Abstract
The purpose of this study was to evaluate the impact of intravitreal dexamethasone implant (Ozurdex) on macular morphology and functions in eyes with macular edema (ME) secondary to retinal vein occlusion. Efficacy outcomes of the treatment were best-corrected visual acuity (BCVA) and central retinal thickness (CRT). Safety outcomes were intraocular pressure and cornea endothelial cell density. The study was conducted by the prospective analysis on 36 patients (17 women and 19 men) aged 28–77 years (the average age was 58±15 years) treated with the injection of dexamethasone implant because of the persistent ME at the Department of Ophthalmology and Ophthalmology Outpatient Clinic of the University Centre of Ophthalmology and Oncology in Katowice. The studied group included 16 patients with central retinal vein occlusion (16 eyes), and 20 patients with branch retinal vein occlusion (20 eyes). We found a significant increase of BCVA after first, second, and third month of treatment. Six months after the treatment, BCVA decreased, although not significantly compared with the value obtained in the third month. Two months after the intravitreal implantation of dexamethasone delivery system, CRT was 338±163 μm and was significantly lower compared with pretreatment value. Between third and sixth month after the treatment, we found insignificant increase of CRT compared with thickness observed in second month. Two months after the treatment, we found an increase in intraocular pressure in 36% of cases and a further decrease during the final visit 6 months after the treatment. During the treatment, there were no significant differences in endothelial cell density in branch retinal vein occlusion and central retinal vein occlusion. We found the intravitreal dexamethasone implant to be safe, well tolerated, and likely to lead to fast morphological and functional improvement of the macula and visual rehabilitation in patients with ME due to retinal vein occlusion.
Introduction
Retinal vein occlusion (RVO) is a sudden obstruction of the retinal venous system and it is an important cause of visual loss.Citation1–Citation3 There are two main types of RVO: central retinal vein occlusion (CRVO) and the branch retinal vein occlusion (BRVO), the latter being more common. Their prevalence equals to 0.6%–1.1% for BRVO and 0.8 per 1,000 patients for CRVO.Citation4–Citation6 The increase in age strongly influences the prevalence of RVO, even 5% of people over 80 may be affected by this disease. In eyes with untreated BRVO, visual acuity may improve over time up to 20/40.Citation7 In untreated CRVO eyes, visual acuity decreases over time.Citation8
The pathogenesis of RVO is influenced by many factors, such as vein compression at an arteriovenous crossing, degenerative changes of vessel walls, and abnormal hematological and hemorheological factors may be distinguished.Citation5,Citation9–Citation12 Some studies have reported connection between BRVO and higher blood viscosity due to high hematocrit and dysregulation of the thrombosis–fibrinolysis balance.Citation13–Citation16
BRVO as well as CRVO are frequently associated with macular edema (ME), which causes visual loss.Citation2,Citation7,Citation8
The formation mechanism of ME in RVO is multifactorial and complex and embraces raised hydrostatic venous pressure, endothelial dysfunction, hypoxia degree in macula center, and inflammation and increased permeability factors in vessels like inflammatory cytokines. All these factors are responsible for the break of the blood–retina barrier due to endothelial cell dysregulation resulting in ME.Citation3,Citation4,Citation10,Citation17,Citation18
In previous studies, the authors found that proangiogenic cytokines (vascular endothelial growth factor [VEGF] and interleukin [IL]-8) and proinflammatory cytokines (IL-6, IL-12, IL-15, IL-17, and IL-23) are elevated in the ocular fluid of the patients with BRVO or CRVO.Citation19–Citation23 In another study, Noma et alCitation24 suggested that VEGF, soluble intercellular adhesion molecule-1, and IL-6 increased vascular permeability and broke the blood–retinal barrier in CRVO patients with ME.
Recently, the standard care for ME secondary to BRVO has been grid laser photocoagulation. Branch Vein Occlusion Study allowed for determination of grid laser as a standard procedure for patients with ME.Citation25,Citation26 Subsequent Central Vein Occlusion Study not only confirmed favorable effects of grid laser on ME but also revealed that there is no statistically important difference in visual acuity.Citation25,Citation27
In recent years, two novel therapies have been applied: anti-inflammatory and antiangiogenic intravitreal strategies.Citation28–Citation32
Three anti-VEGF agents have been recognized as an effective treatment for ME in both types of RVO: intravitreal ranibizumab (Lucentis; Genentech Inc., South San Francisco, CA, USA), aflibercept (EYLEA; Bayer HealthCare, Berlin, Germany), and bevacizumab (Avastin; Genentech Inc.). Bevacizumab has been used as an off-label therapy.Citation28,Citation33–Citation35
In previous studies, investigators found beneficial effects of steroids like triamcinolone acetonide, dexamethasone, and fluocinolone in edema reduction in RVO.Citation31,Citation36,Citation37
In 2009, a sustained-release intravitreal 0.7 mg dexamethasone delivery system, Ozurdex (Allergan Inc., Irvine, CA, USA), was approved for treatment of ME secondary to RVO. Ozurdex has demonstrated efficacy and safety for the treatment of BRVO and CRVO.Citation30,Citation32,Citation38
Furthermore, the authors demonstrated efficacy of the dexamethasone implant in other retinal disorders associated with ME as follows: diabetic retinopathy, Irvine-Gass syndrome, noninfectious vitritis, and age-related macular degeneration.Citation39–Citation42
The implant is fully biodegradable, does not require surgical removal, and if necessary, it allows you to place another implant. Dexamethasone implant is applied 3–4 mm posterior to the limbus. Chang-Lin et alCitation43 distinguished two phases of drug release. The first stage starts immediately after the implantation. It is characterized by 2-month high concentration of the drug release. After 60 days, released drug concentration decreases and the implant becomes smaller and fragmented simultaneously prolonging the therapeutic period. Within 6-month period, drug concentration declines to the nonquantifiable level.
The aim of this study was to evaluate the influence of intravitreal dexamethasone implant (Ozurdex) on macular morphology and function, and its efficacy and safety in eyes with ME secondary to RVO. Efficacy outcomes of the treatment were best-corrected visual acuity (BCVA) and central retinal thickness (CRT) and the safety outcomes were intraocular pressure (IOP) and cornea endothelial cell density (ECD).
Materials and methods
The prospective analysis was conducted on 36 patients (17 women and 19 men) aged 28–77 years (the average age was 58±15 years) treated with the intravitreal injection of dexamethasone implant at the Department of Ophthalmology and Ophthalmology Outpatient Clinic of the University Centre of Ophthalmology and Oncology in Katowice because of ME secondary to RVO. The study was approved by Bioethical Committee of Medical University of Silesia (approval number: KNW/0022/KB1/99/II/13/14).
The group concerned included 16 patients with CRVO (16 eyes) and 20 patients with BRVO (20 eyes). The inclusion criteria for the study were: 1) decrease in BCVA using the Snellen visual acuity chart ≤5/10; 2) CRT >300 μm, as measured by optical coherence tomography (OCT); 3) IOP below 21 mmHg (controlled by no more than one drop); 4) duration of ME shorter than 18 months; 5) age between 18 and 80 years; and 6) consent of the patient. The exclusion criteria were: 1) previous panretinal photocoagulation or pars plana vitrectomy treatment, 2) vitreous hemorrhaging, 3) aphakic eyes, 4) unregulated glaucoma or ocular hypertension, 5) active or suspected eye or eye area infections, and 6) lack of patient’s consent.
Mean time of ME duration in the BRVO group was 6.50±6.50 months () and in the CRVO group was 5.06±4.51 months ().
Figure 1 Histogram showing the distribution of the frequency of particular times of the disease in the branch retinal vein occlusion group.
Note: Obs represents the observed number of patients with branch retinal vein occlusion.
Figure 2 Histogram showing the distribution of the frequency of particular times of the disease in the central retinal vein occlusion group.
Before the intravitreal therapy, all patients underwent ophthalmic examination, which embraced: 1) BCVA using the Snellen visual acuity chart under identical testing conditions; 2) slit lamp examination including anterior and posterior segment of the eye; 3) morphology of the macular area accurately examined by OCT (Cirrus HD-OCT 500, Carl Zeiss Meditec AG, Jena, Germany), foveal thickness was calculated as the average retinal thickness within a circle having a 500-μm radius, which was centered on the fovea; 4) IOP recording with a Goldmann applanation tonometer; and 5) ECD measured by confocal microscopy (Tomey, Erlangen-Tennenlohe, Germany).
For intravitreal therapy, 0.7 mg dexamethasone delivery system, Ozurdex was administered 3–4 mm posterior to the limbus through the pars plana. The implantation was performed with a sterile technique, and prophylactic topical antibiotics were applied for 1 week afterward.
According to the research report, all patients had a 6-month follow-up. Control tests were conducted 1 week, 1, 2, 3, and 6 months after the treatment.
In the research, we analyzed statistical significance of differences between predrug value and subsequent values during the treatment. Because the assumption of normal population distribution (Shapiro–Wilk test) was not met, we used nonparametric Wilcoxon rank-sum test, which is an alternative to the paired Student’s t-test. For all tests, the significance level was α=0.05. All subjects gave a formal written consent before participating in the study. The research followed the tenets of the Declaration of Helsinki.
Results
Best-corrected visual acuity
The average pretreatment BCVA in the studied group (n=36) was 0.166±0.129, with a range of 0.002–0.500.
For the whole group, the parametric analysis of variance tests were calculated. The statistical probability was P=0.00504. In order to verify which of the averages caused the existing differences, the post hoc analysis was performed. We found a significant increase of BCVA after first (0.166±0.129 vs 0.392±0.243, P=0.000857), second (0.166±0.129 vs 0.407±0.247, P=0.000227), and third (0.166±0.129 vs 0.320±0.249, P=0.01489) month of treatment. Six months after the treatment, BCVA decreased, although not significantly compared with the value obtained in the third month (0.298±0.234 vs 0.320±0.249, P=0.992626) ().
Figure 3 Best-corrected visual acuity in studied group (n=36) (mean ± SD).
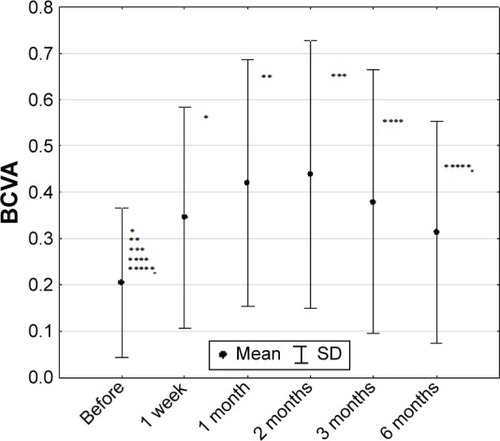
In BRVO (0.107±0.090 vs 0.184±0.137, P=0.02015) and CRVO (0.104±0.106 vs 0.200±0.241, P=0.04864) patients, a statistically significant (Friedman test) increase in BCVA values was observed after the first week of treatment. In the first and second month after the intravitreal implantation of dexamethasone delivery system, BCVA reached plateau and stayed at the similar increased level: in BRVO (0.107±0.090 vs 0.400±0.173, P=0.02015) and in CRVO (0.104±0.106 vs 0.235±0.281, P=0.4864) in first month, and in second month: in BRVO (0.107±0.090 vs 0.410±0.094, P=0.02015) and in CRVO (0.104±0.106 vs 0.232±0.284, P=0.4864). In the BRVO group, plateau was maintained until the third month (0.107±0.090 vs 0.438±0.108, P=0.02015) and after 6 months it decreased (0.107±0.090 vs 0.337±0.152, P=0.02015). In the CRVO group, a decreasing tendency (0.104±0.106 vs 0.199±0.267, P=0.4864 and 0.104±0.106 vs 0.130±0.186, P=0.4864) was revealed in third and sixth months, respectively after injection.
Central retinal thickness
The average preoperative CRT in the studied group was 776±301 μm, with a range of 427–1,362 μm. After the treatment, the OCT examination proved ME reduction. Two months after the intravitreal implantation of dexamethasone delivery system, CRT was 338±163 μm and was significantly lower compared with pretreatment value (P=0.000786). Between third and sixth month after the treatment, we found insignificant increase of CRT compared with thickness observed in the second month (338±163 vs 444±206 μm, P=0.1168 in the third month and 542±182 μm, P=0.210 in the sixth month) ().
Figure 4 Central retinal thickness in studied group of patients (n=36) (mean ± SD).
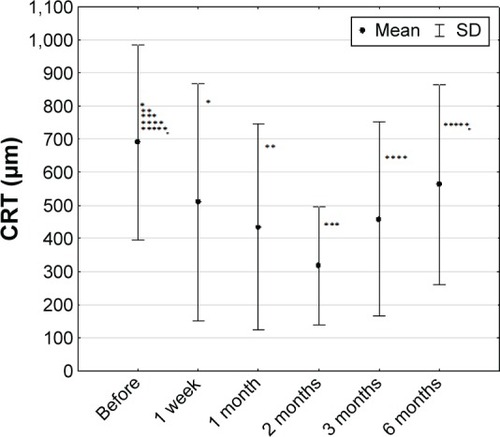
Compared with measurements before injection (872±420 μm in the BRVO group and 979±255 μm in the CRVO group), foveal thickness was decreased to 347±124 μm in the BRVO group (P=0.1296) and 405±252 μm in the CRVO group (P=0.01945).
Nonparametric Friedman test results for whole group were χ2=28.33 and P=0.00003.
During the treatment, there were no significant differences (Friedman test) in the BRVO group (P=0.12964). In the CRVO group, a statistically significant decrease in CRT was observed 2 months after the treatment (978±255 vs 405±252 μm, P=0.01945). An average of 51% decrease in ME was observed 2 months after the treatment and up to 35% decrease in CRT 6 months after the treatment compared with baseline values ().
Intraocular pressure
The average pretreatment IOP in the whole studied group was 16±3 mmHg, with a range of 11–20 mmHg. Two months after the treatment, we found an increase in IOP in 36% of cases (to the 24±7 mmHg and further decrease to 19±5 mmHg during the final visit 6 months after the treatment) (). Nonparametric Friedman test results for whole group were χ2=16.73 and P=0.00504.
Figure 5 Intraocular pressure in studied group of patients (n=36) (mean ± SD).
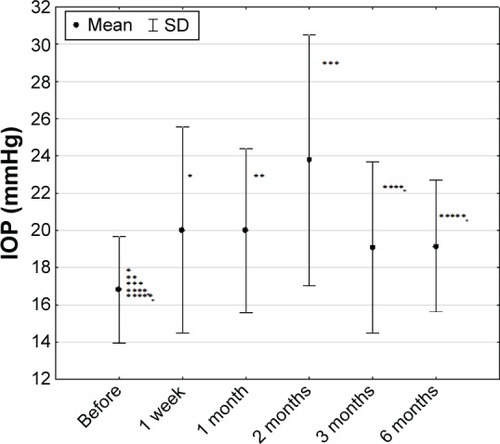
During the final visit, 6 months after injection of the intravitreal dexamethasone delivery system, there were no significant differences (Friedman test) of IOP in BRVO (17±3 vs 17±1 mmHg, P=0.13795) patients compared with pretreatment measurements. In contrast, in the CRVO group we found a significant increase in IOP values compared with pretreatment value (14±3 vs 20±7 mmHg, P=0.01036).
Nonparametric Friedman test results for the whole group were χ2=16.73 and P=0.00504.
The most frequent complications following intravitreal implantation of Ozurdex in our study was increase of IOP observed in 36%, which is comparable with the results presented by other authors. None of the patients in our study needed antiglaucoma surgery. In three cases, IOP was regulated by an application of one and in the next three cases two antiglaucomatous drops. During the final visit 6 months after the implementation, the IOP treatment was below 21 mmHg in all patients concerned but three of them still received local antihypertonic drop treatment.
Endothelial cell density
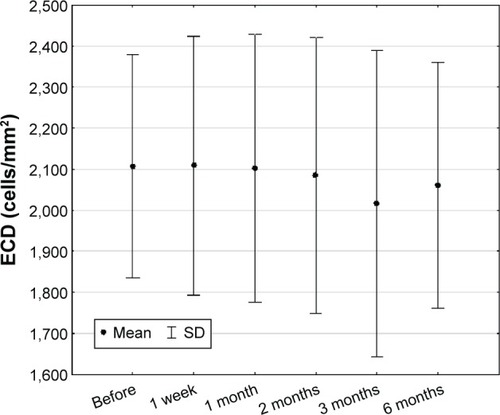
The average ECD in the whole group was 2,154±307 cells/mm2 with a range of 1,704–2,718 cells/mm2. The parametric analysis of variance tests were performed. Statistical probability, P=0.688715, showed that differences between received mean values for the whole group during the time of treatment were not statistically significant (). During the treatment, there were no significant differences (Friedman tests) in ECD in BRVO (2,125±108, P=0.70295) and CRVO (2,008±298, P=0.93047) groups either.
Figure 6 Endothelial cell density in studied group of patients (n=36) (mean ± SD).
In our study, we found 12% of subcapsular cataract progression observed during the final visit, 6 months after the injection of Ozurdex.
Discussion
One of two most common retinal vascular disorders is RVO. Its prevalence varies from 0.7% to 1.6%.Citation4,Citation5
Recently, it has been shown that dexamethasone in a biodegradable drug delivery system (Ozurdex) has a positive influence on patients with RVO: it improves visual acuity as well as reduces ME.Citation40 Results of our study are comparable to previous studies. We have found significant functional (BCVA) and morphological (CRT) improvement of the retina after the Ozurdex intravitreal implantation during the whole time of the observations.
Meyer and SchönfeldCitation44 found that the dexamethasone implant had an immediate effect on ME due to RVO that set in within the first 72 hours following the intravitreal implantation of the drug, which is comparable to the results of our study. The fast effect may be explained by a relatively high concentration of dexamethasone that is released from the implant.
Use of corticosteroids including intravitreal implantation of dexamethasone may cause side effects of treatment, like posterior subcapsular cataracts, increased IOP, and the secondary ocular infections due to bacteria, fungi, or viruses.
In previous studies, which covered nearly 6,000 intravitreal injections, the authors did not observe any endophthalmitis.Citation31,Citation40 In accordance with the results of this study, we did not observe endophthalmitis after intravitreal Ozurdex implantation. Due to lower frequency of Ozurdex intravitreal injection when compared with anti-VEGF, the risk of endophthalmitis seems to be lower.
In SCORE (the Standard Case vs. Corticosteroid for Retinal Vein Occlusion) and GENEVA (Ozurdex GENEVA Study Group) studies, the authors found more frequent IOP elevation when compared with anti-VEGF trial. In the SCORE study, the IOP was dependent on triamcinolone dose (4 mg), with the prevalence of 41% in BRVO eyes and 35% in CRVO eyes.Citation31 In the GENEVA study, the authors found 25% of IOP increase 6 months after intravitreal dexamethasone implantation,Citation40 which is also similar to the results of our study. The incidence of IOP after anti-VEGF agents was not significant as revealed by the HORIZON (Open-Label Extension Trial of Ranibizumab for Choroidal Neovascularization Secondary to Age-Related Macular Degeneration) study.Citation45
In SCORE and GENEVA studies, a subcapsular cataract progression was observed in 20%–35% of patients.Citation31,Citation40 Contrary to our results, Meyer and SchönfeldCitation46 did not notice cataract progression or increase in IOP 6 months after the intravitreal implantation of Ozurdex.
Haller et alCitation30,Citation40 also reported safety of retreatment with Ozurdex for RVO. The authors found the similar incidence of side effects, except for a higher frequency of cataract progression (29.8% compared with 10.5%), and IOP elevation (15.4% compared with 12.6%).
The other side effects following intravitreal implantation of Ozurdex in our study were subconjunctival hemorrhage and vitreous opacity in one case. They were much less frequent and resulted from the procedure itself and the route of administration of the anticoagulant drug and were not side effects of corticosteroid.
What is very important, in the results of our study, is the fact that the treatment had no adverse effects on the amount and function of corneal endothelial cells measured by confocal microscopy. The analysis of density of corneal endothelial cells aimed to evaluate the influence of surgical intervention (intravitreal injection) on this parameter. The analysis revealed no significant changes in ECD after the procedure giving a proof that intravitreal dexamethasone injection does not influence the cornea and, in turn, is a safe treatment method.
The ME duration seems to have an impact on the results, since our study revealed visual acuity improvement in 77% of patients with ME presence ≤6 months and in only 50% of patients with ME presence 7–18 months. It is important to remember that in this disease, visual acuity is also influenced by the degree of macula ischemia, which has not been analyzed in this research.
Limitation
The limitation of this study has been its small size group; nonetheless in Polish conditions, it has been the largest conducted research analyzing the influence of dexamethasone therapy on ME due to BRVO and CRVO.
Conclusion
The dexamethasone implant (Ozurdex) may lead to fast functional improvement and visual rehabilitation in patients with ME due to RVO. The treatment is safe and well tolerated with almost no side effects. High percentage of cases with increasing IOP indicates the necessity of regular checkups of patients treated with Ozurdex.
Disclosure
The authors report no conflicts of interest in this work.
References
- LaouriMChenELoomanMGallagherMThe burden of disease of retinal vein occlusion: review of the literatureEye201125898198821546916
- YauJWLeePWongTYBestJJenkinsARetinal vein occlusion: an approach to diagnosis, systemic risk factors and managementIntern Med J20083890491019120547
- RogersSMcIntoshRLCheungNThe prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia and AustraliaOphthalmology201011731331920022117
- MitchellPSmithWChangAPrevalence and associations of retinal vein occlusion in Australia: the Blue Mountains Eye StudyArch Ophthalmol1996114124312478859084
- KleinRKleinBEMossSEMeuerSMThe epidemiology of retinal vein occlusion: the Beaver Dam Eye StudyTrans Am Ophthalmol Soc20009813314311190017
- BraithwaiteTNanjiAALindsleyKGreenbergPBAnti-vascular endothelial growth factor for macular oedema secondary to central retinal vein occlusion [Review]The Cochrane Library2014518
- RogersSLMcIntoshRLLimLNatural history of branch retinal vein occlusion: an evidence-based systematic reviewOphthalmology20101171094110120430447
- McIntoshRLRogersSLLimLNatural history of central retinal vein occlusion: an evidence-based systematic reviewOphthalmology20101171113112320430446
- TurczyńskiBMichalska-MałeckaKSłowińskaLSzczęsnySRomaniukWCorrelations between the severity of retinopathy in diabetic patients and whole blood and plasma viscosityClin Hemorheol Microcirc200329212913714610308
- RehakJRehakMBranch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalitiesCurr Eye Res20083311113118293182
- TurczyńskiBMichalska-MałeckaKSłowińskaLSzczęsnySRomaniukWNon-proliferative diabetic retinopathy and red blood cells aggregationWiad Lek20045711–1263464015865241
- JefferiesPClemettRDayTAn anatomical study of retinal arteriovenous crossings and their role in the pathogenesis of retinal branch vein occlusionsAust NZ J Ophthalmol199321213217
- TropeGELoweGDMcArdleBMAbnormal blood viscosity and haemostasis in long-standing retinal vein occlusionBr J Ophthalmol1983671371426824618
- JanssenMCden HeijerMCruysbergJRWollersheimHBredieSJRetinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factorsThromb Haemost2005931021102615968383
- TurczyńskiBSzygułaJSłowińskaLMichalska-MałeckaKDrażewskiRWodnieckiJWhole blood and serum viscosity in cardiologic X syndromePol Arch Med Wewn20002847548111303313
- ŚpiewakDReguckaASłowińskaLKabieszAWitekKMichalska-MałeckaKChanges in the hemorheological parameters in age-related macular degenerationOkulistyka201443843
- CampochiaroPAHafizGShahMRanibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulatorMol Ther20081679179918362932
- ChenKHWuCCRoySLeeSMLiuJHIncreased interleukin-6 in aqueous humor of neovascular glaucomaInvest Ophthalmol Vis Sci1999402627263210509659
- FunkMKriechbaumKPragerFIntraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumabInvest Ophthalmol Vis Sci2009501025103219060280
- KanedaSMiyazakiDSasakiSMultivariate analyses of inflammatory cytokines in eyes with branch retinal vein occlusion: relationships to bevacizumab treatmentInvest Ophthalmol Vis Sci2011522982298821273540
- NomaHFunatsuHYamasakiMAqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusionEye (Lond)200822424816826241
- NomaHMinamotoAFunatsuHIntravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusionGraefes Arch Clin Exp Ophthalmol200624430931516133018
- FengJZhaoTZhangYMaYJiangYDifferences in aqueous concentrations of cytokines in macular edema secondary to branch and central retinal vein occlusionPLoS One201387e6814923861862
- NomaHMimuraTTatsugawaMShimadaKAqueous flare and inflammatory factors in macular edema with central retinal vein occlusion: a case seriesBMC Ophthalmol2013111378
- WongTYScottIUClinical practice. Retinal-vein occlusionN Engl J Med20103632135214421105795
- Branch Vein Occlusion Study GroupArgon laser photocoagulation for macular edema in branch vein occlusionAm J Ophthalmol1984982712826383055
- Central Vein Occlusion Study Group M reportEvaluation of grid pattern photocoagulation for macular edema in central vein occlusionOphthalmology1995102142514339097788
- BrownDMCampochiaroPABhisitkulRBSustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III studyOphthalmology20111181594160221684606
- CampochiaroPABrownDMAwhCCSustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III studyOphthalmology2011182041204921715011
- HallerJABandelloFBelfortRJrDexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study resultsOphthalmology20111182453246021764136
- IpMSScottIUVanVeldhuisenPCA randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study report 5Arch Ophthalmol20091271101111419752419
- KuppermannBDBlumenkranzMSHallerJARandomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edemaArch Ophthalmol200712530931717353400
- BrownDMCampochiaroPASinghRPCRUISE Investigators: ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III studyOphthalmology20101171124e11133e120381871
- FigueroaMSContrerasINovalSArruabarrenaCResults of bevacizumab as the primary treatment for retinal vein occlusionBr J Ophthalmol2010941052105620679089
- PacellaEPacellaFLa TorreGTesting the effectiveness of intravitreal ranibizumab during 12 months of follow-up in venous occlusion treatmentClin Ter20121636e413e42223306756
- JonasJBKreissigIDegenringRFIntravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusionGraefes Arch Clin Exp Ophthalmol200224078278312271378
- JainNStinnettSSJaffeGJProspective study of a fluocinolone acetonide implant for chronic macular edema from central retinal vein occlusion: thirty-six-month resultsOphthalmology201211913213721924503
- KuppermannBDChouCWeinbergDVWhitcupSMHallerJABlumenkranzMSDDS Dexamethasone Phase II Study GroupIntravitreous dexamethasone effects on different patterns of diabetic macular edemaArch Ophthalmol2010128564264320212194
- BoyerDSFaberDGuptaSOzurdex CHAMPLAIN Study GroupDexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patientsRetina201131591592321487341
- HallerJABandelloFBelfortRJrOzurdex GENEVA Study GroupRandomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusionOphthalmology20101171134114620417567
- PacellaEVestriARMuscellaRPreliminary results of an intravitreal dexamethasone implant (Ozurdex) in patients with persistent diabetic macular edemaClin Ophthalmol201371423142823901252
- PacellaELa TorreGTurchettiPEvaluation of efficacy dexamethasone intravitreal implant compared to treatment with anti-VEGF in the treatment of diabetic macular edemaSenses Sci201414164168
- Chang-LinJEAttarMAcheampongAPharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implantInvest Ophthalmol Vis Sci2011521808620702826
- MeyerLMSchönfeldCLFast resolution of recurrent pronounced macular edema following intravitreal injection of dexamethasone 0.7 mgCase Rep Ophthalmol20112224625021941500
- SingerMAAwhCCSaddaSHORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degenerationOphthalmol2012119611751183
- MeyerLMSchönfeldCLSecondary glaucoma after intravitreal dexamethasone 0.7 mg implant in patients with retinal vein occlusion: a one-year follow-upJ Ocul Pharmacol Ther201329656056523480270
