Abstract
Background and purpose
Lower-extremity amputations (LEAs) in people with diabetes are associated with reduced quality of life and increased health care costs. Detailed knowledge on amputation rates (ARs) is of utmost importance for future health care and economics strategies. We conducted the present cohort study in order to estimate the incidences of LEA as well as relative and attributable risk due to diabetes and to investigate time trends for the period 2008–2012.
Methods
On the basis of the administrative data from three large branches of German statutory health insurers, covering ~34 million insured people nationwide (about 40% of the German population), we estimated age-sex-standardized AR (first amputation per year) in the populations with and without diabetes for any, major, and minor LEAs. Time trends were analyzed using Poisson regression.
Results
A total of 108,208 individuals (diabetes: 67.3%; mean age 72.6 years) had at least one amputation. Among people with diabetes, we observed a significant reduction in major and minor ARs during 2008–2012 from 81.2 (95% CI 77.5–84.9) to 58.4 (55.0–61.7), and from 206.1 (197.3–214.8) to 177.0 (169.7–184.4) per 100,000 person-years, respectively. Among people without diabetes, the major AR decreased significantly from 14.3 (13.9–14.8) to 11.6 ([11.2–12.0], 12.0), whereas the minor AR increased from 15.8 (15.3–16.3) to 17.0 (16.5–17.5) per 100,000 person-years. The relative risk (RR) comparing the diabetic with the nondiabetic populations decreased significantly for both major and minor LEAs (4% and 5% annual reduction, respectively).
Conclusion
In this large nationwide population, we still found higher major and minor ARs among people with diabetes compared with those without diabetes. However, AR and RR of major and minor LEAs in the diabetic compared with the nondiabetic population decreased significantly during the study period, confirming a positive trend that has been observed in smaller and regional studies in recent years.
Introduction
The global prevalence of diabetes mellitus rose to 8.8% in 2015, which corresponds to 415 million people worldwide.Citation1 Consequently, the number of people with diabetic complications, including foot disease, has increased. Epidemiological studies have shown that up to 75% of lower-extremity amputations (LEAs) are performed in patients with diabetes.Citation2–Citation4 Since LEA reduces quality of lifeCitation5 and increases medical costs,Citation6 various initiatives have been conceived to reduce the number of LEAs among people with diabetes.Citation7,Citation8 In order to improve the quality of medical care among people with diabetes in Germany, disease management programs were introduced for type 2 diabetes in 2003 and for type 1 diabetes in 2005,Citation9 followed by a national guideline for the prevention and treatment of diabetic foot complications.Citation10
Previous studies showed a large variation in incidence rates of LEA and relative risk (RR) among people with and without diabetes, as well as inconsistent results with respect to time trends.Citation2,Citation4,Citation11,Citation12 The German Leverkusen Amputation Reduction Study (LARS)Citation2 began in the early 1990s and covered the population of the city of Leverkusen. It revealed a reduced incidence of LEA in the diabetic population between 1990 and 2005, but an unchanged incidence rate in the nondiabetic population. In the 1990s, the RR comparing people with and without diabetes in the LARS was 26 (95% CI 17–39).Citation13 A more recent study conducted in 2005–2007 using data from one statutory health insurance (SHI) found a considerably lower RR of 7.4 (95% CI 6.3–8.7).Citation3 However, nationwide data on incidence, RRs, and time trends of LEA in the diabetic and nondiabetic populations are lacking to date. Hence, the aim of this study was 1) to estimate the amputation rate (AR) of LEA stratified by amputation level in the population with and without diabetes as well as relative and attributable risk due to diabetes and 2) to investigate time trends for the period 2008–2012.
Methods
Study population and data assessment
We used anonymized nationwide pooled data from three German branches of SHI companies: Allgemeine Ortskran-kenkasse (AOK) (68% of the study population), BARMER Ersatzkasse (25%), and Betriebskrankenkasse (7%), covering ~34 million inhabitants (ie, 42% of the German population). We included data from all individuals who were continuously insured (ie, ≤30 days gap in pairwise subsequent quarters by one of these SHI within the period January 1, 2007, to December 31, 2012) for at least 1 year, which was necessary to define the diabetes status of the insured individuals. Hence, individuals whose insurance period started later (eg, May 1, 2010) and fulfilled the above-mentioned inclusion criteria were also considered. Since diabetes mellitus not only is the leading cause of LEA due to diabetic neuropathy and arterial vascular disease but also contributes to the development of traumatic and cancer-related amputations.Citation14 We have taken into account all amputations between 2008 and 2012 irrespective of the potential causes of LEA, in line with Fosse et al.Citation14 In addition, insurance data from 2007 were used to ensure insurance periods of at least 1 year per person for insured individuals in 2008.
According to an established algorithm,Citation15 a “subject” was classified as having diabetes if at least one of the following criteria was met: 1) diagnosis of diabetes (ICD E10-E14) in at least three out of four consecutive quarters; 2) at least two prescriptions of antihyperglycemic medications (ATC code A10) within 1 year; or 3) at least one diagnosis of diabetes and prescription of an antidiabetic medication, one measurement of HbA1c, or blood glucose in the same quarter (to avoid false-positive cases due to data errors). We also included patients with new-onset diabetes to meet the criteria during the entire observation period. A subject was classified as an “individual with diabetes” from the first quarter wherein the condition was fulfilled, and retained this status throughout the study.
Antihyperglycemic medication was assessed during the 6 months before a first amputation since 2008 (available only for patients with amputations).
We assessed LEA in 2008–2012 using the specific operation procedure codes (OPS) of the hospital discharge documentation.Citation3,Citation16 We differentiated between major (OPS 5-864.x, 5-869.0: any LEA above the ankle joint) and minor amputation (OPS 5-865.x: through or distal to the ankle joint).Citation11,Citation12,Citation17
Neither individual written consent by patients nor ethical approval was required as the data were anonymous and no link to primary data was intended.Citation18
Statistical analysis
The main analyses were conducted for the entire population as well as sex-specifically for any LEAs as well as separately for major and minor amputations.
We estimated AR for each calendar year as follows: the number of any first LEA per individual occurring within this year – as numerator – was divided by the cumulative person-years (PY) at risk, from all insurance quarters of all insured individuals in the respective year – as denominator. Likewise, major and minor ARs were computed counting only first major and first minor amputations occurring within that year. The first major amputation occurring after a first minor amputation within each calendar year (which occurred frequently) was also counted for the analysis of major amputations.
We calculated direct age-sex-standardized AR for the entire population and age-standardized AR for sex-specific analysis using age strata 0–39, 40–49, 50–59, 60–69, 70–79, 80+ years, and the German population in 2010 as standard population. We estimated AR in the total population (ARt), among individuals with diabetes in the population with diabetes (ARd), and in subjects without diabetes in the population without diabetes (ARn). We calculated amputation rate ratios (RR) by dividing the standardized AR of the populations with and without diabetes. Furthermore, we computed attributable risk among the population with diabetes (ARE) and population attributable risk due to diabetes (PAR) along with 95% CI in order to describe what proportion of amputations could theoretically be avoided if the exposure (ie, diabetes) was omitted.Citation19
To test for time trend, we fitted Poisson regression models, since our outcome variable AR is a counting variable per PY, using year of amputation (difference from 2008) to estimate the effect of calendar time. All models were adjusted for over-dispersion, with Pearson scale as correction factor for the total population. The choice of this regression model was justified by the application of goodness-of-fit tests, which showed the best fit for this approach.Citation20 We further adjusted all models for age, sex, and diabetes status as independent variables. Besides this, we stratified all models by diabetes status and sex. In addition, we performed models including interaction between diabetes and year of amputation, age, and sex.
Data were evaluated using the Statistical Analysis System SAS (SAS for Windows 7, Release 9.4 TS1M1; SAS Institute Inc., Cary, NC, USA).
Results
Study population
The description of all insured individuals is shown in . In the period 2008–2012, ~34 million people received SHI continuously from three insurers (2008: 32,916,390 PY, 2012: 34,136,653 PY). Approximately 11% (2008: 10.6%, 2012: 11.9%) of all insured individuals were classified as patients with diabetes with similar numbers among both sexes, with a proportion of insured men of about 46% (2008: 45.4%, 2012: 46.1%).
Table 1 Description of all statutory health insurants with amputationTable Footnotea and the background population in Germany, 2008–2012
In total, 108,208 individuals (66,721 men, 41,487 women) underwent at least one amputation between 2008 and 2012, of whom 77,929 (50,298 men, 27,631 women) underwent at least one minor amputation and 45,414 (26,583 men, 18,831 women) at least one major amputation. The minor-to-major ratio increased from 1.64 in 2008 to 2.13 in 2012 with considerably higher ratios among the men.
Mean age at the time of first any amputation (N=108,208) between 2008 and 2012 was 72.6 years (SD 12.8), with women being considerably older (77.2 years, SD 12.6) than men (69.7 years, SD 12.1). The age at amputation remained nearly stable among both sexes. About two thirds (N=72,782) of all persons with amputation had diabetes (men: 68.9%, women: 64.6%). This proportion remained nearly stable between 2008 and 2012. Among people with diabetes and amputation, insulin therapy was recorded by 40%, oral therapy by 23.0%, and a combination of insulin and oral treatment therapy by 15.9%. Accordingly, 21.5% of these patients had no prescription of any antihyperglycemic medication. This distribution was similar in men and women.
AR, relative and attributable risk
Age- and age-sex-standardized AR as well as RR, ARE, and PAR for each calendar year are shown in and . We observed a consistent reduction in the AR among the population with diabetes over time: 258.6 (95% CI 249.4–267.7) per 100,000 PY in 2008, and 216.2 (208.4–224.1) in 2012. In contrast, the AR in the population without diabetes slightly decreased: 27.9 (27.3–28.5) per 100,000 PY to 26.6 (26.0–27.2). Thus, the RR decreased considerably from 9.3 (8.9–9.7) in 2008 to 8.1 (7.8–8.5) in 2012. Likewise, the ARE decreased from 89.2% (88.8%–89.7%) to 87.7% (87.2%–88.2%), whereas the PAR remained nearly stable at 58%.
Figure 1 Time trend of age-sex-standardized amputation rate of (A) any amputation, (B) major amputation, and (C) minor amputation.
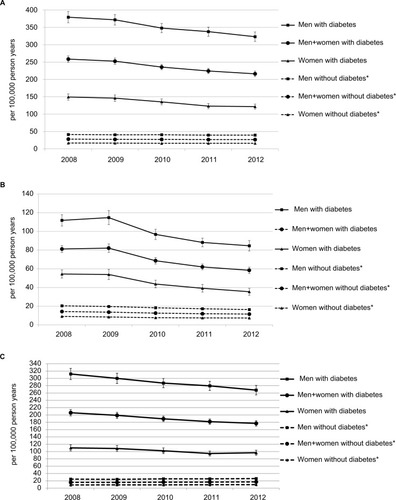
Table 2 Amputation rate among people with and without diabetes in Germany, 2008–2012
The age-standardized AR was two- to threefold higher among men compared with women, which was true for individuals with and without diabetes. With regard to time trend, RR, ARE, and PAR, we observed similar results in both sexes.
The age-sex-standardized major AR decreased strongly in the diabetic population, with a major AR of 81.2 (77.5–84.9) per 100,000 PY in 2008 and 58.4 (55.0–61.7) in 2012. This was also evident in the nondiabetic population, decreasing from 14.3 (13.9–14.8) in 2008 to 11.6 (11.2–12.0) per 100,000 PY in 2012. The RR decreased from 5.7 (5.4–6.0) in 2008 to 5.1 (4.7–5.4) in 2012. A similar pattern was observed for both attributable risks, decreasing from 82% to 80% among the population with diabetes and 48% to 46% among the total population.
We observed more than double the age-standardized major AR in men compared with women in both diabetic and nondiabetic populations, with similar results regarding time trends. The decrease in RR, ARE, and PAR was only prominent among women.
The age-sex-standardized minor AR between 2008 and 2012 in the population with diabetes decreased considerably from 206.1 (197.3–214.8) per 100,000 PY to 177.0 (169.7–184.4), while among the population without diabetes the minor AR increased from 15.8 (15.3–16.3) to 17.0 (16.5–17.5) per 100,000 PY. Hence, we observed a strong reduction in the RR from 13.1 (12.4–13.8) in 2008 to 10.4 (9.9–11.0) in 2012. Likewise, we detected a moderate reduction in ARE (92% to 90%) and PAR (66% to 64%) for the period between 2008 and 2012.
The age-standardized minor AR was about threefold higher in men compared with women, with nearly identical results regarding time trends, RR and ARE. Only PAR was significantly higher among men compared with women ().
Analysis of time trend and other covariates
The results of the fully adjusted Poisson models for the investigation of time trends are presented in . We observed a significant reduction in any AR of 4% per year in the population with diabetes, which was stronger among women compared with men: RR per calendar year was 6% and 3%, respectively. In contrast, among the population without diabetes, this rate remained nearly constant without gender differences (). In the Poisson model, which additionally included interaction terms with diabetes, we observed a significant reduction of 3% per year in RR between the populations with and without diabetes (RR per calendar year 0.97; 95% CI 0.95–0.99) without gender differences. With regard to the whole study period, the RR between the populations with and without diabetes strongly decreased with increasing age (RR 80+ years vs 0–39 years: 0.11; 95% CI 0.08–0.13), with comparable results in both sexes (data not shown).
Table 3 Relative risk (RR) of lower-extremity amputation in people with diabetes, compared with those without diabetes, adjusted for age, sex, and calendar year: results of the Poisson models
When repeating this analysis for major amputation, we observed a substantially stronger significant reduction in the major AR of 9% per year in the population with diabetes with a somewhat stronger decline among women compared with men (11% and 8%, respectively). Likewise, we observed a significant, albeit weaker, reduction in this rate of 6% per year among the population without diabetes, with comparable results in both sexes (). In the model including interaction terms, we observed a significant reduction of 4% per year in RR comparing the populations with and without diabetes (RR per calendar year 0.96; 95% CI 0.94–0.98), which was comparable in both sexes (data not shown).
Regarding the minor AR among people with diabetes, we observed a significantly reduced time trend – by 3% per year with quite similar results in both sexes. In contrast, there was a moderate but significant increase in this rate (2% per year) in the population without diabetes, with similar results across sexes (). Again, we observed a significant reduction of 5% per year in the RR between the populations with and without diabetes (RR per calendar year 0.95; 95% CI 0.94–0.97) when estimated using interaction terms with similar results across sexes.
Discussion
This nationwide study covering >40% of the German population presents the ARs and RRs of LEA in the populations with and without diabetes between 2008 and 2012. The ARs were considerably higher among the population with diabetes compared with the population without diabetes, with particularly high RRs for minor amputation. The major ARs markedly decreased in both populations. In contrast, minor ARs decreased only in the population with diabetes, and even slightly increased in the population without diabetes. RRs between the populations with and without diabetes decreased significantly during the study period for both major and minor LEA. ARs increased strongly with age and were two- to threefold higher in men than in women.
Methodological discrepancies in the study design make any comparison between studies extremely difficult.Citation21 Some studies analyzed any LEA,Citation14,Citation22,Citation23 while another reported the incidence of LEA stratified by anatomical level.Citation4,Citation11,Citation13 Moreover, studies that counted only one LEA per personCitation3,Citation4,Citation14,Citation17 cannot be compared with studies that counted several LEAs per person.Citation11,Citation12 Therefore, some important criteria regarding the interpretation of the study results should be taken into account: anatomic definition of LEA (major/minor LEA); cause of LEA (if traumatic and/or cancer-related, LEA were included or excluded); recording of LEA (one or more LEAs per person were analyzed); and characteristics of the study population and population at risk. In epidemiological studies, often the incidence rate of the first amputation is counted (first lifetime amputation or first amputation during the study period or at least the first amputation of the year) to avoid bias when predictors or time trends are analyzed. Amputations in a person are not independent events; it is well known that the first amputation is a significant predictor of subsequent amputations.Citation24 In this study, we counted the first amputation per person per calendar year, which means that a re-amputation occurring in a subsequent year would also be counted. For this reason, the AR is higher compared with studies that counted only first lifetime LEAs or first LEAs during the study period. Furthermore, we recorded all LEAs regardless of their cause. We were able to define the at-risk diabetic population completely, including people without anti-hyperglycemic medication, and took into account those with new-onset diabetes during the study period.
We found a reduction of 4% per year in the AR in the population with diabetes, with a stronger decline among women. In the nondiabetic population, AR remained almost constant, without significant gender differences. The RR of any LEA between the populations with and without diabetes during the study period decreased substantially, reaching 8.1 in 2012. The observed effect could be explained principally by the reduction in the absolute number of amputations and major AR among people with diabetes (see below).
Our results indicate a further reduction in the incidence of LEA among people with diabetes and in the relative LEA risk that were described previously in the LARS.Citation2 However, our findings regarding AR are somewhat higher compared with the 2005–2007 German study based on SHI data (Gmünder Ersatzkasse [GEK]).Citation3 The main reason for this is that while we counted one LEA per calendar year, the earlier studies counted only one per person, which means that the figures are not comparable. Furthermore, GEK-insured individuals had lower prevalence of diabetes than those from AOK,Citation25 which was the source of some two thirds of the study population. Moreover, the relatively lower number of insured individuals (1.6 million members) and corresponding lower absolute number of LEAs in the previous studies should be taken into account by the comparison. Our results with respect to AR in the diabetic as well as the nondiabetic populations are higher compared with other European countries,Citation4,Citation14 possibly due to a differ ent definition of AR and some older populations with very low socioeconomic status in our study. However, the RRs from our study are somewhat lower in the international comparison with studies from Italy: RR for the entire study period 2001–2010 was 10.95 (95% CI 9.37–12.8)Citation4 and from France: RR in 2003 was 11.8 (95% CI 11.0–12.6).Citation14
Major amputations are performed in cases of excessive tissue loss or sepsisCitation26 or if there are no further surgical or endovascular options for revascularization. They reduce the quality of life considerably and entail high mortality.Citation27,Citation28
In the present study, we observed a considerable reduction of 9% per year in major AR in the population with diabetes between 2008 and 2012, with an even greater reduction among women. This effect may be explained by improvements in the organization of diabetes foot care (diabetes management programs, national guidelines for prevention and treatment strategies for foot complications, nationwide establishment of certified diabetic foot clinics, and constitution of regional networksCitation29). In the nondiabetic population, major AR likewise decreased by 6% per year in both sexes. Also, increased numbers of surgical and endovascular revascularizations may explain in part the positive time trend in the populations with and without diabetes.Citation30,Citation31
The positive time trend toward a significant reduction in major AR among people with diabetes observed in our nationwide study is in line with other epidemiologic studies.Citation2,Citation4,Citation17,Citation32–Citation34 Likewise, the RRs of major LEA, comparing people with and without diabetes, are comparable with the results from an earlier German studyCitation3 and were lower compared with data from Italy (RR 6.36; 95% CI 5.6–7.23)Citation4 and Finland (RR 7.4; 95% CI 7.2–7.7).Citation17 Furthermore, we observed a significant reduction in RRs during the study period, which was also found in the studies by Trautner et alCitation2 and Ikonen et al,Citation17 but not in the study by Lombardo et al.Citation4
Minor amputations are performed in order to remove necrotic tissues, prevent infections, create wounds that can heal under the conditions of modern wound management and offloading, and thus preserve as much of the foot as possible. They allow the person to ambulate with no need for prosthesis and do not affect health-related quality of life any more than conservative treatment.
In the present study, we found a reduction of 3% per year in minor AR among people with diabetes of both sexes, while among people without diabetes, the AR increased slightly – by 2% per year. Comparisons between our results and the findings from other studies were limited because only a few studies have analyzed minor AR considering one LEA per person, and, as mentioned above, study designs are hardly comparable.Citation4,Citation32,Citation35 The Italian study by Lombardo et alCitation4 showed an unchanged minor AR in the population with diabetes and a significant increase in minor LEA incidence in the popula tion without diabetes; whereas a Spanish studyCitation32 described a reduction in one minor LEA among people with diabetes. A study from Denmark showed a significant annual reduction of 9.8% in the population with diabetes and unchanged incidence rates in the population without diabetes during 1996–2011.Citation34
Gender differences in minor LEAs were markedly pronounced: the risk among men for undergoing minor LEA was 2.7 times higher compared with women. This finding coincides with the results of other studies, which, however, were not able to fully elucidate the underlying reasons. Biologic factors seem to contribute to gender differences,Citation36,Citation37 while health care-related and behavioral factors do not.Citation38,Citation39
In the present study, we observed a reduction in the RRs for minor LEA between the populations with and without diabetes during the study period that was quite similar across both sexes. Compared with the RRs found in a study from Italy, the RRs calculated in the Poisson model from our study were lower (RR 9.33 (95% CI: 8.96–9.71) vs 19.37 (16.49–22.7).Citation4 Compared with RRs reported in a study from Denmark, those comparisons were different for men and women: the RR in our study was also lower for men (RR men 10.14 (95% CI 9.67–10.63) vs 14.7 (10.5–20.4), but well in line among women: RR 7.79 (7.34–8.28) vs 7.5 (5.2–10.9).Citation34
Some limitations of this study should be considered. First, the study population included only people with SHI, so the privately insured (11% of the German populationCitation40), which is somewhat younger and healthier than the SHI population,Citation41 could not be considered. Furthermore, persons insured by the included SHIs may differ from those of other companies.Citation41 Second, regarding selection of the person-time of all insured individuals, we used an algorithm that excluded individuals with gaps in SHI of more than 30 days in two consecutive quarters. A previous study demonstrated that people who change their insurance company are younger, better educated, and have a lower prevalence of diabetes and cardiovascular disease.Citation42 As a consequence, our study population was somewhat older than the general German population, which was particularly true for women (proportion in 2010: 5.4% women over 80 years in our data set vs 3.5% women over 80 years in the whole of Germany; 2.1% men over 80 years in our data set vs 1.7% men over 80 years in the whole of Germany). However, the prevalence of diabetes in our study was well in line with recently published nationwide data.Citation43,Citation44 Third, there may be some misclassification concerning the “population at risk,” referring to the population with diabetes. This may be due to undiagnosed diabetes or due to prescription of antihyperglycemic drugs for people with impaired glucose regulation. However, we assume that the latter in particular should be rare. Moreover, in our study, based on the administrative data for the identification of people with diabetes, we have used one established algorithm, which has already been applied successfully by other studies.Citation15,Citation45 Furthermore, within our study, the characteristics of the population are similar to other German data, for example, the relation between insured persons with a diagnosis of diabetes with and without pharmacotherapy is in line with other studies from Germany.Citation44,Citation46–Citation48 Fourth, unfortunately, with our data set, we were not able to estimate the AR of LEA stratified by type 1 and type 2 diabetes. Fifth, only the first amputation per patient and year, and not the all-time first amputation per patient, could be analyzed since we were not able to overlook whether amputations occurred before 2008, in order to estimate the first ever amputation. This might create a bias in the incidence estimations between the populations with and without diabetes. Sixth, adjustment for comorbidity is an important prognostic factor in risk adjustment modeling for different outcomes.Citation49 However, in our study, due to data protection requirements during the process of data extraction, the final study data set lacks related information on individual comorbidities. Thus, we could not adjust our estimates for further potential confounders such as coronary heart disease, cerebrovascular disease, or peripheral arterial disease. These diseases are well-known risk factors for a high incidence of LEA.Citation50–Citation53 The lifetime prevalence of coronary heart disease and stroke among adults aged 40–79 years was 9.3% and 2.9%, respectively, according to the nationwide German Health Interview and Examination Survey for Adults (DEGS1) in 2008–2011.Citation51,Citation53 However, no significant change over time between 1998 and 2010 of the prevalence of these cardiovascular diseases was observed,Citation51,Citation53 potentially indicating that these comorbidities have no impact on the time trend of LEA. Further important factors that could also influence the AR, such as lifestyle variables, are not available in SHI data. However, looking for possible explanatory factors was not the aim of our study. Our aim was to describe the LEA risk and RR according to the St Vincent goals using a data source that enables us to analyze more than 30 million people in Germany, which is in line with several large epidemiological studies.Citation4,Citation14,Citation22 Finally, there were strong multiple interactions between diabetes, age, and sex, which might provoke biased results in stratified Poisson models. However, the results of the latter with regard to time trend were largely in line with those based on age-sex-standardized AR.
This study has a number of strengths. First, this is of the largest studies to cover more than 40% of the German population. Second, we defined the population with diabetes using a uniform algorithm, including people who are not receiving antihyperglycemic treatment.Citation15 This is a compelling prerequisite for a valid estimation of AR in the populations with and without diabetes. Other studies that did not use this tool had to use estimated diabetes prevalence.Citation2,Citation12,Citation32 Third, our study population is a dynamic cohort, including patients with new-onset diabetes mellitus during the study period. Finally, we were able to report the AR and time trend stratified by amputation level.
In conclusion, the present study is an analysis of AR, RR, and corresponding time trends in the largest nationwide population of Germany ever studied. The AR remained higher in the diabetic compared with the nondiabetic population. During the study period, we found a significant continuous reduction in the major and minor ARs among patients with diabetes and reduced major AR among those without diabe tes. In contrast, minor AR slightly increased among people without diabetes. During the study period, RR for both major and minor LEA in the diabetic compared to the nondiabetic population decreased significantly. Our findings point to the need for continuous national monitoring of LEAs.
Author contributions
HC made substantial contributions to the design of the study, conducted the analysis and interpreted the data, and drafted the manuscript. MN made substantial contributions to the conception and design of the study, researched data, and contributed to the concept, design, and drafting of the manuscript. BH and WA researched data, contributed to the data interpretation, and critically commented on the manuscript for scientific content. FH, SM, GR, and TK contributed substantially to the data interpretation and discussion. HF, CG, WU, BW, and AW prepared and extracted data and contributed substantially to the concept, design, and data interpretation. IS and AI contributed substantially to the conception and design of the study, acquisition of data, data interpretation, and discussion. All authors contributed toward data analysis, critically revised the manuscript for important intellectual content, approved the final manuscript, and agreed to be accountable for all aspects of the work.
AI is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Acknowledgments
We acknowledge financial support from the German Federal Ministry of Health (BMG): grant number ZMV I 5-2515VCK005. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- International Diabetes FederationIDF Diabetes Atlas – 7th edition [Internet]2016 [cited May 23, 2016]. Available from: http://www.idf.org/diabetesatlasAccessed February 23, 2018
- TrautnerCHaastertBMaucknerPGatckeLMGianiGReduced incidence of lower-limb amputations in the diabetic population of a German city, 1990–2005: results of the Leverkusen Amputation Reduction Study (LARS)Diabetes Care200730102633263717644615
- IcksAHaastertBTrautnerCGianiGGlaeskeGHoffmannFIncidence of lower-limb amputations in the diabetic compared to the non-diabetic population: findings from nationwide insurance data, Germany, 2005–2007Exp Clin Endocrinol Diabetes2009117950050419629934
- LombardoFLMagginiMDe BellisASeghieriGAnichiniRLower extremity amputations in persons with and without diabetes in Italy: 2001–2010PLoS One201491e8640524489723
- PricePThe diabetic foot: quality of lifeClin Infect Dis200439Suppl 2S129S13115306991
- HoffmannFClaessenHMorbachSWaldeyerRGlaeskeGIcksAImpact of diabetes on costs before and after major lower extremity amputations in GermanyJ Diabetes Complications201327546747223746556
- TuttleKRBakrisGLBilousRWDiabetic kidney disease: a report from an ADA Consensus ConferenceDiabetes Care201437102864288325249672
- Diabetes care and research in Europe: the Saint Vincent declarationDiabet Med1990743602140091
- Gesundheitsberichterstattung des Bundes Gemeinsam Getragen von RKI und Destatis Gesundheit in Deutschland Berlin, November 2015 [Jul 19, 2016] Available from: http://www.gbe-bund.de/pdf/GES-BER2015.pdfAccessed January 19, 2018
- BauerHGermannGGriesFNationale VersorgungsLeitlinie Typ-2-Diabetes: Präventions- und Behandlungsstrategien für Fußkom-plikationen, Langfassung, Version 2.8. February 2010. basierend auf der Fassung von November 20062007 Available from: http://www.deutsche-diabetes-gesellschaft.de/fileadmin/Redakteur/Leitlinien/Evidenzbasierte_Leitlinien/dm-fusskomplikationen-vers2.8-lang.pdfAccessed February 09, 2018
- BuckleyCMO’FarrellACanavanRJTrends in the incidence of lower extremity amputations in people with and without diabetes over a five-year period in the Republic of IrelandPLoS One201277e4149222859991
- AlmarazMCGonzalez-RomeroSBravoMIncidence of lower limb amputations in individuals with and without diabetes mellitus in Andalusia (Spain) from 1998 to 2006Diabetes Res Clin Pract201295339940522133651
- TrautnerCHaastertBSpraulMGianiGBergerMUnchanged incidence of lower-limb amputations in a German City, 1990–1998Diabetes Care200124585585911347743
- FosseSHartemann-HeurtierAJacqueminetSHa VanGGrimaldiAFagot-CampagnaAIncidence and characteristics of lower limb amputations in people with diabetesDiabet Med200926439139619388969
- KosterIvon FerberLIhlePSchubertIHaunerHThe cost burden of diabetes mellitus: the evidence from Germany – the CoDiM studyDiabetologia20064971498150416752168
- HellerGGunsterCSchellschmidtHWie häufig sind Diabetes-bedingte Amputationen unterer Extremitäten in Deutschland? [How frequent are diabetes-related amputations of the lower limbs in Germany? An analysis on the basis of routine data]Dtsch Med Wochenschr20041299429433 German14970914
- IkonenTSSundRVenermoMWinellKFewer major amputations among individuals with diabetes in Finland in 1997-2007: a population-based studyDiabetes Care201033122598260320807872
- SwartEGotheHGeyerSGood Practice of Secondary Data Analysis (GPS): guidelines and recommendationsGesundheitswesen201577212012625622207
- RothmanKJGreenlandSModern EpidemiologyPhiladelphiaLippincott Williams & Wilkins1998
- ClaeskensGStatistical model choiceAnnu Rev Stat Appl201631233256
- NarresMKvitkinaTClaessenHIncidence of lower extremity amputations in the diabetic compared with the non-diabetic population: a systematic reviewPLoS One2017128e018208128846690
- GreggEWLiYWangJChanges in diabetes-related complications in the United States, 1990-2010N Engl J Med2014370161514152324738668
- Van HoutumWHLaveryLAOutcomes associated with diabetes-related amputations in the Netherlands and in the state of California, USAJ Intern Med199624042272318918514
- van HoutumWHLaveryLAMethodological issues affect variability in reported incidence of lower extremity amputations due to diabetesDiabetes Res Clin Pract19973831771839483384
- HoffmannFIcksADiabetes ‘epidemic’ in Germany? A critical look at health insurance data sourcesExp Clin Endocrinol Diabetes2012120741041522441721
- RümenapfGMorbachSWhat can I do with a patient with diabetes and critically impaired limb perfusion who cannot be revascularized?Int J Low Extrem Wounds201413437838925326447
- BoutoilleDFerailleAMaulazDKrempfMQuality of life with diabetes-associated foot complications: comparison between lower-limb amputation and chronic foot ulcerationFoot Ankle Int200829111074107819026199
- HoffstadOMitraNWalshJMargolisDJDiabetes, lower-extremity amputation, and deathDiabetes Care201538101852185726203063
- MorbachSKerskenJLobmannRNobelsFDoggenKVan AckerKThe German and Belgian accreditation models for diabetic foot servicesDiabetes Metab Res Rev201632Suppl 131832526455588
- SchaperNCAndrosGApelqvistJDiagnosis and treatment of peripheral arterial disease in diabetic patients with a foot ulcer. A progress report of the International Working Group on the Diabetic FootDiabetes Metab Res Rev201228Suppl 121822422271741
- ZayedHHalawaMMaillardetLSidhuPSEdmondsMRashidHImproving limb salvage rate in diabetic patients with critical leg ischaemia using a multidisciplinary approachInt J Clin Pract200963685585818248395
- Calle-PascualALGarcia-TorreNMoragaIEpidemiology of nontraumatic lower-extremity amputation in area 7, Madrid, between 1989 and 1999: a population-based studyDiabetes Care20012491686168911522722
- VenermoMManderbackaKIkonenTKeskimakiIWinellKSundRAmputations and socioeconomic position among persons with diabetes mellitus, a population-based register studyBMJ Open201334 piie002395
- RasmussenBSYderstraedeKBCarstensenBSkovOBeck-NielsenHSubstantial reduction in the number of amputations among patients with diabetes: a cohort study over 16 yearsDiabetologia201659112112926590707
- MorrisADMcAlpineRSteinkeDDiabetes and lower-limb amputations in the community. A retrospective cohort study. DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland/Medicines Monitoring UnitDiabetes Care19982157387439589233
- DinhTVevesAThe influence of gender as a risk factor in diabetic foot ulcerationWounds200820512713125942414
- KiziltanMEGunduzAKiziltanGAkalinMAUzunNPeripheral neuropathy in patients with diabetic foot ulcers: clinical and nerve conduction studyJ Neurol Sci20072581–2757917399742
- Correa-de-AraujoRMcDermottKMoyEGender differences across racial and ethnic groups in the quality of care for diabetesWomens Health Issues2006162566516638522
- HjelmKNybergPApelqvistJGender influences beliefs about health and illness in diabetic subjects with severe foot lesionsJ Adv Nurs200240667368412473048
- FlintropJPrivate Krankenversicherung: So viele Versicherte wie nie zuvorDtsch Arztebl Int2012109211107
- HoffmannFIcksAUnterschiede in der Versichertenstruktur von Krankenkassen und deren Auswirkungen für die Versorgungsforschung: Ergebnisse des Bertelsmann-Gesundheitsmonitors [Structural differences between health insurance funds and their impact on health services research: results from the Bertelsmann Health-Care Monitor]Gesundheitswesen2012745291297 German21755492
- HoffmannFIcksADo persons that changed health insurance differ from those who did not? The case of diabetesExp Clin Endocrinol Diabetes2011119956957221811959
- TamayoTBrinksRHoyerAKußORathmannWThe prevalence and incidence of diabetes in germany: an analysis of statutory health insurance data on 65 million individuals from the years 2009 and 2010Dtsch Ärztebl Int20161131117718227118665
- GoffrierBSchulzMBätzing-FeigenbaumJAdministrative Prävalenzen und Inzidenzen des Diabetes mellitus von 2009 bis 2015. Zentralinstitut für die kassenärztliche Versorgung in Deutschland (ZI)Versorgungsatlas-Bericht 17/03Berlin20172017
- KosterIHuppertzEHaunerHSchubertIDirect costs of diabetes mellitus in Germany – CoDiM 2000-2007Exp Clin Endocrinol Diabetes2011119637738521264804
- SchipfSWernerATamayoTRegional differences in the prevalence of known type 2 diabetes mellitus in 45-74 years old individuals: results from six population-based studies in Germany (DIAB-CORE Consortium)Diabet Med2012297e88e9522248078
- BohnBKernerWSeufertJTrend of antihyperglycaemic therapy and glycaemic control in 184,864 adults with type 1 or 2 diabetes between 2002 and 2014: analysis of real-life data from the DPV registry from Germany and AustriaDiabetes Res Clin Pract2016115313827242120
- TamayoTClaessenHRuckertIMTreatment pattern of type 2 diabetes differs in two German regions and with patients’ socioeconomic positionPLoS One201496e9977324915157
- SharabianiMTAylinPBottleASystematic review of comorbidity indices for administrative dataMed Care201250121109111822929993
- CrawfordFCezardGChappellFMA systematic review and individual patient data meta-analysis of prognostic factors for foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations (PODUS)Health Technol Assess201519571210
- GößwaldASchienkiewitzANowossadeckEBuschMAPrävalenz von Herzinfarkt und koronarer Herzkrankheit bei Erwachsenen im Alter von 40 bis 79 Jahren in DeutschlandBundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz201356565065523703482
- LawallHDiehmCHoffmannUReineckeHPeriphere arterielle Verschlusskrankheit: Epidemiologie, Komorbidität und PrognoseDtsch Med Wochenschr2015140241798180226625226
- BuschMASchienkiewitzANowossadeckEGosswaldAPrävalenz des Schlaganfalls bei Erwachsenen im Alter von 40 bis 79 Jahren in Deutschland Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) [Prevalence of stroke in adults aged 40 to 79 years in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)]Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz2013565–6656660 German23703483
