Abstract
Background
Doubling of serum creatinine is often used as a marker for worsening kidney function in nephrology trials. Most people with chronic kidney disease die of other causes before reaching end-stage renal disease. We were interested in the association between doubling of serum creatinine and the risk of a first-time diagnosis of angina pectoris, congestive heart failure (CHF), myocardial infarction (MI), stroke, or transient ischemic attack in patients with chronic kidney disease and with diagnosed type 2 diabetes mellitus.
Methods
We identified all adult patients registered in the “Clinical Practice Research Datalink” between 2002 and 2011 with incident chronic kidney disease and type 2 diabetes mellitus and did a cohort study with a Cox proportional hazard analysis.
Results
We identified in total 27,811 patients, 693 developed angina pectoris, 1,069 CHF, 508 MI, 970 stroke, and 578 transient ischemic attacks. Patients whose serum creatinine doubled during follow-up had increased risks of CHF (hazard ratio [HR] 2.98, 95% confidence interval [CI] 2.27–3.89), MI (HR 2.53, 95% CI 1.62–3.96), and stroke (HR 1.93, 95% CI 1.38–2.69), as compared with patients whose serum creatinine did not double. The relative risks of angina pectoris (HR 1.18, 95% CI 0.66–2.10) or a transient ischemic attack (HR 1.32, 95% CI 0.78–2.22) were similar in both groups.
Conclusion
Diabetic patients with a doubling of serum creatinine were at an increased risk of CHF, MI, or stroke, compared with diabetic patients whose serum creatinine did not double during follow-up.
Background
Chronic kidney disease (CKD) is characterized by changes in kidney structure and function, usually leading to a gradual and permanent loss of kidney function over time. In the UK, awareness for CKD increased in the British National Health Service as the disease was included in the “Quality and Outcomes Framework” in 2006.Citation1 The age-standardized prevalence of CKD stages 3–5 (moderate to severe) was 8.5% for the period 1998–2003.Citation2 Currently, it is estimated that some 13%–14% of people living in the UK have some degree of impaired kidney function, which is determined by the estimated glomerular filtration rate (eGFR).Citation3
Patients with CKD are at an increased risk of cardiovascular diseases and death.Citation4–Citation10 The risks of death or myocardial infarction (MI) have been associated with the extent of kidney impairment and with the level of proteinuria at a given eGFR level.Citation5,Citation11,Citation12 It has been reported that patients with an eGFR <60 mL/min per 1.73 m2 are more likely to die from cardiovascular causes than from progression to end-stage renal disease (ESRD).Citation6 Diabetes mellitus is a leading cause of decreased kidney function and itself a risk factor for cardiovascular outcomes.Citation13
Serum creatinine is a commonly used marker to estimate kidney function. It is used to calculate the eGFR and can easily be monitored.Citation14,Citation15 Doubling of serum creatinine (DSC) has been part of the renal composite end points in clinical nephrology trials since the beginning of the 1990s.Citation16–Citation18 It is assumed that substantial change in serum creatinine is a marker for declining renal function. The concentration of serum creatinine is, however, not only driven by kidney function but also by age, sex, race, and variations in muscle mass, leading to some controversy over the question of whether DSC can be used as a marker for ESRD in nephrology trials.Citation19,Citation20
The purpose of this large observational study was to analyze the association between DSC and the risk of cardiovascular events in patients with CKD and with diagnosed type 2 diabetes mellitus (T2DM), both overall and stratified by sex.
Methods
Data source
We conducted a follow-up study using data from the Clinical Practice Research Datalink (CPRD). The CPRD is a large UK-based database encompassing some eight million people who are enrolled with selected general practitioners (GPs), as described in detail elsewhere.Citation21–Citation23 GPs have been trained to record medical information in a standard manner and to supply it anonymously. The information recorded includes demographics such as sex and year of birth, the location of the general practice, medical diagnoses (based on “Read” codes), drug prescriptions, and a broad range of routine laboratory parameters. Hospital discharge and referral letters can be available for review to validate the diagnoses recorded in the computer record. The information on drug exposure and diagnoses in the CPRD has been validated and proven to be of high quality.Citation24,Citation25 For confidentiality reasons, the database is strictly anonymous. This study has been approved by Independent Scientific Advisory Committee for Medicines and Healthcare Products Regulatory Agency research.
Definition of the study population
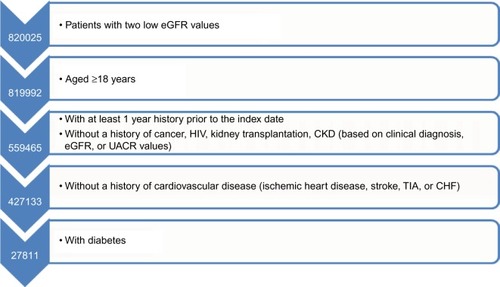
We identified all patients in the CPRD who had at least two eGFR values <90 mL/min per 1.73 m2, separated by at least 90, but no more than 730 days, within the study period which started on January 1, 2002. We calculated the eGFR using the “CDK-EPI Study Equation” using serum creatinine values.Citation15 The start of follow-up for our analyses, the start date, was the date of the first recorded eGFR value <90 mL/min per 1.73 m2 within the study period.
We excluded all patients with one or more of the following criteria at or prior to the start date: age below 18 years, a history of cancer (except nonmelanoma skin cancer) or HIV, a history of donating or receiving a kidney, (full or partial) loss of a kidney, prevalent CKD or ESRD. In addition, we excluded patients with two eGFR values <90 mL/min per 1.73 m2, or two urine albumin-to-creatinine ratio (UACR) values >30 mg/g separated by >90, but no more than 730 days prior to the study period to exclude patients with prevalent kidney disease. We required at least 1 year of previous history in the database prior to the first abnormal eGFR value to increase the likelihood of including patients with an incident kidney problem. In addition, we excluded all patients from the incident CKD population who did not have T2DM, defined by a specific T2DM Read code or an unspecific diabetes code, followed by treatment with oral antidiabetics and/or insulin. We excluded patients whose first insulin prescription was recorded below the age of 30 years in order to reduce the likelihood of including patients with type 1 diabetes mellitus. We also excluded all patients with a history of ischemic heart disease, stroke, transient ischemic attack (TIA), or congestive heart failure (CHF) prior to the start date, as we were only interested in identifying incident cardiovascular outcomes during follow-up ().
Analysis
We investigated the association between DSC and the risk of developing a first diagnosis of angina pectoris, CHF, MI, ischemic or unspecified stroke, or TIA in exposed and unexposed person time using time-dependent Cox proportional regression models. Exposure was classified from the first serum creatinine value within the study period (eGFR values <90 mL/min per 1.73 m2) until the end of follow-up (a study event occurred, the patient died, transferred out of the practice, or the end of data collection whichever came first). Patients were considered nonexposed, that is, without a DSC (the reference group) from the initial creatinine value until they had a recorded serum creatinine value that was at least twice as high as the serum creatinine value at start of follow-up. Once a doubling occurred, patient time was accumulated as exposed time until the end of follow-up. We used Cox proportional regression to calculate crude and adjusted hazard ratios (HRs). The adjusted analyses were adjusted for age (18–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80+ years), sex, body mass index (<18.5, 18.5–24.9, 25.0–29.9, 30+ kg/m2), and smoking status (nonsmoking, ex-smoking, current smoking, unknown), use of angiotensin-converting enzyme (ACE)-inhibitors, angiotensin II receptor blockers, calcium channel blocker, beta-blocker, diuretics, or aldosterone antagonists. All covariates were measured at or prior to the start date.
We performed two sensitivity analyses for the outcomes MI and stroke, in which we restricted the analyses to cases who not only had a recorded Read code for the disease, but who had further evidence in the medical record supporting the diagnosis. For cases with MI, this was a recorded hospitalization or a referral to specialist care, death from MI, or initiation of a new treatment regimen with statins, beta-blockers, ACE-inhibitors, or antiplatelet therapy within 90 days around the MI diagnosis date. We included stroke patients if they were hospitalized or referred to specialist care, if they died, or if they started a new treatment regimen with statins, antiplatelet therapy, or fibrinolytic therapy within 90 days around the stroke diagnosis date. We further conducted a sensitivity analysis restricted to patients whose first eGFR value was below 60 mL/min per 1.73 m2. The statistical analyses were conducted using the software program SAS Version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
We identified 27,811 patients who met our inclusion criteria. Of those, 21,215 had a follow-up time of at least 3 years after the abnormal eGFR value. The mean age of the study population at baseline was 65 (±10) years, and 55% were male. The majority of patients (87%) in the study population had an eGFR ≥60 mL/min per 1.73 m2 at baseline. UACR values were only recorded for 8,470 patients. The baseline characteristics of the study population are displayed in .
Table 1 Baseline characteristics of the study population by DSC status
During follow-up, DSC was observed in 1,262 patients. Overall, 1,069 patients developed CHF, 970 cases stroke, 693 cases angina pectoris, 578 cases had TIA, and 508 patients developed MI. Five percent (5.4%) of all patients with a DSC during follow-up had a CHF diagnosis compared with 3.8% of all patients without DSC during follow-up. An incident angina pectoris diagnosis was recorded for 1% of patients with DSC and for 2.6% without DSC, an incident MI occurred in 1.7% of patients with DSC and in 1.8% of patients with DSC, an incident stroke diagnosis was diagnosed in 3.2% of patients with DSC and in 3.5% without DSC, and 1.2% had a TIA diagnosis among those with DSC and 2.1% among those without DSC.
In the time-varying Cox regression analysis adjusted for age, body mass index, sex, and smoking status and use of ACE-inhibitors, angiotensin II receptor blockers, calcium channel blocker, beta-blockers, diuretics, or aldosterone antagonists at baseline, the risks of developing angina pectoris or TIA were similar for patients with DSC compared with patients without DSC, yielding HRs of 1.18 (95% confidence interval [CI] 0.66–2.10) and of 1.32 (95% CI 0.78–2.22), respectively. The adjusted HRs for CHF, MI, and stroke were 2.98 (95% CI 2.27–3.89), 2.53 (95% CI 1.62–3.96), and 1.93 (95% CI 1.38–2.69), respectively, for patients with or without DSC. When we stratified by sex, the relative risk (RR) of TIA was similar for males and females, while for all other outcomes the risk estimates differed to some degree. The most pronounced difference was observed for MI, with an HR of 3.32 (95% CI 1.85–5.93) for females compared with 1.84 (95% CI 0.90–3.76) in males ().
Table 2 Risk of cardiovascular outcomes in patients with DSC compared with patients without DSC
The sensitivity analyses for MI and stroke in which we restricted the analyses to cases with additional evidence supporting the diagnosis included 459 patients with MI and 763 patients with a stroke diagnosis. In this subgroup, the HR for MI was 2.68 (95% CI 1.67–4.29), again with a substantial difference between males (HR 1.94, 95% CI 0.90–4.18) and females (HR 3.46, 95% CI 1.89–6.33). The overall HR of stroke in this sensitivity analysis was 2.14 (95% CI 1.50–3.06). The HRs for males and females were 1.79 (95% CI 1.02–3.15) and 2.41 (95% CI 1.52–3.84), respectively. In a second sensitivity analysis, we restricted the study population to patients whose first eGFR was lower than 60 mL/min per 1.73 m2. As there were only 3,718 such patients in total, most risk estimates did not reach statistical significance. The results are displayed in .
Table 3 DSC status and risk of cardiovascular outcomes in patients with eGFR<60 mL/min per 1.73 m2
Discussion
DSC has been part of the renal composite end points in clinical nephrology trials since the beginning of the 1990s, as it is assumed that substantial changes in serum creatinine are a marker for declining renal function.Citation16–Citation18 Whether DSC is associated with adverse outcomes such as ESRD, death, or cardiovascular outcomes, and whether it can be used as a marker in clinical trials, is still a matter of controversy.
In this large observational study with patients with T2DM and an eGFR <90 mL/min per 1.73 m2, we observed an increased risk of developing CHF, MI, or stroke for patients with DSC compared with patients without DSC, while the risks of developing angina pectoris and TIA were similar in patients with or without DSC.
The risk of MI was increased in males and females with DSC compared with patients without DSC, while the risk of angina pectoris was only increased in males. Interestingly, the risk in females was almost twice as high as in males, although this difference was not statistically significant and was no longer present when we restricted the analysis to patients with a first eGFR <60 mL/min per 1.73 m2. This overall difference was mainly based on patients with an eGFR of 60–90 mL/min per 1.73 m2. The role of CKD stage has already been discussed in relation to the risk of developing coronary heart disease in previous studies.Citation5,Citation11 A cross-sectional study investigating the association between albuminuria and ischemic heart disease in patients with CKD and with diabetes mellitus reported an increased risk for males (RR 2.84, 95% CI 1.68–4.79), but not for females (RR 0.79, 95% CI 0.51–1.21).Citation26
The risk of stroke was also increased in males and females with DSC compared with patients without DSC. The risk of TIA was not statistically significantly different for patients with or without DSC in the overall population. There was, however, a trend in the sensitivity analysis restricted to patients with an eGFR <60 mL/min per 1.73 m2. A large meta-analysis of prospective studies analyzing the association between low GFR and the risk of stroke reported an increased pooled risk of stroke for patients with an eGFR <60 mL/min per 1.73 m2 of 1.43 (95% CI 1.31–1.57), but not for patients with an eGFR of 60–90 mL/min per 1.73 m2 (RR 1.07, 95% CI 0.98–1.17). The RRs reported in the individual studies included in this meta-analysis varied between 0.85 (95% CI 0.60–1.20) and 3.10 (95% CI 1.80–5.35) for patients with an eGFR <60 mL/min per 1.73 m2 and between 0.74 (95% CI 0.57–0.97) and 1.77 (95% CI 0.98–1.17) for patients with an eGFR of 60–90 mL/min per 1.73 m2.Citation8
The risk of CHF was increased both in males and females with DSC compared with patients without DSC. There was no increased risk when we restricted the analysis to patients with a first eGFR <60 mL/min per 1.73 m2. Thus, the overall increased risk was mainly caused by differences in patients with an eGFR of 60–90 mL/min per 1.73 m2. In previous studies, high creatinine levels as well as renal impairment based on eGFR levels have been associated with an increased risk of heart failure.Citation27,Citation28 In a recent review article, it was further noted that CHF mortality increased in patients with renal impairment, independent from the stage of kidney disease.Citation29
As stated earlier, low eGFR is an independent risk factor for cardiovascular diseases in CKD patients. In patients with renal transplants, it has been shown that graft loss was differentially associated with DSC depending on the starting eGFR.Citation30 Therefore, we cannot rule out that the reported differences were influenced by differences in the reference eGFR values, as patients with DSC were more likely to have lower eGFR values at the start than patients without DSC. The impact of DSC might also depend on whether the patient had an almost normal renal function at the start of follow-up or whether they started with moderate-to-severe impairment of renal function. To assess this, we conducted a sensitivity analysis restricted to patients with an eGFR <60 mL/min per 1.73 m2 at start of follow-up. Go et alCitation4 showed that there was a gradual association between different eGFR strata (<15, 15–29, 30–44, 45–59) and the risk of cardiovascular events in patients with an eGFR <60 mL/min per 1.73 m2. Due to the small number of cases in the sensitivity analysis, we however decided not to further restrict the study population. Not only the level of eGFR is important, but also the amount of proteinuria at a given eGFR level has been associated with cardiovascular outcomes and death.Citation11,Citation31 We were not in a position to adjust for UACR because we had only very limited information; among the 27,811 patients in our study only 8,470 had an UACR value recorded. Information on proteinuria or microalbuminuria might be under-ascertained as results from dipstick tests might be less present in the patient’s record compared with lab analyses performed in the GPs’ office.
An additional limitation of this study is that we did not control for duration of diabetes in the analysis, but diabetes mellitus is itself a risk factor for cardiovascular diseases. We however controlled for hypertension and weight, which are important comorbidities in diabetic patients and also risk factors for cardiovascular disease. We decided to control for hypertension by adjusting for use of different classes of antihypertensive medication because ACE-inhibitors and angiotensin II receptor blockers can have direct effects on proteinuria and on eGFR via the renin–angiotensin–aldosterone system. We further cannot assess whether certain differences observed for males and females are based on differences in renal function or on factors associated with severity or duration of T2DM. It is interesting to note that the majority of patients whose first eGFR was <60 mL/min per 1.73 m2 were females. Whether these females were diagnosed at a later stage, or whether males and females experience clinical adverse effects associated with reduced kidney function at different eGFR levels, can also not be answered with this study.
Our results are based on data from the primary care setting; there is a possibility that we may have missed some cases with cardiovascular outcomes, particularly milder forms of the outcomes of interest. However, as most cardiovascular outcomes in this study represent acute and rather severe diseases, we are confident that few cases with an outcome of interest have been missed. The definition of cardiovascular outcomes was based on Read code, and we know from previous studies that diagnoses in the CPRD are of high quality.Citation25,Citation32,Citation33 In a sensitivity analysis, we searched for further evidence such as newly initiated medication or recorded hospitalizations to validate the MI and stroke diagnoses. In this validation process, we excluded a certain proportion of patients, but the results were similar to the analyses in which we included all potential cases. We restricted the study population to patients 30 years or older with oral antidiabetics and/or insulin-treated diabetes because we were interested in the association between DSC and cardiovascular outcomes in type 2 diabetics. We can, however, not fully exclude that there are nonetheless a few patients with type 1 diabetes in the study population.
The eGFR does vary by race. Information on race is, however, not reliably available in the CPRD, so we could not adjust our eGFR rates for race; we estimated the GFR only with the “white or other” part of the CKD-EPI formula. Most people in the UK are White, so this should not be a material problem in this study.
Our observations and our risk estimates are based on repeated eGFR measurements to identify patients with CKD in 2002–2011. In the twenty-first century, CKD became a disease of interest in the British National Health Service, and GPs were asked to start recording diagnosis and treatment of CKD in more detail. This led to a more comprehensive monitoring of kidney function, which may explain the large number of patients with early stages of CKD.
In summary, in this study including patients with T2DM and an eGFR <90 mL/min per 1.73 m2, the risks of developing an incident diagnosis of CHF, MI, or stroke were higher in patients who had DSC compared with those without DSC. This association might, however, be influenced by the level of renal impairment used as reference value, that is, the baseline creatinine value, at start of follow-up. The presented results need to be interpreted in the context of the above-mentioned limitations.
Author contributions
CS, BC, SSJ, CRM, study concept and design and acquisition, analysis, or interpretation of data; CS, drafting of the manuscript; BC, SSJ, CRM, critical revision of the manuscript for important intellectual content; CS, CRM, statistical analysis; CS, administrative, technical, or material support; CRM, obtained funding and study supervision. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The study was funded by an unconditional grant from AbbVie, North Chicago, IL, USA. Technical support and programming: Pascal Egger (Basel Pharmacoepidemiology Unit, Division of Clinical Pharmacy and Epidemiology, Department of Pharmaceutical Sciences, University of Basel, Basel, Switzerland). The sponsor participated in the design of the study, interpretation of the data, review, and approval of the manuscript.
Disclosure
BC is employed by AbbVie. The authors report no other conflicts of interest in this work.
References
- WiseLHIrvineDSakaguchiMFuscoGImpact of the quality outcomes framework on chronic kidney disease coding in GPRDPharmacoepidemiol Drug Saf201120SupplS323S323
- StevensPEO’DonoghueDJde LusignanSChronic kidney disease management in the United Kingdom: NEOERICA project resultsKidney Int2007721929917440495
- RoderickPRothMMindellJPrevalence of chronic kidney disease in England: findings form the 2009 health survey for EnglandJ Epidemiol Community Health201165A1A40
- GoASChertowGMFanDMcCullochCEHsuCYChronic kidney disease and the risks of death, cardiovascular events, and hospitalizationN Engl J Med2004351131296130515385656
- Di AngelantonioEChowdhuryRSarwarNAspelundTDaneshJGudnasonVChronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort studyBMJ2010341c498620884698
- SchiffrinELLipmanMLMannJFChronic kidney disease: effects on the cardiovascular systemCirculation20071161859717606856
- GansevoortRTCorrea-RotterRHemmelgarnBRChronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and preventionLancet382988933935223727170
- LeeMSaverJLChangK-HLiaoH-WChangS-COvbiageleBLow glomerular filtration rate and risk of stroke: meta-analysisBMJ2010341c424920884696
- SarnakMJLeveyASSchoolwerthACKidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and PreventionHypertension20034251050106514604997
- CoreshJTurinTCMatsushitaKDecline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortalityJAMA2014311242518253124892770
- HemmelgarnBRMannsBJLloydARelation between kidney function, proteinuria, and adverse outcomesJAMA2010303542342920124537
- Chronic Kidney Disease Prognosis ConsortiumMatsushitaKvan der VeldeMAssociation of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysisLancet201037597312073208120483451
- JhaVGarcia-GarciaGIsekiKChronic kidney disease: global dimension and perspectivesLancet2013382988826027223727169
- LeveyASBoschJPLewisJBGreeneTRogersNRothDA more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study groupAnn Intern Med1999130646147010075613
- LeveyASStevensLASchmidCHA new equation to estimate glomerular filtration rateAnn Intern Med2009150960461219414839
- LewisEJHunsickerLGBainRPRohdeRDThe effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study GroupN Engl J Med199332920145614628413456
- MannJFESchmiederREMcQueenMRenal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trialLancet2008372963854755318707986
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathyLancet19973499069185718639217756
- Lambers HeerspinkHJPerkovicVde ZeeuwDIs doubling of serum creatinine a valid clinical ‘hard’ endpoint in clinical nephrology trials?Nephron Clin Pract20111193c195199 discussion c19921832844
- TakeuchiNTakenoshitaEKatoFDoubling of serum creatinine: is it appropriate as the endpoint for CKD? Proposal of a new surrogate endpoint based on the reciprocal of serum creatinineClin Exp Nephrol201115110010721058043
- JickHA database worth savingLancet199735090841045104610213544
- WoodLMartinezCThe general practice research database: role in pharmacovigilanceDrug Saf2004271287188115366975
- LawsonDHShermanVHollowellJThe general practice research database. scientific and ethical advisory groupQJM19989164454529709463
- JickHJickSSDerbyLEValidation of information recorded on general practitioner based computerised data resource in the United KingdomBMJ199130267797667682021768
- HerrettEThomasSLSchoonenWMSmeethLHallAJValidation and validity of diagnoses in the General Practice Research Database: a systematic reviewBr J Clin Pharmacol201069141420078607
- NakhjavaniMMortezaAJenabYGender difference in albuminuria and ischemic heart disease in type 2 diabetesClin Med Res2012102515622031479
- DhingraRGazianoJMDjousseLChronic kidney disease and the risk of heart failure in menCirc Heart Fail20114213814421216838
- ChaeCUAlbertCMGlynnRJGuralnikJMCurhanGCMild renal insufficiency and risk of congestive heart failure in men and women > or =70 years of ageAm J Cardiol200392668268612972106
- IyngkaranPThomasMMajoniWAnavekarNSRoncoCComorbid heart failure and renal impairment: epidemiology and managementCardiorenal Med20122428129723381594
- GataultPAl-NajjarABarbetCDoubling of serum creatinine in clinical trials, cost-effectiveness studies, and individual patients: adequate use in renal transplantationTransplantation20119291012101721941225
- SchmiederREMannJFESchumacherHChanges in albuminuria predict mortality and morbidity in patients with vascular diseaseJ Am Soc Nephrol20112271353136421719791
- HerrettEShahADBoggonRCompleteness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort studyBMJ2013346
- GullifordMCCharltonJAshworthMRuddAGToschkeAMeCRT Research TeamSelection of medical diagnostic codes for analysis of electronic patient records. Application to stroke in a primary care databasePLoS One200949e716819777060