moreover, differences in the gene expression pattern in the PBMCs of patients with AMD after intravitreal injections of ranibizumab were revealed.
In our study, the mean CRT decreased in treated eye and untreated contralateral eye in patients with AMD 3 months after intravitreal injections of ranibizumab, with a sharper decrease in the treatment group. Many previous studies indicated that intravitreal injections of ranibizumab caused a significant reduction of CRT in treated eyes of patients with AMD.Citation18–Citation23 However, the possibility of ranibizumab influence on the untreated eye still needs an explanation. Similar to our results, Wu and Sadda and Rouvas et al observed that intravitreal ranibizumab injections had therapeutic effects on the contralateral, un-injected eyes in patients with neovascular AMD.Citation8,Citation24 Other authors also reported an effect of ranibizumab on the fellow eye of patients with various ocular diseases.Citation25–Citation27 Acharya et al revealed a therapeutic effect on untreated contralateral eyes of patients after the third intravitreal dose of 0.5 mg ranibizumab but with bilateral uveitis-related cystoid macular edema.Citation7 Similar to our results, these authors observed a reduction in CRT in treated and contralateral untreated eyes. However, the limitation of Acharya et al’s study is the small sample size. Likewise, Al-Dhibi and Khan revealed a bilateral reduction of uveitic cystoid macular edema following unilateral intravitreal bevacizumab injection in an 8-year-old child.Citation28 In turn, Sharma et al observed that an intravitreal dexamethasone implantation also seems to have a bilateral effect after unilateral injection in a 54-year-old patient with macular edema.Citation29 In another study, Hosseini et al observed that intravitreal injection of bevacizumab significantly changed ocular hemodynamic parameters of both the injected and the untreated fellow eyes of AMD patients.Citation30 It seems that this effect may be due to systemic spread of bevacizumab after intravitreal injections.
The decrease in CRT in the contralateral eyes may suggest that intravitreal injections of anti-VEGF agents can have systemic effects. It is known that after intravitreal injections bevacizumab can be detected in higher serum concentrations than ranibizumab and also has a systemically longer half-life.Citation31,Citation32 Xu et al revealed that ranibizumab serum half-life is only approximately 2 hours. One reason for this may be that ranibizumab, an antibody fragment, lacks an Fc domain.Citation33 However, pharmacokinetics and bioavailability of ranibizumab are not yet fully known. Previous animal studies revealed small amounts of bevacizumab and ranibizumab in the serum and in the untreated contralateral eye after intravitreal administration.Citation34,Citation35 Moreover, Christoforidis et al demonstrated in an animal model that anti-VEGF agent intravitreal injections can have an influence on the cutaneous wound healing.Citation36,Citation37 However, only few published reports suggested that low serum levels of anti-VEGF agents may induce a therapeutic effect in the fellow eye of patients.Citation7,Citation8,Citation24,Citation28 Additionally, the disruption of blood–retinal barrier in retinal diseases can also increase systemic absorption of anti-VEGF agents after intravitreal injections.Citation7,Citation24
Another major issue related to anti-VEGF agents is understanding the molecular mechanism of action of these drugs. Golan et al, in their in vitro study, suggested that anti-VEGF agents may have an influence on gene expression involved in VEGF signaling pathways in different ways.Citation38 Therefore, the second part of this study focused on assessing the gene expression profile in PMBCs in AMD patients before and after intravitreal administration of ranibizumab. When we were launching our study there were only few published data regarding gene expression/protein level differences in blood samples of patients with AMD.Citation13–Citation15 Mo et al determined serum cytokine levels in patients with AMD and suggested that IFN-γ-inducible protein-10 and eotaxin may be early biomarkers in this disease.Citation39 In turn, Falk et al did not observe significant differences in the blood expression levels of chemokine receptor CCR3 and chemokine CCL11 in patients with neovascular AMD.Citation15 Moreover, these authors suggested that intravitreal injections of ranibizumab do not cause systemic alterations of expression of analyzed genes. In contrast, our results revealed changed expression of 3,097 transcripts after ranibizumab treatment. Among all these transcripts, 1,339 were up-regulated, whereas 1,758 were down-regulated. Similarly, Dabir et al, using microarray technique, analyzed systemic gene expression profile but in patients with diabetic macular edema.Citation40 These authors revealed that five genes were up-regulated and 105 genes were down-regulated among all studied groups, including diabetic, treatment responder, and treatment non-responder groups. Moreover, there was only one gene up-regulated between the diabetic and treatment responder groups. These authors also suggested that systemic levels of selected genes may be used to classify the treatment responders and non-responders. In addition, classification of differentially expressed genes based on functional category and pathways revealed that these genes play an important role in cancer, metabolism, ECM-receptor interaction, tricarboxylic acid cycle, retinol metabolism, TGF-β metabolism, VEGF pathway, cell adhesion molecules, p53 signaling, Jak-Stat signaling, and MAPKs pathways.Citation40 Similarly, our results revealed that differentially expressed genes before and after ranibizumab treatment in PBMCs of AMD patients play an important role in many of the aforementioned pathways and biological processes, including cellular, metabolic, cell communication, cell–cell signaling, cell adhesion, immune system, and angiogenesis.
In another study, Nassar et al determined levels of many serum cytokines in patients with AMD before and after ranibizumab treatment.Citation13 These authors showed that IL-17 and TNF-α levels statistically significantly differ among patients with improvement, no change, and deterioration of CRT after anti-VEGF treatment, respectively. In turn, previous studies revealed that there were no statistically significant differences between serum/plasma VEGF concentrations before and after intravitreal ranibizumab injection measured by enzyme-linked immunosorbent assay.Citation41–Citation43 However, the slightly increased serum VEGF concentration in 1 week after ranibizumab treatment may suggest systemic effects of intraocular anti-VEGF therapy.Citation41 On the contrary, other authors observed a reduced VEGF level in serum of AMD patients after bevacizumab intravitreal injections.Citation31,Citation44 Similarly, our results revealed inhibition of VEGF expression among differentially expressed genes in PBMCs of intravitreal ranibizumab treatment patients. In conclusion, our results suggest the potential systemic effects of anti-VEGF therapy for AMD. Our study revealed the reduction of CRT after intravitreal ranibizumab injections in patients with neovascular AMD both in treated and contralateral untreated eye.Citation45 Moreover, our study indicated a differential gene expression in PMBCs obtained from AMD patients before and after the intravitreal ranibizumab treatment. These results may contribute to a better understanding of the molecular mechanisms involved in the drug response of patients with AMD. Moreover, the differentiating genes determined by oligonucleotide microarray technique may also be the target of further research. Unfortunately, the major limitation of our study is a relatively small number of samples. Therefore, there is a need to study a larger population and carry out further analysis in order to better clarify the systemic effects of anti-VEGF-agents. Moreover, detailed examination of AMD therapy will also help to identify better treatment strategies for this disease.
Acknowledgments
This research was supported in part by PL-Grid Infrastructure (http://www.plgrid.pl/en).
Disclosure
The authors declare that there are no conflicts of interest.
References
- CheungCMWongTYIs age-related macular degeneration a manifestation of systemic disease? New prospects for early intervention and treatmentJ Intern Med2014276214015324581182
- EmersonMVLauerAKCurrent and emerging therapies for the treatment of age-related macular degenerationClin Ophthalmol20082237738819668729
- RégnierSMalcolmWAllenFWrightJBezlyakVEfficacy of anti-VEGF and laser photocoagulation in the treatment of visual impairment due to diabetic macular edema: a systematic review and network meta-analysisPLoS One201497e10230925029255
- BrownDMWykoffCCWongTPRanibizumab in preproliferative (ischemic) central retinal vein occlusion: the rubeosis anti-VEGF (RAVE) trialRetina20143491728173524914476
- CouturierABousquetEZhaoMAnti-vascular endothelial growth factor acts on retinal microglia/macrophage activation in a rat model of ocular inflammationMol Vis20142090892024966662
- CiullaTARosenfeldPJAnti-vascular endothelial growth factor therapy for neovascular ocular diseases other than age-related macular degenerationCurr Opin Ophthalmol200920316617419381089
- AcharyaNRSittivarakulWQianYHongKCLeeSMBilateral effect of unilateral ranibizumab in patients with uveitis-related macular edemaRetina20113191871187621716165
- WuZSaddaSREffects on the contralateral eye after intravitreal bevacizumab and ranibizumab injections: a case reportAnn Acad Med Singapore200837759159318695773
- LimLSCheungCMMitchellPWongTYEmerging evidence concerning systemic safety of anti-VEGF agents – should ophthalmologists be concerned?Am J Ophthalmol2011152332933121855670
- HwangDJKimYWWooSJParkKHComparison of systemic adverse events associated with intravitreal anti-VEGF injection: ranibizumab versus bevacizumabJ Korean Med Sci201227121580158523255862
- WongTYAge-related macular degeneration and cardiovascular disease in the era of anti-vascular endothelial growth factor therapiesAm J Ophthalmol2009148332732919703607
- CampbellRJBellCMPatersonJMStroke rates after introduction of vascular endothelial growth factor inhibitors for macular degeneration: a time series analysisOphthalmology201211981604160822717458
- NassarKGrisantiSElfarELükeJLükeMGrisantiSSerum cytokines as biomarkers for age-related macular degenerationGraefes Arch Clin Exp Ophthalmol2015253569970425056526
- NitaMMichalska-MałeckaKMazurekUInfluence of ranibizumab treatment on the extracellular matrix in patients with neovascular age-related macular degenerationMed Sci Monit20142087588324866589
- FalkMKSinghAFaberCNissenMHHviidTSørensenTLBlood expression levels of chemokine receptor CCR3 and chemokine CCL11 in age-related macular degeneration: a case-control studyBMC Ophthalmol2014142224575855
- OrchelJWitekLKimsaMExpression patterns of kinin-dependent genes in endometrial cancerInt J Gynecol Cancer201222693794422706224
- AveryRLWhat is the evidence for systemic effects of intravitreal anti-VEGF agents, and should we be concerned?Br J Ophthalmol201498Suppl 1i7i1024326326
- ChavanRPanneerselvamSAdhanaPNarendranNYangYBilateral visual outcomes and service utilization of patients treated for 3 years with ranibizumab for neovascular age-related macular degenerationClin Ophthalmol2014871772324748766
- CananHSızmazSAltan-YaycıoğluRSarıtürkCYılmazGVisual outcome of intravitreal ranibizumab for exudative age-related macular degeneration: timing and prognosisClin Interv Aging2014914114524453484
- MoisseievEKatzGMoisseievJSwitching treatment for neovascular age-related macular degeneration from bevacizumab to ranibizumab: who is likely to benefit from the switch?Retina20153571323133026102434
- CATT Research Group; MartinDFMaguireMGRanibizumab and bevacizumab for neovascular age-related macular degenerationN Engl J Med2011364201897190821526923
- SubramanianMLAbediGNessSBevacizumab vs ranibizumab for age-related macular degeneration: 1-year outcomes of a prospective, double-masked randomised clinical trialEye (Lond)201024111708171520885427
- KrebsISchmettererLBoltzAA randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degenerationBr J Ophthalmol201397326627123292928
- RouvasALiarakosVSTheodossiadisPThe effect of intravitreal ranibizumab on the fellow untreated eye with subfoveal scarring due to exudative age-related macular degenerationOphthalmologica2009223638338919602910
- LükeJNassarKGrisantiSLükeMRegression of rubeosis in the fellow eye after intravitreal ranibizumab injectionGraefes Arch Clin Exp Ophthalmol2013251137137323132337
- RotsosTSymeonidisCTriantafillopoulouIKanellopoulosSKourisASignificant reduction of diabetic macular edema following intravitreal ranibizumab injection in the fellow eyeInt Ophthalmol20143461271127425192913
- PescosolidoNFazioSRuscianoDTherapeutic improvement in the contralateral eye after ranibizumab intravitreal treatment in a patient affected by bilateral subfoveal choroidal neovascularizationJSM Biotechnol Bioeng2014221038
- Al-DhibiHKhanAOBilateral response following unilateral intravitreal bevacizumab injection in a child with uveitic cystoid macular edemaJ AAPOS200913440040219482498
- SharmaAShethJMadhusudanRJSundaramoorthySKEffect of intravitreal dexamethasone implant on the contralateral eye: a case reportRetin Cases Brief Rep20137321721925391109
- HosseiniHLotfiMEsfahaniMHEffect of intravitreal bevacizumab on retrobulbar blood flow in injected and uninjected fellow eyes of patients with neovascular age-related macular degenerationRetina201232596797122146127
- AveryRLCastellarinAASteinleNCSystemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMDBr J Ophthalmol201498121636164125001321
- MoisseievEWaisbourdMBen-ArtsiEPharmacokinetics of bevacizumab after topical and intravitreal administration in human eyesGraefes Arch Clin Exp Ophthalmol2014252233133724170282
- XuLLuTTuomiLPharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approachInvest Ophthalmol Vis Sci20135431616162423361508
- BakriSJSnyderMRReidJMPulidoJSEzzatMKSinghRJPharmacokinetics of intravitreal bevacizumab (Avastin)Ophthalmology2007114585585917467524
- GaudreaultJFeiDRusitJSubocPShiuVPreclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administrationInvest Ophthalmol Vis Sci200546272673315671306
- ChristoforidisJRickettsRPrattCThe effect of intravitreal anti-VEGF agents on peripheral wound healing in a rabbit modelClin Ophthalmol20126616922275809
- ChristoforidisJBWangJJiangAThe effect of intravitreal bevacizumab and ranibizumab on cutaneous tensile strength during wound healingClin Ophthalmol2013718519123378736
- GolanSEntin-MeerMSemoYGene profiling of human VEGF signaling pathways in human endothelial and retinal pigment epithelial cells after anti VEGF treatmentBMC Res Notes2014761725201034
- MoFMProiaADJohnsonWHCyrDLashkariKInterferon gamma-inducible protein-10 (IP-10) and eotaxin as biomarkers in age-related macular degenerationInvest Ophthalmol Vis Sci20105184226423620220052
- DabirSSDasDNallathambiJMangaleshSYadavNKSchoutenJSDifferential systemic gene expression profile in patients with diabetic macular edema: responders versus nonresponders to standard treatmentIndian J Ophthalmol2014621667324492504
- WangXSawadaTSawadaOSaishinYLiuPOhjiMSerum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degenerationAm J Ophthalmol2014158473874424973606
- CarneiroAMCostaRFalcãoMSVascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumabActa Ophthalmol2012901e25e3021958440
- ZehetnerCKralingerMTModiYSSystemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trialActa Ophthalmol2015932e154e15925488124
- WangDChoiKSLeeSJSerum concentration of vascular endothelial growth factor after bilateral intravitreal injection of bevacizumabKorean J Ophthalmol2014281323824505199
- SizmazSKucukerdonmezCKalAPinarciEYCananHYilmazGRetinal and choroidal thickness changes after single anti-VEGF injection in neovascular age-related macular degeneration: ranibizumab vs bevacizumabEur J Ophthalmol201424690491024803153
