Abstract
Serrated polyps (SPs) of the colorectum pose a novel challenge to practicing gastroenterologists. Previously thought benign and unimportant, there is now compelling evidence that SPs are responsible for a significant percentage of incident colorectal cancer worldwide. In contrast to conventional adenomas, which tend to be slow growing and polypoid, SPs have unique features that undermine current screening and surveillance practices. For example, sessile serrated polyps (SSPs) are flat, predominately right-sided, and thought to have the potential for rapid growth. Moreover, SSPs are subject to wide variations in endoscopic detection and pathologic interpretation. Unfortunately, little is known about the natural history of SPs, and current guidelines are based largely on expert opinion. In this review, we outline the current taxonomy, epidemiology, and management of SPs with an emphasis on the clinical and public health impact of these lesions.
Introduction
Colorectal cancer (CRC) remains the second leading cause of cancer-related mortality in the US despite widespread screening protocols.Citation1 Importantly, serrated polyps (SPs) have been identified as a unique pathway to CRC that may account for up to 35% of sporadically occurring CRCs.Citation2 These lesions have distinct molecular features that set them apart from the traditional “Fearon–Vogelstein” or “adenoma–carcinoma” model of tumorigenesis.Citation3 In contrast to conventional adenomas, premalignant SPs are more prevalent in females, more frequently located in the proximal colon, and carry a novel genetic signature characterized by BRAF mutations, CpG island methylation, and microsatellite instability.Citation4 Of particular concern, serrated pathway cancers represent a disproportionate number of interval CRC (i.e., cancers occurring after a negative screening test) and have appropriately become a target of public health investigation.Citation5
The purpose of this review is to provide an overview of the taxonomy, epidemiology, and management of SPs.
Overview of SPs
History
Much of the uncertainty surrounding SPs is driven by the fact that, historically, these polyps were all classified as hyperplastic polyps (HPs) and were considered innocuous, without malignant potential, and thus clinically unimportant. However, a series of case reports in the 1970s and 1980s began to question this long-held convention.Citation6,Citation7 The term “serrated adenoma” was officially coined in 1990 when Longacre and Fenoglio-Preiser used the name to characterize a series of premalignant lesions that had a “serrated glandular pattern simulating that seen in hyperplasia”.Citation8 Further progress was made in 2008, when Torlakovic et al successfully differentiated sessile serrated polyps (SSPs) and traditional serrated adenomas (TSAs) on the basis of crypt architecture and molecular markers, setting the groundwork for modern classification systems.Citation9 Currently, the World Health Organization (WHO) recognizes three major types of SPs: HPs, SSPs, and TSAsCitation10 ().
Taxonomy and histology
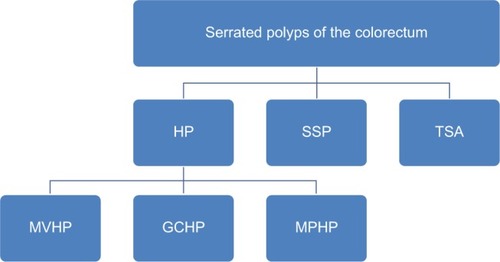
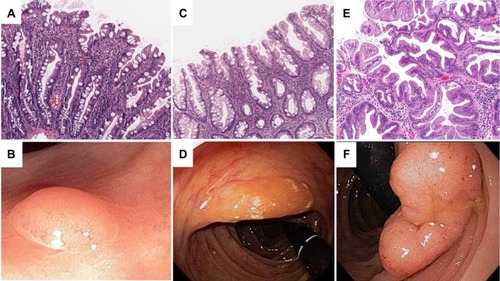
HPs
HPs are the most indolent of SPs and are characterized by straight crypts that rise perpendicularly from the muscularis mucosae. HPs have a jagged infolding crypt epithelium that is more pronounced near the luminal surface, which gives them a “serrated” appearanceCitation11 (). Endoscopically, these lesions are smooth, symmetric, pale, and tend to be distally locatedCitation12 (). HPs are subclassified histologically by the mucin content of their epithelial cells. Microvesicular hyperplastic polyps (MVHPs) exhibit cells with vacuolated cytoplasm containing numerous small mucin droplets. Goblet cell hyperplastic polyps (GCHPs) are composed almost entirely of goblet cells with large mucin-containing apical vesicles, and mucin-poor HPs have scant cytoplasmic mucin.Citation13
SSPs
SSPs are distinguished from HPs by crypt distortion.Citation9,Citation14 In these lesions, the zone of proliferation migrates to the side of the crypt, causing disorganization and dilatation of crypt architecture.Citation2 Classically, these configurations are referred to as “boot” or “anchor-shaped” crypt basesCitation2,Citation15,Citation16 (). On endoscopic examination, SSPs tend to be pale, larger than 5 mm, flat or only slightly raised, and smooth with irregular bordersCitation17–Citation19 (). Many of these lesions excrete excessive quantities of mucin and are often covered with a thin, yellow, mucinous cap and/or surrounded by a “rim of debris”.Citation19 Of note, there is some controversy about the terminology for these lesions, and other authors use different terms such as sessile serrated adenoma (SSA), SSA or SSP, or sessile serrated lesion (SSL). Herein, we use the term SSP to avoid confusion with conventional adenomas.
Sessile serrated polyps with dysplasia (SSPDs) have similar crypt architecture and gross appearance to SSPs, but have dysplastic features including pseudostratification, hyperchromatic nuclei, and mitotic figures.Citation20 Approximately 15% of SSPs will have dysplastic features, and these lesions disproportionately affect women.Citation15
TSAs
Of all SPs, TSAs are the least prevalent, representing approximately 1% of SPs.Citation15 Histologically, they represent a hybrid of serrated and conventional adenomas with “sawtooth” crypts haphazardly arranged in a tubulovillous patternCitation19,Citation21 (). The defining feature of TSAs is ectopic crypt formation. Ectopic crypts lose their orientation to the muscularis mucosae and branch out at obtuse angles, creating villous projections into the lumen of the large intestine.Citation2,Citation21 A significant number of these lesions are frankly dysplastic and capable of malignant transformation, albeit through a different molecular pathway than SSPs.Citation4,Citation8,Citation22 As TSAs are typically more polypoid in form and located within the distal colorectum, they are more easily detected on endoscopy than SSPsCitation23,Citation24 (). All participants provided written informed consent for this study including publication of photography and image captured during colonoscopy.
The molecular pathways of serrated carcinogenesis
Conventional adenomas arise by the accumulation of a well-studied sequence of mutations involving APC, KRAS, and p53.Citation25 As the malignant potential of SPs has only recently been appreciated, the molecular underpinnings of serrated carcinogenesis are the subject of active research. From this, two primary pathways are emerging.
SSP pathway
A mutation in the BRAF oncogene is thought to be the inciting event of the SSP pathwayCitation4 (). When present, BRAF mutations trigger downregulation of apoptosis and promote cellular proliferation.Citation26 Secondly, hypermethylation of promoter regions causes epigenetic silencing of key regulatory genes.Citation27,Citation28 While some degree of methylation is present in nearly all types of cancer, serrated neoplasms in this pathway demonstrate global methylation of CpG islands and are thus classified as CpG island methylation phenotype-high (CIMP-H).Citation29 Epigenetic silencing of MLH1, a critical DNA mismatch repair gene, is thought to trigger the microsatellite instability (MSI) seen in serrated adenocarcinoma.Citation30,Citation31 As MVHPs have high rates of BRAF mutations, they are the favored precursors of SSPs.Citation32,Citation33 From this stage, increasing levels of epigenetic silencing facilitate progression to SSPD and, ultimately, MSI-High (MSI-H) CRC.Citation4,Citation34,Citation35
Figure 3 Serrated carcinogenesis.
Abbreviations: CIMP, CpG island methylation phenotype; GCHP, goblet cell hyperplastic polyp; MSI, microsatellite instable; MSS, microsatellite stable; MVHP, microvesicular hyperplastic polyp; SSP, sessile serrated polyp; TSA, traditional serrated adenoma.
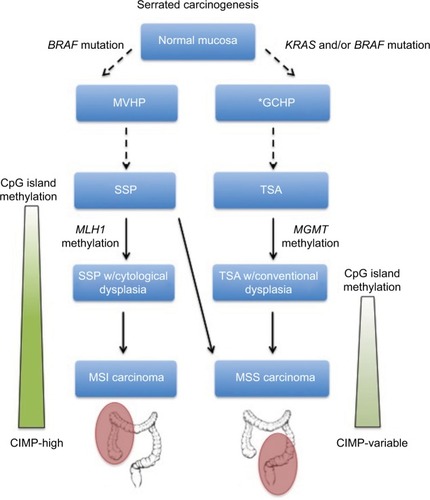
TSA pathway
The mechanism by which TSAs progress to CRC is less well understood, but has important differences with the SSP pathway (). To begin with, TSAs are much more genetically diverse than SSPs. They may or may not have BRAF mutations and can be either CIMP-H or CIMP-low.Citation22 Of importance, TSAs do have a high frequency of KRAS mutations, a key oncogene implicated in many types of malignancy, including the traditional adenoma–carcinoma sequence.Citation22,Citation36,Citation37 Initially, activating KRAS and/or BRAF mutations causes uncontrolled cellular proliferation.Citation4,Citation38 Epigenetic silencing of the DNA repair gene MGMT then allows for the accumulation of subsequent mutations, ultimately leading to a subtype of CRC that is microsatellite stable.Citation39 As GCHPs have higher frequencies of KRAS mutations, they are hypothesized to be the precursors to TSAs, although no definitive link has been elucidated.Citation35 Given the genetic and phenotypic diversity of TSAs, it has been proposed that multiple distinct pathways exist.Citation40
Serrated polyposis syndrome (SPS)
SPS is a phenotypically diverse condition characterized by multiple concurrent SPs. The WHO defines SPS as: 1) at least five SPs proximal to the sigmoid colon, at least two of which are >10 mm in diameter; 2) any number of SPs occurring proximal to the sigmoid colon in an individual with a first-degree relative who has been diagnosed with SPS; or 3) >20 SPs of any size throughout the colon of a single individual.Citation10 Based on a series of case studies, males and females appear to be equally affected and the mean age at presentation is in the sixth decade of life.Citation41 While the true incidence of CRC in SPS is unknown, estimates from small case series are as high as 70%.Citation42–Citation44 Also, importantly, first-degree relatives of those who carry a diagnosis of SPS have an increased risk of CRC.Citation45 There is growing evidence of a genetic etiology of SPS, but no proven hereditary basis, and routine genetic testing is not currently recommended.Citation46–Citation48 More research is needed to separate what is likely a number of molecularly distinct disease processes.
Epidemiology of SPs
Prevalence
Population-based studies estimate that roughly 40% of adults harbor at least one SP.Citation17,Citation33,Citation49 Of these, HPs are by far the most common, representing 70%–90% of SPs.Citation17,Citation33,Citation50 SSPs (10%–25%) and TSAs (~1%) make up a smaller proportion of SPs, respectively.Citation15,Citation17,Citation33,Citation51
The prevalence of SSPs is estimated to be anywhere between 2% and 15% in average risk patients.Citation15,Citation17,Citation52,Citation53 However, the true prevalence may be even higher, as SPs are often subtle and likely underdetected on routine colonoscopy. The median age at presentation of SSPs is 61,Citation15 and they are at least as common in females as males.Citation15,Citation33 In contrast to HPs, SSPs are predominately located on the right side of the colon.Citation15,Citation33,Citation50
Risk factors
Much of the epidemiologic data on serrated colorectal lesions predate the current classification system. However, important risk factors have emerged. Tobacco, alcohol, and obesity have consistently been identified as risk factors for SPs.Citation54–Citation60 These results were recently confirmed in a meta-analysis by Bailie et al, which reported increased risk for tobacco smoking (relative risk [RR], 2.47; 95% confidence interval [CI], 2.12–2.87), alcohol intake (RR, 1.33; 95% CI, 1.17–1.52), and body mass index (RR, 1.40; 95% CI, 1.22–1.61) when comparing the highest and lowest categories of exposure.Citation61 While studies on protective factors are mixed,Citation55,Citation58,Citation59 pooled data suggest that nonsteroidal anti-inflammatory drug use as well as diets high in folate, calcium, or fiber significantly reduce the risk of SPs.Citation61 Interestingly, Caucasians appear to have a higher prevalence of SPs than do African-Americans or Hispanics.Citation62
With regard to SSPs, multiple studies have documented female sex as a significant risk factorCitation15,Citation33,Citation51 (). Aggregate data on modifiable risk factors from Bailie et al suggest that smoking and alcohol are more strongly associated with SSPs than SPs as a whole.Citation61 The role of obesity is less clear, and the available data are conflicting.Citation59,Citation61,Citation63 A recent study by Davenport et al identified diets high in red meat and fat as important risk factors for SSPs, while simultaneously reporting that folate and fiber have a protective effect.Citation64 Interestingly, Burnett-Hartman et al found an independent association between a higher level of education and SSPs, but whether this represents a true association or is confounded by other factors (e.g., differences in bowel preparation) has yet to be resolved.Citation59 Given their relative scarcity, there is a paucity of data on TSAs and their risk factors are largely unknown.
Table 1 Summary of literature on the risk factors for SSPs, including data from individual studies and meta-analysis where available
When interpreting epidemiologic studies on SPs, it is important to remember that early investigations did not differentiate between subtypes of SPs, and even more recently reported data are hindered by pathologic misclassification, particularly the distinction between large proximal HPs and SSPs.
Natural history
True HPs, especially those that are small and located in the distal colon and rectum, are thought to have little or no malignant potential.Citation20 However, MVHPs and GCHPs may serve as important intermediaries in serrated carcinogenesis, as previously discussed.
In contrast, there is substantial evidence that both SSPs and TSAs have malignant potential. Various studies have documented foci of high-grade dysplasia and/or invasive adenocarcinoma developing within these lesions.Citation15,Citation16,Citation65 Moreover, there are multiple case reports of SSPs found adjacent to MSI-H colorectal tumors.Citation66–Citation68 Overall, the rate of high-grade dysplasia in SSPs ranges from 1% to 16%,Citation24,Citation69–Citation73 and it is estimated that around 6% of SSPs will develop into MSI-H CRC.Citation74
Of great debate is the concept of dwell time or the rate at which SSPs progress to CRC. Multiple observational studies have shown higher rates of serrated cancers than SSPDs, suggesting a rapid transition phase or a relatively truncated dwell time.Citation34,Citation75,Citation76 Also, there are a few case reports of SSPs left in situ transforming into invasive CRC in less than a year.Citation77,Citation78
Alternatively, Lash et al reported that the median age of patients with SSPs, SSPDs, and SSPs with foci of adenocarcinoma was 61, 66, and 76 years, respectively.Citation15 The authors concluded that these results imply a slow, stepwise progression along the pathway of serrated carcinogenesis over a period of 15+ years. In addition, a retrospective case series of MSI-H carcinoma diagnosed at the site of previous polypectomy reported a mean of 7.3 years between initial polypectomy and cancer resection.Citation16
Importantly, these studies are limited by the fact that SSPs are notoriously difficult to detect on endoscopy, are often incompletely resected, and were historically thought benign and unimportant. The true behavior of SSPs in vivo is extremely challenging to study for ethical and logistical reasons. Nevertheless, optimal screening algorithms demand a better understanding of the natural history of SPs and SSPs, in particular.
The risk of synchronous and metachronous neoplasia
SPs increase the risk for both synchronous (concurrent) and metachronous (future or interval) neoplasia. In a cross-sectional analysis involving nearly 5000 patients, Li et al found that the presence of large (>10 mm) SPs was a strong predictor of synchronous advanced colorectal neoplasia (odds ratio [OR], 3.24; 95% CI, 2.05–5.13).Citation79 These results were later reinforced by two large multicenter studies reporting similar risks of synchronous neoplasia associated with large SPs.Citation80,Citation81 With respect to metachronous lesions, Schreiner et al reported an elevated risk of future advanced neoplasia in patients with proximal SPs on baseline colonoscopy (OR, 3.14; 95% CI, 1.59−6.20).Citation80 As previously highlighted, many of these large and/or proximal SPs would now be classified as SSPs.
Using the current pathologic classification, Hazewinkel et alCitation82 and Ng et alCitation83 found increased risk for synchronous advanced neoplasia in large and proximal HPs as well as SSPs. These results were confirmed by a large, population-based case–control study from Denmark, which highlighted the risk for metachronous CRC in patients with SSPs, particularly those located in the proximal colon (OR, 12.42; 95% CI, 4.88–31.58).Citation84 A recent article by Melson et al suggests that the risk of metachronous advanced neoplasia in low-risk SPs is comparable to that of high-risk tubular adenomas.Citation85 Moreover, small observational studies estimate the risk of metachronous CRC in patients harboring SSPs to be as high as 12.5%.Citation86,Citation87
Rates of synchronous and metachronous SSPs also appear to be elevated,Citation87,Citation88 and the observation that metachronous SSPs may be limited to those with preexisting SPs suggests a “field effect” phenomenon even in patients who do not meet the formal criteria for SPS.Citation88 Meanwhile, for those who carry a diagnosis of SPS, the presence of SSPDs, advanced adenomas, or combined WHO phenotypes 1 and 3 appears to increase the risk for CRC.Citation89
Taken together, these studies suggest that the risk of advanced neoplasia increases as one moves from distal to proximal colon, from small to large polyp size, and from HP to more advanced serrated lesions such as SSPs and TSAs. Due to these complexities, many experts have advocated for surveillance intervals specific to SPs.
Detection of SPs
Endoscopic detection
Notably, there is significant variation in the endoscopic detection rates of SPs (0%–22%) even among experienced gastroenterologists,Citation17,Citation90,Citation91 and those practicing today may miss more than half of the SPs.Citation17,Citation90 Adequate bowel preparation is associated with better detection of flat lesions, in general, and SSPs, in particular.Citation92,Citation93 This stands to reason, given their subtle endoscopic appearance and typical location in the right colon, which is more often affected by suboptimal bowel cleansing. Wijkerslooth et al showed that SSP detection rates were also associated with withdrawal times.Citation91 Kahi et al found that the detection rate of SPs is correlated to that of conventional adenomas.Citation90 However, despite this correlation, it should be noted that there are endoscopists with high adenoma detection rates and low SSP detection rates and vice versa; this implies that overlapping, but not interchangeable skills are needed to detect clinically important SPs. Fortunately, the detection rate of SPs appears to be increasing over time, as their clinical significance gains recognition by endoscopists and pathologists alike.Citation17 An emphasis on tracking the adequacy of bowel preparation, cecal intubation rates, and polyp detection rates is helping to bridge disparities in practice.Citation94
Alternative methods for detection
Standard colonoscopy is superior to other readily available CRC screening modalities in detecting SPs, as the use of blood-based stool tests, flexible sigmoidoscopy, and computed tomography (CT) enterography have obvious limitations. To begin with, SSPs are less likely to undergo spontaneous hemorrhageCitation14,Citation95 and their relatively low profile decreases the probability of trauma-associated injury.Citation96 Visualization of SSPs on CT enterography is complicated by their sessile or flat morphology, and flexible sigmoidoscopy simply does not reach the right side of the colon where SSPs predominate. In support of this, Chang et al found that fecal immunochemical testing (a more specific test of fecal occult blood from colonic source) has poor sensitivity for detecting even large SSPs.Citation97 A recent randomized controlled trial (RCT) comparing standard colonoscopy to CT colonography reported a superior SSP detection rate (4.3% vs. 0.8%) in the standard colonoscopy arm,Citation98 and Kahi et al revealed that more than half of the proximal advanced SPs had no distal lesions, highlighting the limitations of flexible sigmoidoscopy in CRC surveillance.Citation99
Of interest, fecal DNA studies have shown promise as a novel tool for CRC screening.Citation100,Citation101 While these assays reliably detect CRC, their sensitivity for SSPs >10 mm is in the range of 42%–66%.Citation100,Citation102,Citation103 Fecal DNA tests are limited by their lack of molecular markers specific to serrated neoplasms, poor specificity when compared to other noninvasive tests (i.e., fecal immunochemical testing), and the need for follow-up invasive testing for positive results. Notwithstanding, preliminary studies of BRAF stool assays have shown potential,Citation104 leaving the door open for the development of fecal DNA tests with higher sensitivity for precancerous SPs.
Highlighting concerning areas of mucosa with dye, a technique known as chromoendoscopy, has proven to be a useful tool in the detection of SPs.Citation19,Citation33 Multiple studies have demonstrated that this technique can improve detection of both conventional adenomas and SPs.Citation105–Citation108 However, use of chromoendoscopy substantially lengthens procedure times, which is the major limitation of this technique and undermines its usefulness as a screening modality. In the largest RCT to date, Kahi et al reported a modest increase in the detection of flat and small adenomas in the chromoendoscopy arm. However, specific data on SSP detection were not reported, and the authors concluded that the additional yield was modest and did not justify the routine use of screening chromoendoscopy.Citation108
Devices that allow for real-time histologic assessment of colonic mucosa during colonoscopy have also shown promise in the detection of SPs. Narrow-band imaging (NBI) with and without magnification are the most popular of these, and both have been proven to reliably differentiate adenomas with malignant potential from benign hyperplastic lesions.Citation109–Citation111 Several groups have developed and validated standard criteria by which to identify SPs utilizing NBI.Citation112,Citation113 However, a recent meta-analysis did not find strong evidence for the benefit of image-enhanced colonoscopy for detection of SSPs, and thus, studies specifically designed to assess SSP detection rates are needed before these modalities can be widely accepted.Citation114
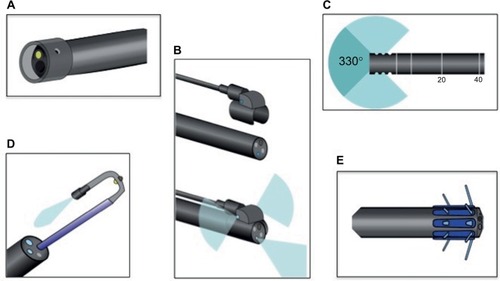
More recently, a number of devices aimed at exposing additional colonic mucosa have been developed, such as wide-angled lenses and retroscopes (). These devices are designed to broaden the operator’s visual field and help image the backs of colonic folds. Panoramic or wide-angled colonoscopy devices employ multiple lenses to nearly double the standard visual field, and a recent RCT showed significant decreases in adenoma miss rates over standard colonoscopy.Citation115 Retroscope devices provide a continuous retroflexed view of the colonic mucosa as the scope is withdrawn and may also improve detection of adenomas and SSPs.Citation116,Citation117
Figure 4 Novel colonoscopic technologies.
Endoscopic removal
In the absence of formal guidelines, there are limited data available to aid practicing gastroenterologists. However, most experts recommend that all SPs with the exception of small (<5 mm) distal HPs be removed.Citation4,Citation11,Citation14,Citation20,Citation34 Even then, many suggest that these diminutive, rectosigmoid HPs be sampled randomly for histologic evaluation.Citation20
SPs are notoriously challenging to resect, given their sessile morphology, indistinct borders, and predominance for the right colon, and the rates of incomplete resection are high. Pohl et al found that rates of incomplete resection were much higher for SSPs than for conventional adenomas (31.0% vs. 7.2%) and that SSP histology was an independent risk factor for incomplete resection (RR, 3.74; 95% CI, 2.04–6.84).Citation118 Of most concern, nearly half of large (>10 mm) SSPs were incompletely resected in this study, and there is evidence that incomplete polypectomy plays an important role in the development of interval CRCs.Citation119
The optimal strategy for the resection of SPs depends on the location, size, and morphology of the lesion as well as the skill set of the individual endoscopist and the tools available with him or her. For smaller lesions, cold snare polypectomy has been found to be safe, while allowing for appropriate histologic evaluation of tissue margins.Citation120,Citation121 Larger lesions may require piecemeal resection with or without mucosal “lifting” with the injection of saline or another tissue expander to facilitate delineation and removal.Citation122,Citation123
Pathologic interpretation
As the malignant potential of SPs is a relatively new concept, consistent pathologic interpretation remains a challenge.Citation124 Hetzel et al exposed significant variation in the classification of HPs and SSPs among practicing pathologists at a single academic medical center.Citation17 In a large retrospective Canadian study, substantial numbers of proximal HPs (20%) and HPs >5 mm (17%) were reclassified as SSPs upon review by trained gastrointestinal pathologists, and these results were replicated in a recent European study.Citation125 Importantly, misclassification of SPs makes interpreting older studies of SPs (particularly those published prior to 2008) challenging, and even contemporary studies may include patients with older pathology readings that are not consistent with contemporary criteria. While there is evidence that the pathologic diagnosis of SSPs is increasing with time,Citation126 educational outreach and seamless communication between gastroenterologists and pathologists are needed to improve diagnostic accuracy and ensure appropriate management.
Surveillance
Of critical importance to the management of SPs is the establishment of appropriate surveillance intervals. Both the US Multisociety Task Force and an international consensus panel have outlined a detailed strategy for the management of HPs, SSPs, SSPDs, and TSAsCitation20,Citation127 (). The latest European guidelines include no specific recommendations for SPs.Citation128 Of note, the consensus panel recommends more frequent surveillance in patients with proximal and/or large HPs, reflecting an appreciation of the aforementioned challenges in pathologic diagnosis. For patients diagnosed with SPS, annual colonoscopy is recommended.Citation20 An interval of 3–6 months is suggested for SPs requiring piecemeal resection or with positive margins on routine pathologic examination to ensure adequate resection.Citation20,Citation74,Citation127
Table 2 Current recommendations for surveillance intervals after colonoscopy with serrated polyps
Future directions
Improving our understanding of serrated carcinogenesis
The genetic and epigenetic drivers of the serrated pathway are incompletely understood. Identifying both the cause and the downstream effects of CpG island methylation may reveal additional tumor markers and/or novel therapeutic approaches. Also, a better understanding of the genetic basis of SPS would undoubtedly aid in the detection, classification, and management of these challenging cases. As our molecular diagnostics improve, we may come to recognize the separate phenotypes of SPS as distinct clinical entities.
Optimizing detection and resection
Perhaps the first obstacle to overcome with regard to improving the detection of SPs is the variation that currently exists between practicing endoscopists. Operators should strive to meet published standards for conventional adenomas. Based on available data, a detection rate of at least 1%–2% for SSPs is reasonable.Citation129 However, this likely underestimates the true prevalence of these lesions, as trials utilizing formal training programs and advanced endoscopic techniques report much higher numbers.Citation33,Citation130 Fecal DNA tests have shown promising results, but inclusion of serrated pathway markers is necessary to improve their ability to detect advanced precancerous lesions. Further advances in endoscopic imaging may lead to improvement in SSP detection, but this remains to be seen.
There is uncertainty with respect to the optimal methods to resect SPs. As previously mentioned, rates of incomplete resection are currently above acceptable thresholds.Citation17,Citation94 Clarification with regard to which lesions may benefit from submucosal lifting, chromoendoscopy, hot vs. cold snare technique, and/or underwater resection is necessary.Citation131–Citation133 Whether or not certain snare designs (e.g., crescent-shaped, stiff, or braided wire snares) are superior to others for the resection of SSPs and other flat polyps is also an area where additional data are needed.
Clarifying surveillance intervals
Understanding the natural history of SPs is essential to outlining appropriate surveillance intervals. Current guidelines vary and are not based on robust data. Well-designed epidemiologic studies of MSI-H, proximal, and interval CRCs may provide useful information about the dwell time of SPs. In addition, the identification of molecular and histologic features associated with rapid progression would be invaluable.
As our power to detect even the subtlest colonic lesions increases, there are appropriate concerns about the added risk of consequent resections and surveillance. Especially in the arena of cancer screening, gastroenterologists must be careful not to upset the delicate balance between benefit and harm. Surveillance recommendations are fluid and likely to change as our understanding of serrated carcinogenesis improves, and this is a rapidly evolving field. Therefore, current practice must continually be reappraised to ensure the optimal care of patients harboring SPs.
Conclusion
Serrated neoplasms are responsible for a third of newly diagnosed CRC, a disproportionate number of which are interval cancers and occur despite recommended screening. Despite progress, pathologic misclassification and endoscopic under-detection of SPs remain significant challenges. Innovations such as NBI, wide-angle colonoscopy, and fecal DNA testing are promising, but additional study is needed to ensure these technologies improve SP detection and decrease CRC incidence. Future investigations should focus on understanding the natural history of SPs, identifying risk factors for rapid progression and optimizing the detection and resection of these lesions.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- SnoverDCUpdate on the serrated pathway to colorectal carcinomaHum Pathol201142111020869746
- FearonERVogelsteinBA genetic model for colorectal tumorigenesisCell19906157597672188735
- LeggettBWhitehallVRole of the serrated pathway in colorectal cancer pathogenesisGastroenterology201013862088210020420948
- SawhneyMSFarrarWDGudisevaSMicrosatellite instability in interval colon cancersGastroenterology200613161700170517087932
- CooperHSPatchefskyASMarksGAdenomatous and carcinomatous changes within hyperplastic colonic epitheliumDis Colon Rectum1979223152156446245
- JassJRRelation between metaplastic polyp and carcinoma of the colorectumLancet Lond Engl198318314–83152830
- LongacreTAFenoglio-PreiserCMMixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasiaAm J Surg Pathol19901465245372186644
- TorlakovicEEGomezJDDrimanDKSessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA)Am J Surg Pathol2008321212918162766
- SnoverDAhnenDJBurtRWSerrated polyps of the colon and rectum and serrated (“hyperplastic”) polyposisBozmanFTTN CarneiroFHrubanRHWHO Classification of Tumours Pathology and Genetics Tumours of the Digestive System4th ednBerlin, GermanySpringer-Verlag2010
- HuangCSO’brienMJYangSFarrayeFAHyperplastic polyps, serrated adenomas, and the serrated polyp neoplasia pathwayAm J Gastroenterol200499112242225515555008
- WayeJDBilottaJJRectal hyperplastic polyps: now you see them, now you don’t – a differential pointAm J Gastroenterol19908512155715592252015
- TorlakovicESkovlundESnoverDCTorlakovicGNeslandJMMorphologic reappraisal of serrated colorectal polypsAm J Surg Pathol2003271658112502929
- EastJESaundersBPJassJRSporadic and syndromic hyperplastic polyps and serrated adenomas of the colon: classification, molecular genetics, natural history, and clinical managementGastroenterol Clin North Am2008371254618313538
- LashRHGentaRMSchulerCMSessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patientsJ Clin Pathol201063868168620547691
- GoldsteinNSBhanotPOdishEHunterSHyperplastic-like colon polyps that preceded microsatellite-unstable adenocarcinomasAm J Clin Pathol2003119677879612817424
- HetzelJTHuangCSCoukosJAVariation in the detection of serrated polyps in an average risk colorectal cancer screening cohortAm J Gastroenterol2010105122656266420717107
- LambertRKudoSEViethMPragmatic classification of superficial neoplastic colorectal lesionsGastrointest Endosc20097061182119919879563
- JaramilloETamuraSMitomiHEndoscopic appearance of serrated adenomas in the colonEndoscopy200537325426015731942
- RexDKAhnenDJBaronJASerrated lesions of the colorectum: review and recommendations from an expert panelAm J Gastroenterol201210791315132922710576
- YantissRKOhKYChenYTRedstonMOdzeRDFiliform serrated adenomas: a clinicopathologic and immunophenotypic study of 18 casesAm J Surg Pathol20073181238124517667549
- KimKMLeeEJKimYHChangDKOdzeRDKRAS mutations in traditional serrated adenomas from Korea herald an aggressive phenotypeAm J Surg Pathol201034566767520305537
- LiSCBurgartLHistopathology of serrated adenoma, its variants, and differentiation from conventional adenomatous and hyperplastic polypsArch Pathol Lab Med2007131344044517516746
- MoritaTTamuraSMiyazakiJHigashidaniYOnishiSEvaluation of endoscopic and histopathological features of serrated adenoma of the colonEndoscopy200133976176511558029
- VogelsteinBFearonERHamiltonSRGenetic alterations during colorectal-tumor developmentN Engl J Med198831995255322841597
- GarnettMJMaraisRGuilty as charged: B-RAF is a human oncogeneCancer Cell20046431331915488754
- SamowitzWSAlbertsenHHerrickJEvaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancerGastroenterology2005129383784516143123
- HawkinsNNorrieMCheongKCpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instabilityGastroenterology200212251376138711984524
- ToyotaMAhujaNOhe-ToyotaMHermanJGBaylinSBIssaJPCpG island methylator phenotype in colorectal cancerProc Natl Acad Sci U S A199996158681868610411935
- KaneMFLodaMGaidaGMMethylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell linesCancer Res19975758088119041175
- WeisenbergerDJSiegmundKDCampanMCpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancerNat Genet200638778779316804544
- O’BrienMJYangSMackCComparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end pointsAm J Surg Pathol200630121491150117122504
- SpringKJZhaoZZKaramaticRHigh prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopyGastroenterology200613151400140717101316
- O’BrienMJHyperplastic and serrated polyps of the colorectumGastroenterol Clin North Am200736494796817996799
- YangSFarrayeFAMackCPosnikOO’BrienMJBRAF and KRAS mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation statusAm J Surg Pathol200428111452145915489648
- FuBYachidaSMorganRZhongYMontgomeryEAIacobuzio-DonahueCAClinicopathologic and genetic characterization of traditional serrated adenomas of the colonAm J Clin Pathol2012138335636622912351
- BosJLRas oncogenes in human cancer: a reviewCancer Res19894917468246892547513
- YamashitaNMinamotoTOchiaiAOndaMEsumiHFrequent and characteristic K-ras activation in aberrant crypt foci of colon. Is there preference among K-ras mutants for malignant progression?Cancer199575Suppl 6152715337889486
- WhitehallVLWalshMDYoungJLeggettBAJassJRMethylation of O-6-methylguanine DNA methyltransferase characterizes a subset of colorectal cancer with low-level DNA microsatellite instabilityCancer Res200161382783011221863
- TsaiJLiauJLinYTraditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesisMod Pathol201427101375138524603588
- GuarinosCSanchez-FortunCRodriguez-SolerMAlendaCPayaAJoverRSerrated polyposis syndrome: molecular, pathological and clinical aspectsWorld J Gastroenterol201218202452246122654442
- RubioCAStemmeSJaramilloELindblomAHyperplastic polyposis coli syndrome and colorectal carcinomaEndoscopy200638326627016528654
- LeggettBADevereauxBBidenKSearleJYoungJJassJHyperplastic polyposis: association with colorectal cancerAm J Surg Pathol200125217718411176066
- BuchananDDSweetKDriniMPhenotypic diversity in patients with multiple serrated polyps: a genetics clinic studyInt J Colorectal Dis201025670371220213458
- BoparaiKSReitsmaJBLemmensVIncreased colorectal cancer risk in first-degree relatives of patients with hyperplastic polyposis syndromeGut20105991222122520584785
- YoungJJassJRThe case for a genetic predisposition to serrated neoplasia in the colorectum: hypothesis and review of the literatureCancer Epidemiol Biomark Prev2006151017781784
- YoungJJenkinsMParrySSerrated pathway colorectal cancer in the population: genetic considerationGut200756101453145917566021
- SyngalSBrandREChurchJMGiardielloFMHampelHLBurtRWACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromesAm J Gastroenterol2015110222325645574
- WilliamsARBalasooriyaBADayDWPolyps and cancer of the large bowel: a necropsy study in LiverpoolGut198223108358427117903
- HiguchiTSugiharaKJassJRDemographic and pathological characteristics of serrated polyps of colorectumHistopathology2005471324015982321
- CarrNJMahajanHTanKLHawkinsNJWardRLSerrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenomaJ Clin Pathol200962651651819126563
- BudaABonaMDDottiIPrevalence of different subtypes of serrated polyps and risk of synchronous advanced colorectal neoplasia in average-risk population undergoing first-time colonoscopyClin Transl Gastroenterol20123e623238028
- KahiCJLiXEckertGJRexDKHigh colonoscopic prevalence of proximal colon serrated polyps in average-risk men and womenGastrointest Endosc201275351552022018551
- LiebermanDAPrindivilleSWeissDGWillettW380VCSGRisk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individualsJama2003290222959296714665657
- MartinezMEMcPhersonRSLevinBGloberGAA case-control study of dietary intake and other lifestyle risk factors for hyperplastic polypsGastroenterology199711324234299247459
- JiBTWeissfeldJLChowWHHuangWYSchoenREHayesRBTobacco smoking and colorectal hyperplastic and adenomatous polypsCancer Epidemiol Biomark Prev2006155897901
- KearneyJGiovannucciERimmEBDiet, alcohol, and smoking and the occurrence of hyperplastic polyps of the colon and rectum (United States)Cancer Causes Control CCC19956145567718735
- MorimotoLMNewcombPAUlrichCMBostickRMLaisCJPotterJDRisk factors for hyperplastic and adenomatous polyps: evidence for malignant potential?Cancer Epidemiol Biomark Prev20021110 Pt 110121018
- Burnett-HartmanANPassarelliMNAdamsSVDifferences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical siteAm J Epidemiol2013177762563723459948
- OmataFBrownWRTokudaYModifiable risk factors for colorectal neoplasms and hyperplastic polypsIntern Med Tokyo Jpn2009483123128
- BailieLLoughreyMBColemanHGLifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysisGastroenterology201715219210427639804
- WallaceKGrauMVAhnenDThe association of lifestyle and dietary factors with the risk for serrated polyps of the colorectumCancer Epidemiol Biomark Prev200918823102317
- AndersonJCRangasamyPRustagiTRisk factors for sessile serrated adenomasJ Clin Gastroenterol201145869469921325950
- DavenportJRSuTZhaoZModifiable lifestyle factors associated with risk of sessile serrated polyps, conventional adenomas and hyperplastic polypsGut2016 pii: gutjnl-2016-312893. [Epub ahead of print]
- SheridanTBFentonHLewinMRSessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions “caught in the act”Am J Clin Pathol2006126456457116938659
- PatilDTShadrachBLRybickiLALeachBHPaiRKProximal colon cancers and the serrated pathway: a systematic analysis of precursor histology and BRAF mutation statusMod Pathol201225101423143122684223
- MakinenMJGeorgeSMJernvallPMakelaJVihkoPKarttunenTJColorectal carcinoma associated with serrated adenoma – prevalence, histological features, and prognosisJ Pathol2001193328629411241406
- Garcia-SolanoJPerez-GuillermoMConesa-ZamoraPClinicopathologic study of 85 colorectal serrated adenocarcinomas: further insights into the full recognition of a new subset of colorectal carcinomaHum Pathol201041101359136820594582
- MatsumotoTMizunoMShimizuMManabeTIidaMClinicopathological features of serrated adenoma of the colorectum: comparison with traditional adenomaJ Clin Pathol199952751351610605404
- JaramilloEWatanabeMBefritsRde LeonEPRubioCSlezakPSmall, flat colorectal neoplasias in long-standing ulcerative colitis detected by high-resolution electronic video endoscopyGastrointest Endosc199644115228836711
- RubioCAJaramilloEFlat serrated adenomas of the colorectal mucosaJpn J Cancer Res Gann19968733053098613434
- SongSYKimYHYuMKComparison of malignant potential between serrated adenomas and traditional adenomasJ Gastroenterol Hepatol200722111786179017914951
- ChinoAYamamotoNKatoYThe frequency of early colorectal cancer derived from sessile serrated adenoma/polyps among 1858 serrated polyps from a single institutionInt J Colorectal Dis201631234334926510850
- HuangCSFarrayeFAYangSO’BrienMJThe clinical significance of serrated polypsAm J Gastroenterol2011106222924021045813
- GoldsteinNSSmall colonic microsatellite unstable adenocarcinomas and high-grade epithelial dysplasias in sessile serrated adenoma polypectomy specimens: a study of eight casesAm J Clin Pathol2006125113214516483002
- JassJRSerrated route to colorectal cancer: back street or super highway?J Pathol2001193328328511241405
- OonoYFuKNakamuraHProgression of a sessile serrated adenoma to an early invasive cancer within 8 monthsDig Dis Sci200954490690918688718
- NakamuraHFuKParra-BlancoAA sessile colonic polyp showing striking morphological changes within a 2-month periodEndoscopy200739 Suppl 1E279E8017957624
- LiDJinCMcCullochCAssociation of large serrated polyps with synchronous advanced colorectal neoplasiaAm J Gastroenterol2009104369570219223889
- SchreinerMAWeissDGLiebermanDAProximal and large hyper-plastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasiaGastroenterology201013951497150220633561
- HiraokaSKatoJFujikiSThe presence of large serrated polyps increases risk for colorectal cancerGastroenterology201013951503151020643134
- HazewinkelYde WijkersloothTRStoopEMPrevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopyEndoscopy201446321922424254386
- NgSCChingJYLChanVCWAssociation between serrated polyps and the risk of synchronous advanced colorectal neoplasia in average-risk individualsAliment Pharmacol Amp Ther2015411108115
- ErichsenRBaronJAHamilton-DutoitSJIncreased risk of colorectal cancer development among patients with serrated polypsGastroenterology2016150489590226677986
- MelsonJMaKArshadSPresence of small sessile serrated polyps increases rate of advanced neoplasia upon surveillance compared with isolated low-risk tubular adenomasGastrointest Endosc201684230731426855297
- LuFIde W vanNOwenDThaSPTurbinDAWebberDLLongitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinomaAm J Surg Pathol201034792793420551824
- TeriakyADrimanDKChandeNOutcomes of a 5-year follow-up of patients with sessile serrated adenomasScand J Gastroenterol201247217818322229626
- MacaronCVuHTLopezRPaiRKBurkeCARisk of metachronous polyps in individuals with serrated polypsDis Colon Rectum201558876276826163955
- IJspeertJEGRanaSAQAtkinsonNSSClinical risk factors of colorectal cancer in patients with serrated polyposis syndrome: a multicentre cohort analysisGut201566227828426603485
- KahiCJHewettDGNortonDLEckertGJRexDKPrevalence and variable detection of proximal colon serrated polyps during screening colonoscopyClin Gastroenterol Hepatol201191424620888435
- de WijkersloothTRStoopEMBossuytPMDifferences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal timeGastrointest Endosc201377461762323321338
- Parra-BlancoANicolas-PerezDGimeno-GarciaAThe timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized studyWorld J Gastroenterol200612386161616617036388
- ClarkBTLaineLHigh-quality bowel preparation is required for detection of sessile serrated polypsClin Gastroenterol Hepatol20161481155116227060426
- LasisiFRexDKImproving protection against proximal colon cancer by colonoscopyExpert Rev Gastroenterol Hepatol20115674575422017701
- WaldockAEllisIOArmitageNCTurnerDRHardcastleJDHis-topathological assessment of bleeding from polyps of the colon and rectumJ Clin Pathol19894243783822715351
- SobinLHThe histopathology of bleeding from polyps and carcinomas of the large intestineCancer19855535775812578088
- ChangLCShunCTHsuWFFecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivityClin Gastroenterol Hepatol2016 Epub201684
- IJspeertJENoltheniusCJTKuipersEJCT-colonography vs. colonoscopy for detection of high-risk sessile serrated polypsAm J Gastroenterol2016111451652227021193
- KahiCJVemulapalliKCSnoverDCJawadKHACummingsOWRexDKFindings in the distal colorectum are not associated with proximal advanced serrated lesionsClin Gastroenterol Hepatol201513234535125083562
- ImperialeTFRansohoffDFItzkowitzSHMultitarget stool DNA testing for colorectal-cancer screeningN Engl J Med2014370141287129724645800
- RedwoodDGAsayEDBlakeIDStool DNA testing for screening detection of colorectal neoplasia in Alaska native peopleMayo Clin Proc201691617026520415
- HeighRIYabTCTaylorWRDetection of colorectal serrated polyps by stool DNA testing: comparison with fecal immunochemical testing for occult blood (FIT)PLoS One201491e8565924465639
- LidgardGPDomanicoMJBruinsmaJJClinical performance of an automated stool DNA assay for detection of colorectal neoplasiaClin Gastroenterol Hepatol201311101313131823639600
- JinYMLiBJQuBDuYJBRAF, K-ras and BAT26 mutations in colorectal polyps and stoolWorld J Gastroenterol200612325148515216937524
- KiesslichRvon BerghMHahnMHermannGJungMChromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colonEndoscopy200133121001100611740641
- BrookerJCSaundersBPShahSGTotal colonic dye-spray increases the detection of diminutive adenomas during routine colonoscopy: a randomized controlled trialGastrointest Endosc200256333333812196768
- LapalusMGHelbertTNapoleonBDoes chromoendoscopy with structure enhancement improve the colonoscopic adenoma detection rate?Endoscopy200638544444816767577
- KahiCJAndersonJCWaxmanIHigh-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screeningAm J Gastroenterol201010561301131720179689
- RexDKNarrow-band imaging without optical magnification for histologic analysis of colorectal polypsGastroenterology200913641174118119187781
- KanaoHTanakaSOkaSHirataMYoshidaSChayamaKNarrow-band imaging magnification predicts the histology and invasion depth of colorectal tumorsGastrointest Endosc2009693 Pt 263163619251003
- EastJESuzukiNBassettPNarrow band imaging with magnification for the characterization of small and diminutive colonic polyps: pit pattern and vascular pattern intensityEndoscopy2008401081181718828077
- HewettDGKaltenbachTSanoYValidation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imagingGastroenterology2012143359960722609383
- IJspeertJEGBastiaansenBAJvan LeerdamMEDevelopment and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polypsGut201665696397025753029
- ParikhNDChaptiniLNjeiBLaineLDiagnosis of sessile serrated adenomas/polyps with image-enhanced endoscopy: a systematic review and meta-analysisEndoscopy201648873173927223636
- GralnekIMSiersemaPDHalpernZStandard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trialLancet Oncol201415335336024560453
- WayeJDHeighRIFleischerDEA retrograde-viewing device improves detection of adenomas in the colon: a prospective efficacy evaluation (with videos)Gastrointest Endosc201071355155620018280
- LeufkensAMDeMarcoDCRastogiAEffect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE studyGastrointest Endosc201173348048921067735
- PohlHSrivastavaABensenSPIncomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) studyGastroenterology20131441748023022496
- FarrarWDSawhneyMSNelsonDBLederleFABondJHColorectal cancers found after a complete colonoscopyClin Gastroenterol Hepatol20064101259126416996804
- UnoYObaraKZhengPCold snare excision is a safe method for diminutive colorectal polypsTohoku J Exp Med199718342432499549824
- TapperoGGaiaEGiuliPDMartiniSGubettaLEmanuelliGCold snare excision of small colorectal polypsGastrointest Endosc19923833103131607081
- HoltBAJayasekeranVSonsonRBourkeMJTopical submucosal chromoendoscopy defines the level of resection in colonic EMR and may improve procedural safety (with video)Gastrointest Endosc201377694995323472997
- Dolz-AbadiaCVilella-MartorellASubmucosal chromoendoscopy. A technique that highlights epithelia and differentiates histological components, and renders colon polypectomy easier and saferRev Esp Enferm Dig2015107743043526140636
- FarrisABMisdrajiJSrivastavaASessile serrated adenoma: challenging discrimination from other serrated colonic polypsAm J Surg Pathol2008321303518162767
- SchachschalGSehnerSChoschzickMImpact of reassessment of colonic hyperplastic polyps by expert GI pathologistsInt J Colorectal Dis201631367568326847619
- GillPWangLMBaileyAEastJELeedhamSChettyRReporting trends of right-sided hyperplastic and sessile serrated polyps in a large teaching hospital over a 4-year period (2009–2012)J Clin Pathol201366865565823576460
- LiebermanDARexDKWinawerSJGiardielloFMJohnsonDALevinTRGuidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US multi-society task force on colorectal cancerGastroenterology2012143384485722763141
- AtkinWSValoriRKuipersEJEuropean guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition – colonoscopic surveillance following adenoma removalEndoscopy201244Suppl 3SE151SE16323012119
- SanakaMRGohelTPoduguAAdenoma and sessile serrated polyp detection rates: variation by patient sex and colonic segment but not specialty of the endoscopistDis Colon Rectum20145791113111925101608
- RajuGSVadyalaVSlackRAdenoma detection in patients undergoing a comprehensive colonoscopy screeningCancer Med20132339140223930215
- SweetserSBaronTHOptimizing resection of sessile serrated polypsEndoscopy201446 Suppl 1 UCTN:E231-0034-1365379. Epub201457
- RaadDTripathiPCooperGFalck-YtterYRole of the cold biopsy technique in diminutive and small colonic polyp removal: a systematic review and meta-analysisGastrointest Endosc201683350851526545637
- YenAWLeungJWLeungFWA novel method with significant impact on adenoma detection: combined water-exchange and cap-assisted colonoscopyGastrointest Endosc201377694494823473001