Abstract
Objective
Earlier research suggests that birth weight may be associated with celiac disease (CD), but the direction of association has been unclear potentially due to confounding effect from genetic and intrafamilial factors. Through within-twin analyses, we aimed to minimize confounding effects such as twins that share genetic and early environmental exposures.
Materials and methods
Using the Swedish Twin Registry, we examined the birth weight of 146,830 twins according to the CD status. CD was defined as having villous atrophy according to a small intestinal biopsy reports.
Results
The prevalence of diagnosed CD was 0.5% (n=669), and we included 407 discordant pairs of CD–non-CD twins. Comparing the 669 CD patients with non-CD twins, the association between birth weight and future CD was not statistically significant (odds ratio [OR] per 1000 g increase in birth weight: 1.16; 95% confidence interval [CI]=0.97–1.38). In males, the association was positive and statistically significant (OR=1.50; 95% CI=1.11–2.02). However, the association was not significant in within-pair analyses for both dizygotic and monozygotic twins and for both sexes.
Conclusion
This population-based study found that in male twins, higher birth weight was associated with higher risk of CD. However, when comparing discordant twin pairs in within-twin pair analyses, there was no statistically significant association between birth weight, intrauterine growth, and future risk of CD.
Introduction
Celiac disease (CD) is a chronic immune-mediated disorder triggered by the exposure to gluten in genetically predisposed individuals. On gluten exposure, the individual typically develops small intestinal villous atrophy (VA) and mucosal inflammation.Citation1 Individuals with CD are at increased risk of a number of disordersCitation2 including lymphoproliferative diseaseCitation3 and adverse pregnancy outcome,Citation4 and in most populations also death.Citation5 Up until recently, both age at gluten introduction and breastfeeding were regarded as important risk factors for future CD,Citation6,Citation7 but two large randomized clinical trials have reported a null association between duration of breastfeeding, age at gluten introduction, and the risk of later CD.Citation8,Citation9 Since heritability cannot explain all the variance in risk for CD,Citation10 other environmental factor(s) are likely to contribute to the celiac pathogenesis.Citation11
Considering that CD often debuts at young age, perinatal conditions may contribute to CD pathogenesis. In one of the first studies on newborn characteristics and later CD, Sandberg-Bennich et alCitation12 found a negative association between birth weight and CD (low birth weight [≤2499 g] increased the risk of CD [odds ratio {OR}=1.27; 95% confidence interval {CI}=1.07–1.52]). In contrast, two recent studies have reported a positive association between birth weight and CD. In the Norwegian study by Emilsson et al,Citation13 both low birth weight (<2500 g; OR=0.79) and “very” low birth weight (<1500 g; OR=0.45) were linked to a lower risk of future CD. However, none of the risk estimates reached statistical significance (the analysis of very low birth weight only contained one case of CD), and z-scores for birth weights were similar in patients with CD and their controls. In a Swedish dataset, Namatovu et alCitation14 found an inverse relationship between very low birth weight and CD, but this relationship failed to reach statistical significance after adjustment for covariates (OR=0.8; 95% CI=0.5–1.2). Meanwhile, Mårild et alCitation15 found that a very low birth weight may protect against future CD (OR=0.87; 95% CI=0.63–1.21), while their study showed a neutral relationship between low birth weight and CD (adjusted OR=1.02). Interestingly, Mårild et alCitation15 observed an increased risk of CD in small for gestational age (SGA) children (OR=1.21; 95% CI=1.09–1.35).
The Swedish Twin Registry (STR) collects data on all twins in Sweden.Citation16 Through zygosity data (where monozygotic [MZ] twin pairs share 100% of their genes and dizygotic [DZ] twin pairs on average 50% of the genome), it allows researchers to disentangle genetic and environmental factors in the etiology of complex diseases. In this study, we examined the association between birth weight and CD in twins with biopsy-verified CD.
Materials and methods
CD
In 2006–2008, we contacted all 28 pathology departments in Sweden and obtained data on small intestinal biopsies performed in 1969–2008. We then updated the data collection in 2013 to include individuals undergoing biopsy up until this year. Data included date of biopsy, biopsy site (duodenum and jejunum), VA (Marsh grade III), and personal identity number.Citation17 We defined CD as having VA in the duodenum or jejunum at histopathology examination. This definition has been validated, and in a Swedish setting,Citation18 95% of all individuals with VA have CD (this is in fact higher than having a physician-assigned diagnosis in the National Patient RegisterCitation19). The histopathology examination was based on an average of three small intestinal specimensCitation20 which should detect 95% of all CD patients. Additional details on the collection of biopsy data have been published previously. In total, 39,935 individuals with CD were identified and then matched to the STR. This study was approved by the regional ethics review board in Stockholm on June 14, 2006 (2006/633-31/4). This was a registry-based study, and for this reason, no participant was contacted.Citation26 The study did not include any identifying information.
The STR
The STR started in the 1950s and contains information about twins born in Sweden since 1886.Citation16 Zygosity is determined through questionnaire data about intrapair similarities in childhood, being of opposite sex, or DNA analyses, and has an estimated accuracy of >98%.Citation21
In this study, we identified all twins alive in 2007 (N=146,830 from 80,296 pairs) and born since 1906 (so that participants were ≤100 years at the end of the study) whose birth year and sex were known, out of whom 669 (0.5%) had a diagnosis of CD (VA; ). In addition to birth weight, we obtained information on sex, birth length, birth year, and zygosity from the STR. The study was not restricted to Caucasians. Swedish regulations prohibit registers on religion or race.
Table 1 Descriptive information, number (percentage of non-missing values within each variable) if not else stated
Swedish Medical Birth Registry
This registry started in 1973 and contains data on >98% of all births. Data were collected on standardized forms. Only birth weights ≥300 g and ≤7000 g were accepted in the registry to decrease errors. Through this register, we obtained data on birth weight and gestational length (in days). When the information was not available in the Medical Birth Registry, we retrieved the corresponding data from the STR, collected through questionnaires. The Medical Birth Registry is regularly audited and has a high quality.Citation22
Birth weight and z-scores
Data on birth weight were available, from either the Medical Birth Registry or the STR, for 102,384 twins. We calculated gestational age-standardized birth weight, henceforth called z-scores; separately for males and females, we standardized birth weight for each gestational day by subtracting the gestational day-specific mean and dividing by the gestational day-specific SD. Where fewer than five observations were available in a gestational day, we combined adjoining days until at least five observations were accumulated before calculating the z-score.
Descriptive
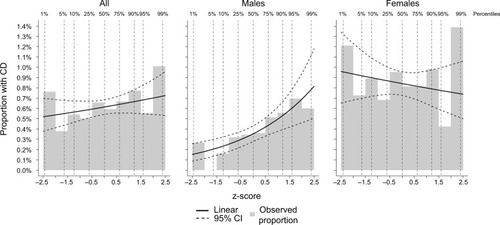
We summarized all variables in the groups with and without CD. Specifically, we counted the number of twins of each sex, calculated the mean birth weight, categorized birth weight into very low (≤1499 g), low (1500–2499 g), normal (2500–3499 g), and high (≥3500 g), and calculated mean z-scores. We calculated the mean birth length and gestational age, as well as categorized according to predefined groups. Furthermore, we categorized twins as birth year, zygosity, and based on whether the twin had an observed co-twin (i.e., representing a complete pair). We plotted observed proportions of CD by birth weight (as a histogram in 250 g increments from 1200 to 3950 g) and added a modeled effect from a logistic regression model, where birth weight was included as a linear predictor on the log-odds scale – first on the total sample, then for males and females separately (). Finally, we plotted a similar plot using the z-scores (in 0.5 increments from −2.5 to 2.5 SD; ).
Figure 1 Observed proportion with CD by birth weight.
Abbreviations: CD, celiac disease; CI, confidence interval.
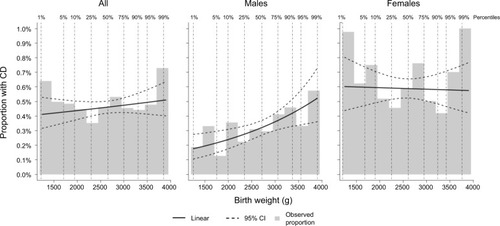
Figure 2 Observed proportion with CD by gestational age-standardized birth weight (z-scores).
Abbreviations: CD, celiac disease; CI, confidence interval.
Statistical analyses
Birth weight: all twins
We performed logistic regression with CD as outcome and birth weight as exposure, where birth weight was assumed to have a linear effect on the log-odds scale. We then fitted a model where we adjusted for the potentially confounding effect of the covariates sex and birth year (in the same categories as for the descriptive; ).
Intrauterine growth: all twins
We added the covariate gestational age (same categories as given in ) to the abovementioned model. We then continued to perform logistic regression using z-scores (gestational age-standardized birth weight), first crudely and then with adjustment for sex and birth year.
Low birth weight
We then performed all mentioned analyses using low birth weight (≤2499 g, including individuals with very low birth weight) as predictor in a logistic regression model.
Within-twin pair analyses
To investigate whether potential associations seen were due to factors shared by the two twins within each pair, we performed the so-called within-pair analyses. In these analyses, each pair is considered a cluster in a conditional logistic regression; thus, each twin’s co-twin acts as a control and is matched on (and thus adjusted for) all factors shared between the twins; specifically shared confounders and shared mediators are adjusted for in the analyses.Citation23 We analyzed birth weight, z-scores, and low birth weight, separately for DZ and MZ twins, representing increased adjustment of genetic factors.
Finally, we performed all statistical analyses stratified by sex.
Sensitivity analyses
To investigate whether the association between birth weight and CD differed between MZ and DZ twins, we estimated the associations separately, and by sex, with continuous birth weight as predictor. We used this approach since causes of differences in birth weight between twins who are MZ and DZ may differ due to differences in intrauterine environment – where MZ twins may share placenta and/or amniotic sac, while DZ twins do not. If MZ and DZ twins do not have a similar association between birth weight and CD, an analysis combining the subpopulations may not be advisable.
Since birth weight has increased with time, and practice in diagnosing CD has changed, we performed analyses on the subsample born in 1980–2004 – a population where CD was primarily diagnosed in childhood since CD used to be regarded as a pediatric diagnosis. In this subsample, we plotted the observed proportion of CD by birth weight and analyzed the crude and sex and birth year-adjusted association between birth weight and CD using logistic regression, for both sexes combined and stratified by sex.
To ensure that inclusion of opposite-sexed DZ twin pairs did not bias the results, we performed an analysis of birth weight and CD excluding twins from opposite-sexed pairs, stratified by sex. Finally, we stratified our analyses according to the following: if twin pairs were complete or incomplete.
All statistical analyses were performed in a generalized estimation equation setting, using a cluster–robust sandwich estimator to adjust the precision of the estimates (CIs) by eliminating distributional assumptions and accounting for dependencies between twins in pairs. To this end, we used the gee function from the drgee packageCitation24 in R.Citation25
Results
Descriptive
Descriptive information on the study population is presented in . Of the 146,830 twins included in the study, 669 (0.5%) had biopsy-verified CD, with an overrepresentation of females (66.7% of CD cases were females versus 50.7% of non-CD twins; p<0.001). The mean birth weight was 2662 g in CD twins and 2638 g in non-CD twins. The z-scores did not differ significantly (p=0.252). The lengths at birth, 47.3 cm and 47.2 cm, respectively, were also similar (p=0.689). The gestational age did not differ significantly between CD and non-CD twins (p=0.741). While the majority of twins with CD were born in 1985–2004, likely reflecting that the diagnosis of CD used to take place primarily in childhood and computerized data on VA are scarce before this period, the birth years of non-CD twins were more evenly distributed. Zygosity was similar in CD and non-CD twins. When plotting the proportions of CD cases against categorized birth weight, no clear pattern was observed in the total sample and among females, but a positive association was detectable among males (); the pattern was similar when plotting against z-scores ().
Main results
Birth weight: all twins
For each 1000 g increase in birth weight, the odds of CD increased by a factor of 1.08 (crude OR=1.08; 95% CI=0.91–1.30; ). This association was stronger, and statistically significant in males (OR=1.51; 95% CI=1.12–2.04) and practically null for females (OR=0.98; 95% CI=0.79–1.23). Adjustments for sex and birth year changed the association somewhat (OR=1.16; 95% CI=0.97–1.38), but not notably so in the sex-specific analyses.
Table 2 Analyses of the association between birth weight and CD, estimate (95% CI)
Intrauterine growth: all twins
The association with birth weight remained similar when adjusting for gestational age (OR=1.15; 95% CI=0.85–1.54), while it became stronger in males (OR=2.09; 95% CI=1.39–3.15) and with a lower risk for CD in females (OR=0.86; 95% CI=0.59–1.24; ). However, the crude estimates were covered by the CIs from the adjusted analyses and vice versa. In the analysis of z-scores, the association of a one SD increase in z-score was not significant, in both crude (OR=1.07; 95% CI=0.95–1.21; ) and adjusted (OR=1.06; 95% CI=0.93–1.19) analyses. Similar to analyses of birth weight, the association was positive and significant for males (OR=1.40; 95% CI=1.17–1.67) and close to null for females (OR=0.95; 95% CI=0.82–1.10).
Table 3 Analyses of the association between z-score (birth weight standardized per gestational age) and CD, estimate (95% CI)
Low birth weight
In analyses with low birth weight (≤2499 g) as binary exposure, the patterns of results were similar; low birth weight was associated with a lower risk of CD, particularly in males (). However, the power was lower in this analysis, and no estimate was significantly different from zero.
Table 4 Analyses of the association between low birth weight (≤2499 g) and CD, estimate (95% CI)
Within-twin pair analyses
In the within-twin pair analyses, no ORs differed significantly from 1, neither in combined nor in sex-specific analyses and neither in DZ nor in MZ twin pairs (–). This may reflect that genetic and/or shared environmental factors confound the association between birth weight and CD, at least among males. However, the CIs were wide since the informative pairs for within analysis are those who are discordant on outcome, as well as on exposure, making it difficult to draw any decisive inferences from the analyses.
Sensitivity analyses
The association appeared similar in DZ and MZ twins as indicated by the CI of the estimate in one zygosity overlapping the CI of the other ().
Results from analyses of subsample born in 1980–2004 are presented in and . The results were very similar as in the full cohort.
Analyses excluding the opposite-sexed DZ twins showed similar estimates as in the main analysis, but with wider CIs ().
Data stratified for complete versus incomplete twin pairs were similar to those in the main analyses ().
Discussion
In this nationwide cohort of twins, we found no overall association between birth weight, intrauterine growth, or low birth weight (≤2499 g) and later CD. While a statistically significant association was found between birth weight and intrauterine growth and later CD among males, that association disappeared in within-twin analyses. Within-twin analyses allowed us to consider shared environmental and genetic factors, but with limited statistical power. While several individuals in our study may also have been included in earlier Swedish studiesCitation14,Citation15 (all three studies took place in Sweden), the current study used a larger dataset, identifying cases through biopsy registers, and used an innovative twin method to minimize the confounding effect from genetic and early environmental factors.
In a recent Swedish study by Namatovu et alCitation14 based on 6596 children with CD, low birth weight (≤1499 g) was inversely associated with later CD (p=0.01), but this association was not statistically significant after adjustment for potential confounders.Citation14 Furthermore, that study also found protective effects of high maternal age, high income, and average body mass index of the pregnant woman (25–29 years). Several of these factors may covary with child birth weight, and therefore confound relationships with CD. Comparing twins with the same mother, and especially MZ twins with identical genetic background, allowed us to eliminate the influence of maternal background characteristics.
As opposed to Namatovu et al,Citation14 two other Scandinavian studiesCitation13,Citation15 have explored the influence of SGA. In the first and so far largest study on pregnancy outcome and CD,Citation15 Mårild et al found a small excess risk of CD in SGA children. No similar increase was seen in the Norwegian study by Emilsson et al.Citation13 Although the authors of these studies used slightly different definitions, risk estimates were actually similar in the two most similar analyses on SGA (Sweden: OR=1.21; 95% CI=1.09–1.35, and Norway: OR=1.12; 95% CI=0.70–1.80).Citation13,Citation15 None of the two studies did however find any association between birth weight and future CD.
We used two approaches when examining birth weight and intrauterine growth and risk of CD. First, we examined our “full twin cohort” and observed a borderline increased risk of CD with increasing birth weight (a positive association among males). A positive association between birth weight and CD in males was also seen in the study by Namatovu et al.Citation14 Several studies have demonstrated sex differences with regard to prevalence,Citation27,Citation28 clinical presentation,Citation29 and histopathological featuresCitation30 of autoimmune disease, and the immune system is not identical in males and femalesCitation31 potentially due to differences in sex hormones.Citation32 Either or several of these factors may contribute to an increased susceptibility to CD in males with high birth weight. But the association could also be a chance finding due to multiple comparisons and should be explored in future larger analyses.
Second, we examined birth weight within twins to control for potential shared genetic and environmental confounders. Considering that an increase in birth weight was not associated with CD in our male within-twin analyses (neither among DZ nor among MZ twins), we find it unlikely that birth weight plays more than a marginal role for CD pathogenesis in either sex, although we acknowledge the limited power of our within-twin analyses. Median birth weights are similar within Europe,Citation33 while the prevalence of CD varies substantially.Citation34 This supports our findings that, on a population level, birth weight is unlikely to influence the prevalence of CD more than marginally, if at all.
Among the strengths of our study is the large number of affected twins (n=669), of which 140 belonged to 106 MZ twin pairs. By comparison, the study by Nistico et alCitation35 on twin genetics was based on 23 MZ twin pairs, but neither that studyCitation35 nor any other twin study has to our knowledge evaluated the importance of perinatal factors in CD development. In our study, data on zygosity, birth weight, and CD status were collected independently, thereby decreasing the risk of bias.
We used biopsy record data to identify CD. Biopsies were up until 2012 the reference standard for diagnosis in both adults and childrenCitation36 and has remained so in adultsCitation1,Citation37 (but with an option to abstain from biopsy in a subset of children). Earlier validation has shown that small intestinal biopsies have a high sensitivity for CD (96% of gastroenterologists and 100% of pediatricians reported performing a biopsy before assigning the CD diagnosis during the study period),Citation18 and when examining patient charts of 114 patients with VA, 108 had CD (95%).Citation18 Even though we did not require a positive serology for the CD diagnosis, 88% of VA individuals with available data were positive for transglutaminase, endomysium, or gliadin antibodies.Citation17
Among the limitations of our study is the risk of misclassification. Undiagnosed CD (now classified as absent) in discordant twin pairs will drive the difference in birth weight toward null. This is similar to all studies based on diagnosed CD, and our prevalence of diagnosed CD (0.5%) is consistent with previous reports from other European countries. A second limitation is our limited statistical power. Despite taking advantage of the nationwide STR with almost 150,000 twins, CIs for within-twin analyses were wide.
Conclusion
Our population-based study found that in male twins higher birth weight was associated with higher risk of CD. However, when comparing discordant twin pairs in within-twin pair analyses, there was no association between birth weight, intrauterine growth, and future risk of CD.
Author contributions
Agreed with the manuscript’s results and conclusion and approved the final version of the manuscript: JFL, RK-H, BL, JH, LE, and PKM. Designed the study: JFL, RK-H, LE, and PKM. Analyzed the data: RK-H. Wrote the first draft of the manuscript: JFL and RK-H. Contributed to the revision of the paper: BL, JH, LE, and PKM. Contributed to the design of the study and interpretation of the data analyses: BL and JH. Responsible for data integrity: JFL, RK-H, and PKM. Obtained funding: JFL. All the authors approved the final version of the manuscript.
Acknowledgments
JFL was supported by grants from the Swedish Society of Medicine and the Swedish Research Council. None of the funders had any influence on this study.
Supplementary materials
Figure S1 Observed proportion with CD by birth weight in subsample born in 1980–2004.
Notes: Percentiles refer to the percentage of individuals with a birth weight lower than indicated. Linear refers to a modeled proportion in a logistic regression with an effect that is linear on the log-odds scale. 95% CI refers to 95% bootstrap CIs of modeled proportion with CD based on 10,000 repeats.
Abbreviations: CD, celiac disease; CI, confidence interval.
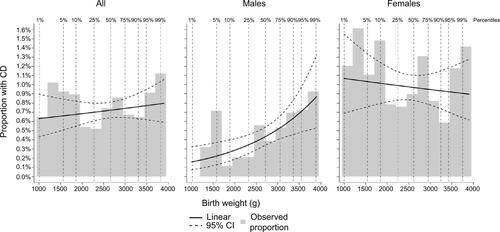
Table S1 Analyses of the association between birth weight and CD, estimate (95% CI)
Table S2 Analyses of the association between birth weight and CD in subsample born in 1980–2004 (N=58,771), estimate (95% CI)
Table S3 Analyses of the association between birth weight and CD among same-sexed twin pairs, estimate (95% CI)
Table S4 Analyses of the association between birth weight and CD stratified by whether twin pair was complete, estimate (95% CI)
Disclosure
The authors report no conflicts of interest in this work.
References
- LudvigssonJFLefflerDABaiJCThe Oslo definitions for coeliac disease and related termsGut2013621435222345659
- AbenavoliLDelibasicMPetaVTurkulovVDe LorenzoAMedic-StojanoskaMNutritional profile of adult patients with celiac diseaseEur Rev Med Pharmacol Sci201519224285429226636515
- ElfstromPGranathFEkstrom SmedbyKRisk of lymphoproliferative malignancy in relation to small intestinal histopathology among patients with celiac diseaseJ Natl Cancer Inst2011103543644421289299
- LudvigssonJFMontgomerySMEkbomACeliac disease and risk of adverse fetal outcome: a population-based cohort studyGastroenterology2005129245446316083702
- LudvigssonJFMontgomerySMEkbomABrandtLGranathFSmall-intestinal histopathology and mortality risk in celiac diseaseJAMA2009302111171117819755695
- IvarssonAHernellOStenlundHPerssonLABreast-feeding protects against celiac diseaseAm J Clin Nutr200275591492111976167
- AkobengAKRamananAVBuchanIHellerRFEffect of breast feeding on risk of coeliac disease: a systematic review and meta-analysis of observational studiesArch Dis Child2006911394316287899
- VriezingaSLAuricchioRBraviERandomized feeding intervention in infants at high risk for celiac diseaseN Engl J Med2014371141304131525271603
- LionettiECastellanetaSFrancavillaRSIGENP (Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition) Working Group on Weaning and CD RiskIntroduction of gluten, HLA status, and the risk of celiac disease in childrenN Engl J Med2014371141295130325271602
- Kuja-HalkolaRLebwohlBHalfvarsonJWijmengaCMagnussonPKLudvigssonJFHeritability of non-HLA genetics in coeliac disease: a population-based study in 107 000 twinsGut201665111793179827207974
- LebwohlBLudvigssonJFGreenPHThe unfolding story of celiac disease risk factorsClin Gastroenterol Hepatol201412463263524211288
- Sandberg-BennichSDahlquistGKallenBCoeliac disease is associated with intrauterine growth and neonatal infectionsActa Paediatr2002911303311883814
- EmilssonLMagnusMCStordalKPerinatal risk factors for development of celiac disease in children, based on the prospective Norwegian Mother and Child Cohort StudyClin Gastroenterol Hepatol201513592192725459557
- NamatovuFOlssonCLindkvistMMaternal and perinatal conditions and the risk of developing celiac disease during childhoodBMC Pediatr20161617727267234
- MårildKStephanssonOMontgomerySMurrayJALudvigssonJFPregnancy outcome and risk of celiac disease in offspring: a nationwide case-control studyGastroenterology201214213945.e321995948
- MagnussonPKAlmqvistCRahmanIThe Swedish Twin Registry: establishment of a biobank and other recent developmentsTwin Res Hum Genet201316131732923137839
- LudvigssonJFOtterblad-OlaussonPPetterssonBUEkbomAThe Swedish personal identity number: possibilities and pitfalls in healthcare and medical researchEur J Epidemiol2009241165966719504049
- LudvigssonJFBrandtLMontgomerySMGranathFEkbomAValidation study of villous atrophy and small intestinal inflammation in Swedish biopsy registersBMC Gastroenterol2009911919284576
- LudvigssonJFAnderssonEEkbomAExternal review and validation of the Swedish national inpatient registerBMC Public Health201111145021658213
- LudvigssonJFBrandtLMontgomerySMSymptoms and signs in individuals with serology positive for celiac disease but normal mucosaBMC Gastroenterol200995719624815
- LichtensteinPDe FaireUFloderusBSvartengrenMSvedbergPPedersenNLThe Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studiesJ Intern Med2002252318420512270000
- The Swedish Medical Birth Register: A Summary of Content and QualityStockholm, SwedenSwedish National Board of Health and Welfare2003 Available from: http://www.socialstyrelsen.se/Lists/Artikelk-atalog/Attachments/10655/2003-112-3_20031123.pdfAccessed October 13, 2017
- SjölanderAZetterqvistJConfounders, mediators or colliders – what types of shared covariates does the sibling comparison design control for?Epidemiology201728454054728575894
- ZetterqvistJSjölanderAPlonerADrgee: Doubly Robust Generalized Estimating Equations1.1.0 ed. R Package2015
- R Core TeamR: A Language and Environment for Statistical ComputingVienna, AustriaR Foundation for Statistical Computing20133-900051-07-0
- LudvigssonJFHabergSEKnudsenGPEthical aspects of registry-based research in the Nordic countriesClin Epidemiol2015749150826648756
- EatonWWRoseNRKalaydjianAPedersenMGMortensenPBEpidemiology of autoimmune diseases in DenmarkJ Autoimmun20072911917582741
- NgoSTSteynFJMcCombePAGender differences in autoimmune diseaseFront Neuroendocrinol201435334736924793874
- CiacciCCirilloMSollazzoRSavinoGSabbatiniFMazzaccaGGender and clinical presentation in adult celiac diseaseScand J Gastroenterol19953011107710818578167
- FairweatherDFrisancho-KissSRoseNRSex differences in autoimmune disease from a pathological perspectiveAm J Pathol2008173360060918688037
- McCombePAGreerJMMackayIRSexual dimorphism in autoimmune diseaseCurr Mol Med2009991058107919747114
- TrigunaiteADimoJJorgensenTNSuppressive effects of androgens on the immune systemCell Immunol20152942879425708485
- HemmingKHuttonJLGlinianaiaSVJarvisSNPlattMJDifferences between European birthweight standards: impact on classification of “small for gestational age”Dev Med Child Neurol2006481190691217044959
- MustalahtiKCatassiCReunanenACoeliac EU Cluster, Project EpidemiologyThe prevalence of celiac disease in Europe: results of a centralized, international mass screening projectAnn Med201042858759521070098
- NisticoLFagnaniCCotoIConcordance, disease progression, and heritability of coeliac disease in Italian twinsGut200655680380816354797
- HusbySKoletzkoSKorponay-SzaboIRESPGHAN Working Group on Coeliac Disease DiagnosisESPGHAN Gastroenterology CommitteeEuropean Society for Pediatric Gastroenterology, Hepatology, and NutritionEuropean Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac diseaseJ Pediatr Gastroenterol Nutr201254113616022197856
- LudvigssonJFBaiJCBiagiFBSG Coeliac Disease Guidelines Development GroupBritish Society of GastroenterologyDiagnosis and management of adult coeliac disease: guidelines from the British Society of GastroenterologyGut20146381210122824917550
