Abstract
Objective
We studied the effect of sustained virologic response (SVR) after treatment with pegylated-interferon (PEG-IFN) plus ribavirin on the development of liver cirrhosis in elderly patients with chronic hepatitis C (CHC).
Patients and methods
This retrospective study enrolled 145 elderly CHC patients (aged ≥65 years) who were treatment-naïve and were treated with PEG-IFN plus ribavirin for 6 months between January 2005 and December 2011. Abdominal sonography was performed and liver biochemistry was studied at baseline, at the end of treatment, and every 3–6 months thereafter. The development of liver cirrhosis and related complications was evaluated at the follow-ups. The aspartate aminotransferase-to-platelet ratio index was used as a noninvasive maker for fibrosis.
Results
The mean patient age was 69.1±3.3 years, and the average follow-up time was 5.5 years (standard deviation: 2.5 years, range: 1.1–12.3 years). Ninety-five patients (65.5%) achieved SVR, and 26 (17.9%) discontinued treatment. Twenty-seven patients (18.6%) developed liver cirrhosis after treatment. Patients without SVR had significantly greater risk of liver cirrhosis than those with SVR (hazard ratio [HR]: 3.39, 95% confidence interval [CI]: 1.312–8.761, P=0.012). The difference in 3-year cumulative incidence of liver cirrhosis was 24.8% greater for patients without SVR (35.2%, 95% CI: 13.0–57.5, P=0.012) compared with those with SVR (10.4%, 95% CI: 3.1–17.7). There was a trend of a higher baseline aspartate aminotransferase-to-platelet ratio index score in patients who progressed to liver cirrhosis compared with those who did not progress (2.1±1.2 vs 1.6±1.3, P=0.055), but the difference failed to reach significance by Cox regression (adjusted HR: 1.285, 95% CI: 0.921–1.791, P=0.14).
Conclusion
An SVR following PEG-IFN combination treatment can reduce the risk of liver cirrhosis in elderly CHC patients.
Background
Chronic hepatitis C (CHC) is a major cause of chronic liver disease and affects >185 million people worldwide.Citation1 The most serious complications of CHC are high mortality from decompensated liver cirrhosis and hepatocellular carcinoma (HCC).Citation2,Citation3 Age and age at infection are two of the most important risk factors for the progression to liver cirrhosis during chronic hepatitis C virus (HCV) infection.Citation4,Citation5 Patient age seems to have a greater effect than age at infection, and is especially significant for patients older than 65 years.Citation6 Many countries have aging cohorts of people with HCV infections because of previous peaks in the incidence of HCV infections.Citation2,Citation4 A sustained virologic response (SVR), achieved by interferon-based therapies, can slow the progression of liver fibrosisCitation7–Citation10 and reduce the development of HCC and liver-related mortality.Citation11–Citation13 Thus, antiviral treatments may provide the greatest benefit for elderly patients.
However, the high prevalence of cirrhosis and comorbidities in elderly patients leads to higher rates of treatment discontinuation and dose reduction.Citation14,Citation15 In particular, the SVR rate is lower in elderly patients than young patients due to their poor tolerance to treatment.Citation15 Thus, the decision of treatment for an elderly patient must consider the long-term benefits and the patient’s life expectancy. Few studies have examined the occurrence rate of liver cirrhosis after achieving SVR in elderly patients with CHC.
The aspartate aminotransferase-to-platelet ratio index (APRI) is a noninvasive marker that has been validated for the diagnosis of both significant fibrosis and cirrhosis.Citation16,Citation17 APRI is a useful marker for the prognosis in CHC patients. However, the prognostic value of APRI in elderly patients for predicting the occurrence of liver cirrhosis is uncertain.
Objectives
The aim of this study was to evaluate the risk of the progression to liver cirrhosis after combined therapy consisting of pegylated interferon (PEG-IFN) plus ribavirin (RBV) in elderly patients (≥65 years old) with CHC.
Patients and methods
Patient selection
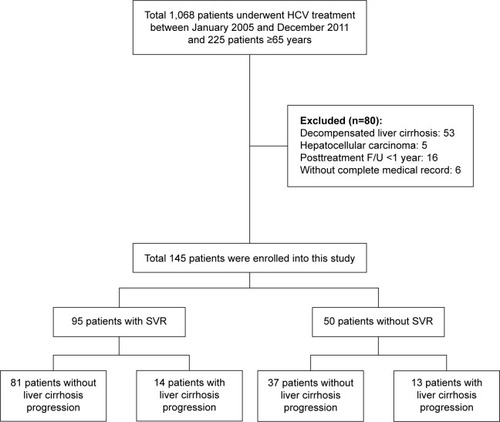
Patients with HCV infection who were aged ≥65 and underwent treatment with either PEG-IFN-α-2a or PEG-IFN-α-2b plus RBV from January 2005 to December 2011 were enrolled from the Dalin Tzu Chi General Hospital in this retrospective study. All the patients were positive for anti-hepatitis C antibody for >6 months, had alanine aminotransferase (ALT) levels higher than the upper limit of normal (ULN), and had detectable serum HCV RNA. Patients with decompensated liver cirrhosis, malignant neoplasms, incomplete medical records, autoimmune diseases, HIV infection, neutropenia (<1,500 neutrophils/mL), thrombocytopenia (<75,000 platelets/mL), anemia (<12 g hemoglobin/dL in females and <13 g/dL in males), or poorly controlled psychiatric diseases were excluded. Two hundred and twenty-five patients were initially enrolled (). Fifty-three patients with compensated liver cirrhosis, five patients with HCC, 16 patients with posttreatment follow-up times <1 year, and six patients without complete medical records were excluded. The remaining 145 patients were finally included. There were 801 person-years of follow-ups, and the average follow-up time was 5.5 years (standard deviation [SD]: 2.5 years, range: 1.1–12.3 years). This study was approved by the Ethics Committee of Dalin Tzu Chi General Hospital (B10303008), all patients provided written consent, and the local ethics committee approved the consent process.
Treatment regimen
Oral RBV plus subcutaneous PEG-IFN-α-2a (180 μg; Pegasys®; Hoffmann-La Roche, Basel, Switzerland) or subcutaneous PEG-IFN-α-2b (1.5 μg/kg; PegIntron®; Schering-Plough Corp., Kenilworth, NJ, USA) was administered to eligible patients for 6 months. The fixed duration (6 months) without consideration of HCV genotypes is due to restrictions imposed by the reimbursement policy of the Bureau of National Health Insurance in Taiwan. The dose of oral RBV was 800 mg/day for patients who weighed <55 kg, 1,000 mg/day for patients who weighed 55–75 kg, and 1,200 mg/day for patients who weighed >75 kg. Dose adjustments of all drugs and administration of supplemental erythropoietin or blood transfusion were determined according to published practice guidelines.Citation18–Citation20
Clinical monitoring
The primary outcome was time to liver cirrhosis. All patients were given liver function tests (serum aspartate aminotransferase [AST], ALT, total bilirubin, creatinine, hemoglobin, white blood cell count, and platelets), and abdominal sonography was performed at the gastrointestinal outpatient clinic at baseline, end of treatment (6 months), 24 weeks after the end of treatment, and every 3–6 months thereafter. Liver cirrhosis and associated liver complications were evaluated every 3–6 months after the end of treatment. Liver cirrhosis was diagnosed by liver biopsy or radiologic cirrhosis. Radiologic cirrhosis was defined as two documented ultrasonograms of liver cirrhosis (included coarse liver echotexture with nodularity and small liver size) combined with features of portal hypertension (splenomegaly, ascites, hepatic encephalopathy, or varices).Citation21 Liver biopsy was an optional procedure and was performed in 108 patients (74.5%) at baseline with consent.
HCV RNA was quantified at baseline and at 24 weeks after the end of treatment. A diagnosis of fatty liver was based on results from a biopsy and/or abdominal ultrasound. Other clinical factors, including diabetes mellitus, chronic hepatitis B, and alcoholism, were recorded by chart review. Chronic hepatitis B was diagnosed if a patient had seropositivity for hepatitis B surface antigen for at least 6 months. The APRI score was calculated as: [(AST/ULN)/platelet count (109/L)] ×100.Citation22 The APRI was used as a noninvasive maker for fibrosis.
HCV quantification and genotyping
Serum HCV RNA was quantified at baseline and at 24 weeks after the end of treatment by use of real-time polymerase chain reaction, which had a detection limitation of 15 IU/mL.Citation23 The threshold for discriminating low and high baseline HCV RNA was 400,000 IU/mL.Citation16 HCV genotyping was performed by melting curve analysis (Roche LightCycler; Biotronics Tech Corp., Lowell, MA, USA).Citation24
Sustained virologic response
SVR was defined as undetectable HCV RNA for at least 24 weeks after completion of the combined treatment. Patients who were positive for HCV RNA at week 24 after the end of treatment were considered non-SVR.
Statistical analysis
SPSS 19.0 for Windows (SPSS Inc, Chicago, IL, USA) was used for all statistical analyses. The chi-square test or Fisher’s exact test was used for nominal variables. Continuous variables were compared using Student’s t-test for two independent groups. Age, sex, diabetes, SVR, other risks of hepatitis (alcoholism, hepatitis B virus [HBV] coinfection, and fatty liver), HCV RNA load, HCV genotype, and baseline APRI score were considered as potential confounders for cirrhosis progression. In order to identify risk factors for cirrhosis, the proportional hazards Cox regression model was used to control for those possible confounding factors, regardless of whether they were significant predictors in the univariate analyses.Citation25 Results were shown as hazard ratios (HRs) with 95% confidence intervals (CIs). A P-value <0.05 was considered significant in all analyses.
Results
Demographic and clinical characteristics
There were 64 males and 81 females, and the mean patient age was 69.1±3.3 years (). Eighty-nine of 145 patients (61.4%) were infected with HCV genotype-1, and 56 patients (38.6%) were infected with other HCV genotypes. Ninety-five patients (65.5%), including 45 with HCV genotype-1 and 50 with other HCV genotypes, achieved SVR. Twenty-six patients (17.9%) did not complete the full 6-month treatment, and the patients had mean treatment duration of 84 days. Two patients stopped treatment due to lack of virological response. Twenty-four patents did not accept complete treatment due to treatment side effect. Among those patients, anemia (11/24) was the most common side effect reported. There were no deaths or severe treatment-related complications during the treatment period.
Table 1 Baseline characteristics and outcomes of patients with chronic hepatitis C who progressed or did not progress to liver cirrhosis after the end of treatment
Baseline characteristics and treatment outcomes
shows the baseline characteristics and treatment outcomes of patients who did and did not progress to liver cirrhosis. There were 801 person-years of follow-up data, and the average follow-up time was 5.5 years (SD: 2.5 years, range: 1.1–12.3 years). During the follow-up, 27 patients (18.6%) developed liver cirrhosis, 12 patients developed HCC, three patients developed varices, two patients developed ascites, and one patient developed spontaneous bacterial peritonitis. One death that was unrelated to liver disease occurred during the follow-up period. The average age of patients with liver cirrhosis was 70.2±3.3 years, and 17 of these patients (62.9%) were females. Among patients who progressed to cirrhosis (n=27), 14 patients (51.9%) were infected with HCV genotype-1, and 14 patients (51.9%) achieved SVR. Univariate analysis of patients who did and did not progress to cirrhosis indicated no significant differences in age, sex, diabetes, smoking, other risks of hepatitis (alcoholism, HBV coinfection, and fatty liver), HCV RNA load, HCV genotype, baseline APRI, and SVR. There was a trend of a higher baseline APRI score in patients who progressed to liver cirrhosis compared with those who did not progress (2.1±1.2 vs 1.6±1.3, P=0.055).
Baseline characteristics and progression to cirrhosis
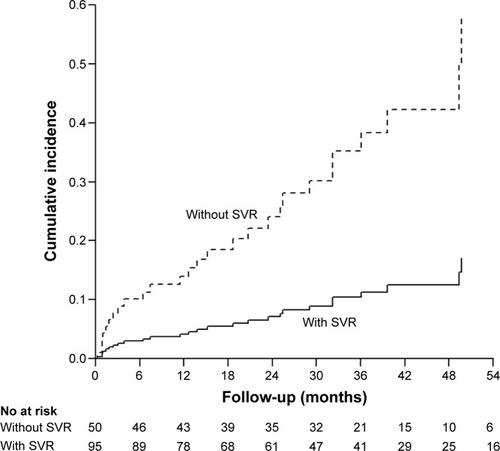
shows the result of Cox regression analysis with adjustment for age, sex, diabetes, other risks of hepatitis (alcoholism, HBV coinfection, and fatty liver), HCV RNA load, HCV genotype, and baseline APRI. The results show a significant association between progression to liver cirrhosis and no SVR (HR: 3.39, 95% CI: 1.312–8.761, P=0.012). There was also a trend of an association between liver cirrhosis and low HCV RNA load (HR: 0.392, 95% CI: 0.152–1.009, P=0.052). There was no significant association between progression to liver cirrhosis and high baseline APRI by Cox regression (adjusted HR: 1.285, 95% CI: 0.921–1.791, P=0.14). shows the cumulative incidences of liver cirrhosis after treatment. The risk of liver cirrhosis was significantly greater in patients without SVR (3-year cumulative incidence: 35.2%, 95% CI: 13.0–57.5, P=0.012) than in those with SVR (3-year cumulative incidence: 10.4%, 95% CI: 3.1–17.7). This corresponds to a difference in the 3-year cumulative incidence of 24.8%. Decrease of progression to liver cirrhosis occurred in one patient for every four CHC patients who achieved SVR within 3 years.
Table 2 Adjusted HRs for progression from chronic hepatitis C to liver cirrhosis with adjustment for sex, DM, other risks of hepatitis (hepatitis B virus coinfection, alcoholism, and fatty liver), and HCV genotype
Discussion
Patients older than 65 years are often excluded in clinical trials of HCV therapies because they often have significant comorbidities and experience adverse effects during or after treatment. Therefore, few studies have examined the progression of CHC in elderly patients.Citation6,Citation14,Citation15,Citation26–Citation28
Previous research indicated that the progression of CHC to liver cirrhosis was associated with certain baseline host factors and time-dependent risk factors.Citation2,Citation3,Citation29,Citation30 The cumulative nature of fibrosis progression and the potential for more rapid progression in patients older than 40 years mean that cirrhosis is increasingly significant in elderly patients.Citation2,Citation5 In fact, previous research has documented that a longer duration of CHC infection and advanced patient age are associated with more rapid progression of CHC to liver cirrhosis.Citation2,Citation31 Our findings support the rapid progression of liver cirrhosis in patients aged 65 years and older. In particular, the 3-year cumulative risk of liver cirrhosis was 35.2% in our elderly patients who had persistent viremia.
Previous studies examined the efficacy of PEG-IFN plus RBV for treatment of CHC in elderly patients.Citation4,Citation15,Citation26,Citation28,Citation32–Citation35 Although such patients have higher rates of dose reduction and adverse effects, some of them achieve SVR.Citation4,Citation15,Citation32,Citation35 A recent meta-analysis indicated that the overall rate of SVR in elderly patients was significantly lower than in young patients based on intention-to-treat analysis (42.0% vs 60.1%, P<0.00001) and per-protocol analysis (54.4% vs 67.4%, P=0.002).Citation15 The rates of drug discontinuation and RBV dose reduction in elderly patients were significantly higher than in younger patients.Citation15 The poor SVR rate in elderly patients may be related to the high prevalence of liver cirrhosis and treatment intolerance.Citation4,Citation14,Citation33 Our study demonstrated the efficacy of PEG-IFN plus RBV treatment in elderly patients. In particular, 65.5% of patients overall achieved SVR, and this included 50.6% of those with HCV genotype-1 and 89.3% of those with other HCV genotypes. These data support the use of our treatment regimen in elderly patients with CHC who do not have liver cirrhosis or HCC. Furthermore, there were no deaths or severe treatment-related complications, and most patients who could not tolerate treatment stopped treatment near the beginning of the regimen (mean treatment duration: 84 days). This suggests that the standard PEG-IFN plus RBV treatment is safe in elderly patients if they are closely monitored. From our previous study, the patients who had absence of liver cirrhosis, low baseline HCV RNA levels, high baseline ALT level, no HCV genotype-1, or were treated with PEG-IFN-α-2a were good responders to PEG-IFN plus RBV treatments.Citation36 Thus, it seems that treatment will be effective in selected elderly patients with CHC, such as those with good response predictors (genotype 2/3, low viral load, IL28B-CC), good performance status, and without major concomitant diseases.
Previous long-term follow-up studies showed the benefits of SVR in patients with CHC.Citation7–Citation11 The achievement of an SVR through interferon-based therapy slows the progression from chronic hepatitis to liver fibrosisCitation7–Citation10 and reduces the subsequent development of HCC and liver-related mortality.Citation11–Citation13 These effects are universal and were stronger in patients with advanced fibrosis.Citation11
A large-scale study that followed 1,386 patients with CHC but not liver cirrhosis over 5 years reported that the annual incidence of liver cirrhosis in untreated patients and patients treated with interferon-based therapy (n=892) was 2.26% and 1.11% (nonresponders: 1.99%, sustained responders: 0.74%), respectively.Citation9 In addition, the 14.5-year cumulative incidence of cirrhosis was significantly lower in sustained responders (4.8%) than in nonresponders (21.6%, P=0.0007) and in untreated patients (36.6%, P<0.0001). The present study showed that cirrhosis progressed rapidly and that an SVR slowed the progression to fibrosis in elderly patients with CHC. In particular, patients without SVR had a significantly higher risk of liver cirrhosis than those with SVR, and a significantly higher 3-year cumulative incidence of liver cirrhosis than those with SVR. These results suggest that suppression of HCV RNA can dramatically reduce the risk of liver cirrhosis in elderly patients.
A previous study reported that high APRI score was associated with significant fibrosis or cirrhosis.Citation22 Our patients also had elevated APRI scores (mean ± SD: 1.7±1.3), confirming the presence of advanced fibrosis at baseline and consistent with the rapid progression of fibrosis in elderly patients. In addition, there was a trend of a higher baseline APRI score in patients who progressed to liver cirrhosis than those who did not progress (2.1±1.2 vs 1.6±1.3, P=0.055). Nonetheless, after adjusting for APRI score by Cox regression, SVR remained the most important factor for progression to liver cirrhosis.
Antiviral treatments for CHC have evolved since 2011, and several new drugs, including direct-acting antivirals and host-targeted agents, are marketed or under clinical development. Interferon-free regimens are expected to cure >90% of patients with CHC,Citation37 and the newly available drugs have fewer side effects than interferon-based regimens. This may improve compliance and reduce dropout rates. However, the new HCV therapies are very expensive and have not been thoroughly evaluated in elderly patients. Thus, additional research is needed to evaluate the cost-effectiveness of these new therapies in elderly patients. Before adoption of the new therapies in elderly patients, the PEG-IFN plus RBV regimen should continue to be used to prevent the rapid progression to cirrhosis.
There are several limitations in this study. First, this was a retrospective observational study. Some information such as age at infection, the number of patients who underwent therapy evaluation, the excluded patient number, and the reasons for excluding cannot be obtained from this study. However, it is difficult to perform a prospective controlled trial in elderly patients, so our findings nonetheless provide important information regarding treatment decisions in this population. Second, only 108 patients (74.5%) underwent liver biopsy at baseline, and we cannot provide paired biopsy data. Thus, most patients with cirrhosis were diagnosed using clinical criteria. However, our diagnostic criteria for cirrhosis included two documented ultrasonograms of liver cirrhosis with solid clinical end points (splenomegaly, ascites, hepatic encephalopathy, or varices).Citation9 Although ultrasound is inaccurate in detecting the early stages of liver cirrhosis, combination of the morphologic finding can improve the performance of ultrasound to detect cirrhosis, reaching an accuracy of over 80%.Citation38
In conclusion, liver fibrosis progressed rapidly in elderly patients. An SVR following PEG-IFN combination treatment can reduce the risk of liver cirrhosis in elderly CHC patients.
Author contributions
Chih-Wei Tseng contributed to statistical analysis and drafting of the manuscript, and provided material support. Ting-Tsung Chang and Shu-Fen Wu were involved in critical revision of the manuscript for important intellectual content. Shinn-Jia Tzeng performed statistical analysis. Yu-Hsi Hsieh, Tsung-Hsing Hung, and Hsiang-Ting Huang provided material support. Kuo-Chih Tseng provided material support and was involved in critical revision of the manuscript for important intellectual content. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank their nursing departments for their assistance in procuring record. This study was funded by Dalin Tzu Chi General Hospital (DTCRD99(2)-E-13).
Disclosure
The authors declare no conflicts of interest in this work.
References
- Mohd HanafiahKGroegerJFlaxmanADWiersmaSTGlobal epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalenceHepatology2013571333134223172780
- HajarizadehBGrebelyJDoreGJEpidemiology and natural history of HCV infectionNat Rev Gastroenterol Hepatol20131055356223817321
- MaasoumyBWedemeyerHNatural history of acute and chronic hepatitis CBest Pract Res Clin Gastroenterol20122640141223199500
- HuangCFChuangWLYuMLChronic hepatitis C infection in the elderlyKaohsiung J Med Sci20112753353722208535
- TheinHHYiQDoreGJKrahnMDEstimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regressionHepatology20084841843118563841
- ThabutDLe CalvezSThibaultVHepatitis C in 6,865 patients 65 yr or older: a severe and neglected curable disease?Am J Gastroenterol20061011260126716771947
- BrunoSBattezzatiPMBellatiGLong-term beneficial effects in sustained responders to interferon-alfa therapy for chronic hepatitis CJ Hepatol20013474875511434622
- GeorgeSLBaconBRBruntEMClinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patientsHepatology20094972973819072828
- HuangJFYuMLLeeCMSustained virological response to interferon reduces cirrhosis in chronic hepatitis C: a 1,386-patient study from TaiwanAliment Pharmacol Ther2007251029103717439503
- ShiratoriYImazekiFMoriyamaMHistologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapyAnn Intern Med200013251752410744587
- NgVSaabSEffects of a sustained virologic response on outcomes of patients with chronic hepatitis CClin Gastroenterol Hepatol2011992393021699815
- Trapero-MaruganMMendozaJChaparroMLong-term outcome of chronic hepatitis C patients with sustained virological response to peginterferon plus ribavirinWorld J Gastroenterol20111749349821274379
- MorganRLBaackBSmithBDEradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studiesAnn Intern Med201315832933723460056
- FreiPLeuchtAKHeldUElderly age is not a negative predictive factor for virological response to therapy with pegylated interferon-alpha and ribavirin in chronic hepatitis C virus patientsLiver Int20143455155724034338
- YangZZhuangLYangLEfficacy and safety of peginterferon plus ribavirin for patients aged ≥65 years with chronic hepatitis C: a systematic review and meta-analysisClin Res Hepatol Gastroenterol20143844045024176812
- GonzalezHCJafriSMGordonSCRole of liver biopsy in the era of direct-acting antiviralsCurr Gastroenterol Rep20131530723319086
- LurieYWebbMCytter-KuintRShteingartSLederkremerGZNoninvasive diagnosis of liver fibrosis and cirrhosisWorld J Gastroenterol201521115671158326556987
- European Association for the Study of the LiverEASL Clinical Practice Guidelines: management of hepatitis C virus infectionJ Hepatol20146039242024331294
- FriedMWSide effects of therapy of hepatitis C and their managementHepatology200236S237S24412407599
- OmataMKandaTYuMLAPASL consensus statements and management algorithms for hepatitis C virus infectionHepatol Int2012640943526201405
- WongGLChanHLMakCWEntecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosisHepatology2014581537154723389810
- WaiCTGreensonJKFontanaRJA simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis CHepatology20033851852612883497
- RatgeDScheiblhuberBNitscheMKnabbeCHigh-speed detection of blood-borne hepatitis C virus RNA by single-tube real-time fluorescence reverse transcription-PCR with the LightCyclerClin Chem2000461987198911106331
- BullockGCBrunsDEHaverstickDMHepatitis C genotype determination by melting curve analysis with a single set of fluorescence resonance energy transfer probesClin Chem2002482147215412446470
- KanwalFKramerJRIlyasJDuanZEl-SeragHBHCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of US Veterans with HCVHepatology2014609810524615981
- AntonucciGLongoMAAngelettiCThe effect of age on response to therapy with peginterferon alpha plus ribavirin in a cohort of patients with chronic HCV hepatitis including subjects older than 65 yrAm J Gastroenterol20071021383139117403072
- MindikogluALMillerRRHepatitis C in the elderly: epidemiology, natural history, and treatmentClin Gastroenterol Hepatol20097128134 quiz 12419084480
- OzeTHiramatsuNYakushijinTIndications and limitations for aged patients with chronic hepatitis C in pegylated interferon alfa-2b plus ribavirin combination therapyJ Hepatol20115460461121145907
- SeeffLBThe history of the “natural history” of hepatitis C (1968–2009)Liver Int200929899919207971
- McCombsJMatsudaTTonnu-MiharaIThe risk of long-term morbidity and mortality in patients with chronic hepatitis C: results from an analysis of data from a Department of Veterans Affairs Clinical RegistryJAMA Intern Med201417420421224193887
- FreemanAJDoreGJLawMGEstimating progression to cirrhosis in chronic hepatitis C virus infectionHepatology20013480981611584380
- HondaTKatanoYShimizuJEfficacy of peginterferon-alpha-2b plus ribavirin in patients aged 65 years and older with chronic hepatitis CLiver Int20103052753719523048
- HuangCFYangJFDaiCYEfficacy and safety of pegylated interferon combined with ribavirin for the treatment of older patients with chronic hepatitis CJ Infect Dis201020175175920102281
- MonicaFLirussiFNassuatoGHepatitis C virus infection and related chronic liver disease in a resident elderly population: the Silea StudyJ Viral Hepat199853453519795919
- NishikawaHIguchiEKoshikawaYThe effect of pegylated interferon-alpha2b and ribavirin combination therapy for chronic hepatitis C infection in elderly patientsBMC Res Notes2012513522405406
- TsengCWChenCYChangTTPeginterferon alfa-2a is associated with elevations in alanine aminotransferase at the end of treatment in chronic hepatitis C patients with sustained virologic responsePLoS One20149e10020724937007
- PawlotskyJMNew hepatitis C therapies: the toolbox, strategies, and challengesGastroenterology20141461176119224631495
- BerzigottiACasteraLUpdate on ultrasound imaging of liver fibrosisJ Hepatol20135918018223333447