Abstract
Purpose
The purpose of this study was to examine why Norway has the highest rate of mortality due to cutaneous melanoma (CM) in Europe. The Norwegian Malignant Melanoma Registry (NMMR) enables the study of clinical and histopathological characteristics of patients who die due to CM.
Results
The NMMR and the Norwegian Cause of Death Registry provided data on the clinical and histopathological factors as well as the date and cause of death, through June 2015 for all first invasive CMs diagnosed in 2008–2012 (n=8087). Cox regression was used to estimate associations between clinical and pathological factors and CM-specific death. Multiple imputation was used to handle missing data.
Results
The CMs were equally distributed between men (49.9%) and women (50.1%), and the median follow-up was 4.0 years (range: 0.08–7.5 years). Trunk was the most common anatomic site (48%), superficial spreading melanoma was the dominant melanoma subtype (68.2%), median Breslow thickness was 1.0 mm, ulceration was present in 23% of CMs, and 91.8% of cases were in a local clinical stage at diagnosis. Compared to women, men were diagnosed at a higher age, with thicker and more-often-ulcerated tumor, and more often were in advanced clinical stages. During follow-up, 1015 patients died due to CM, representing 52.8% of all deaths. The nodular subtype made up the dominant proportion of fatal CM cases (55.3% in women, 64.6% in men). Sex, age, anatomic site (trunk), T-stage, ulceration, clinical stage, and having a second primary CM were associated with increased risk of CM-specific death.
Conclusion
Our data suggest that the high rate of mortality due to CM observed in Norway is attributable to the more advanced stage of the disease at diagnosis. Most high-risk cases occurred in male patients ≥70 years of age. Efforts to improve awareness and secondary prevention of CM, including warning signs of all melanoma subtypes, are required urgently and should be targeted toward men in particular.
Plain language summary
We were curious why Norway has the highest rate of mortality due to cutaneous melanoma (CM) in Europe. Data from the Norwegian Malignant Melanoma Registry (NMMR) enabled us to study patient and tumor characteristics among Norwegian patients, their relations to melanoma-specific death, and compare our results with those from other countries. We included all patients diagnosed with their first CM in 2008–2012 (n=8087) and linked information on the date and cause of death. We found that trunk was the most common tumor site, superficial spreading melanoma (SSM) was the dominant subtype, median tumor thickness was 1.0 mm, about 20% of the tumors were ulcerated, and about 10% of patients had metastases at diagnosis. Compared to women, men had thicker tumors and more advanced disease at diagnosis. During follow-up, 1015 patients died from melanoma. Factors that increased the risk of death were male sex, age ≥70 years, tumor site at the trunk, tumor thickness >1 mm, ulceration, metastases, and having a second tumor. Compared to other countries’ data, Norwegian patients have more advanced disease at diagnosis, which can explain our high mortality rate. Our results underline the need for improved public awareness, especially in men.
Introduction
The incidence of and mortality rates due to cutaneous melanoma (CM) in the Scandinavian countries are among the highest in Europe.Citation1 In Norway, CM is the cancer with the steepest increase in incidence after 2000, with an annual increase of ~3.5%, and is now the fifth and fourth most frequent cancer in men and women, respectively (all ages).Citation2 The age-adjusted rate of mortality due to CM has also increased in both sexes, with the highest rate observed in men.Citation2 In 2012, the rate of mortality due to CM in Norway was 3.5 per 100,000 (world standard population), which is ranked third worldwide and the highest in Europe (http://globocan.iarc.fr/Pages/bar_sex_site_sel.aspx), although several European countries have higher or comparable incidence.
Tumor thickness (Breslow), ulceration, and presence of regional or distant metastasis at diagnosis are the most important prognostic factors in CM.Citation3–Citation5 In the Nordic countries, most cases are diagnosed in an early stage of the disease, and in the period 1999–2003, the 5-year relative survival after CM waŝ80% in men and 90% in women.Citation6 In Sweden, increasing CM incidence has been accompanied by a decrease in tumor thickness and improvement in survival in men.Citation7 In Denmark and Norway, the largest increase in incidence has occurred for local-stage tumors.Citation8,Citation9 In Northwestern European countries, USA, and Australia, increasing incidence of CM is accompanied by a stable rate of mortality,Citation10 raising the question whether the increase in incidence represents a true increase in risk or whether it is due to better detection, higher awareness, and potentially overdiagnosis of CM.Citation11 In Norway, however, the high and increasing mortality rate raises the question whether the CM diagnoses are delayed, particularly in men.
The NMMR was established in 2008 and now provides the opportunity to study clinical and histopathological characteristics of all primary invasive CM cases, diagnosed in 2008–2012, and the association of these characteristics with CM-specific death. Furthermore, we aimed to discuss the findings in relation to results reported from comparable countries and gain new knowledge to help target secondary prevention in the Norwegian population.
Patients and methods
Patients
The Cancer Registry of Norway (CRN) has recorded all cancer diagnoses nationwide since 1953. Mandatory reporting from independent sources ensures completeness and high-quality data.Citation12 After 2000, >99% of all CM cases are morphologically verified.Citation2,Citation12 In 2008, the NMMR was established under the CRN, registering additional clinical and histopathological information related to each CM case.
In total, 8120 patients with a first primary invasive CM diagnosis in the period 2008–2012 were identified and included in the study. Sex, age at diagnosis, region of residence, anatomic site, melanoma subtype, Breslow thickness, T-stage, Clark level, presence of ulceration, clinical stage at diagnosis, and information on second primary CM during the study period were obtained from the NMMR.
Information on death and emigration was obtained by linkage to the Causes of Death Registry and the National Population Registry. The end of follow-up was on June 30, 2015. The cause of death is set by the doctor, and reporting to the Causes of Death Registry is mandatory by law (since January 1, 1951). The 10th edition of the World Health Organization’s International Classification of Diseases is used. The unique 11-digit personal identification number system, implemented in Norway in 1964, secures the linkages. In all, 33 (0.4%) cases were lost to follow-up, leaving 8087 cases for analyses.
All data were de-identified before analyses. Reporting from cancer-specific registries, with de-identified data, is regulated by the Norwegian law on health registries, needing no further ethical approval.
Clinical and histopathological variables
Based on age at diagnosis, the CM cases were divided into age groups (<50, 50–69, and ≥70 years). Codes for residential municipality at the time of diagnosis were categorized in accordance with the Norwegian Regional Health Authority regions (South-Eastern, Western, Central, and Northern Norway Regional Health Authorities). As an indicator of dermatologist availability, residential codes were categorized as urban and rural areas.
The anatomic site of the primary tumor was classified according to the International Classification of Diseases for Oncology, Third Edition (ICDO-3)Citation13 and categorized as head/neck (190.0), trunk (190.1/190.7), upper extremity (190.2), lower extremity (190.3/190.4), and other (190.5/190.6/190.8). Cases without information on the anatomic site (190.9) were categorized as unspecified (n=477).
Melanoma subtype is registered according to the following ICDO-3 codes:Citation13 SSM (M87433), nodular melanoma (NM; M87213), lentigo maligna melanoma (LMM; M87423), acral lentiginous melanoma (ALM; M87443), melanoma unspecified (M87203), and other (M87453/M87803/M87613). For 2126 cases, information on subtype was not given.
The Norwegian guidelines for the period 2008–2015 advised reporting of Breslow thickness in millimeters to 1 decimal point.Citation14 Breslow thickness was categorized as the following T-stages: T1 (≤1.0 mm), T2 (1.01–2.0 mm), T3 (2.01–4.0 mm), and T4 (>4.0 mm). Cases without information on Breslow thickness (n=857) were categorized as unspecified.
Information on the Clark level (I–V) and ulceration (present, not present) was used as registered in the NMMR. For a large proportion of cases (n=3638), information on ulceration was not specified. The CRN coding and classification system follows international standards,Citation12 with some modifications for stage as described in the CRN annual report;Citation2 based on information on clinical and histopathological notifications for each CM case, metastasis is coded by trained medical coders according to strict rules and local coding practice at the CRN. Clinical stage at diagnosis was categorized as follows: local disease (no metastases), regional metastasis (metastases in regional lymph nodes, satellites, and in-transit metastases), and distant metastasis (organ metastases and nonregional lymph node metastases). Cases without information about metastasis (n=1116) were categorized as unspecified.
Criteria for a new primary CM during the study period (0, 1, or >1) were different melanoma subtype, different anatomic site, or date of diagnosis at least 4 months after the first CM diagnosis.
Statistical analysis
Descriptive data are presented as frequencies (%) and medians (ranges). Differences between men and women were tested by the Mann–Whitney test for continuous variables and the chi-square test for categorical variables.
The CM cases were followed from the date of diagnosis until death, emigration, or end of follow-up (June 30, 2015), whichever occurred first. The traditional Kaplan–Meier estimator overestimates cumulative incidence in the presence of competing risk due to the incorrect assumption of non-informative censoring;Citation15–Citation17 thus, cumulative incidence of CM-specific death and that of death from other causes were estimated by a nonparametric method, taking competing events into account.Citation15
Cox regression, with the time since date of CM diagnosis as the time scale, was used to study the association between the clinical and histopathological variables and CM-specific death. Results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). The melanoma subtype ALM was included in the “other” category due to low numbers, and the Clark level was not included due to the high correlation with T-stage. The incidence of a new primary CM during the study period was modeled as a time-dependent covariate. Interaction between sex and age has previously been reportedCitation18 and was, thus, evaluated by a likelihood ratio test (with age dichotomized as <70/≥70 in complete-case data).
We imputed missing data (anatomic site, melanoma subtype, Breslow thickness, ulceration, and clinical stage) using multiple imputation with chained equations.Citation19 A combined result was set after running the imputation model 30 times.Citation19 In addition, analyses were conducted based on complete-case data.
All statistical analyses were performed using Stata 14 (StatCorp LP, College Station, TX, USA).
Results
shows the characteristics of the total 8087 CM cases, in total and stratified by sex; the cases comprised 50.1% women and 49.9% men. The mean age at diagnosis was 64 years, and men were older (65 years) than the women (62 years). The majority of the CM cases (61.2%) resided in areas under the South-Eastern Norway Regional Health Authority, and both sexes were similarly distributed in the regions.
Table 1 Characteristics of the 8087 CM cases (2008–2012), total and stratified by sex
The distribution of CM with regard to the anatomic site was different for men and women () and varied by age (). For both sexes, trunk was the most common location (48.0%), but it was more common in men (60.4%) than in women (35.8%); women had more leg tumors (34.0% vs 13.0%, respectively). Head/neck tumors were most frequent in patients ≥70 years of age in both sexes ().
Figure 1 (A) Anatomic site, (B) melanoma subtype, (C) T-stage, and (D) ulceration in CM cases diagnosed in Norway in 2008–2012, according to sex and age (n=8087).
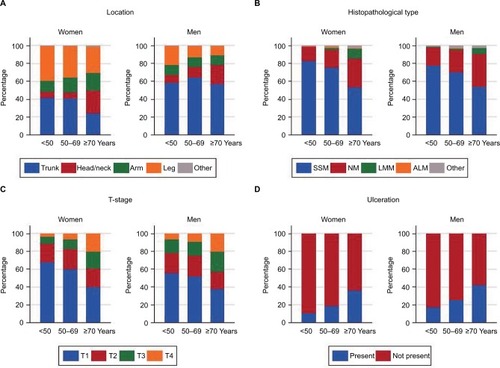
The dominant melanoma subtype was SSM (70.6% in women and 65.8% in men), followed by NM (22.8% in women and 28.6% in men; ). The proportion of NM was highest in men for all anatomic sites (data not shown), with increasing proportion by age in both sexes (34.7% being NM in patients ≥70 years; ).
Median Breslow thickness was 1.0 mm in women and 1.1 mm in men (). SSM tumors were thinner (median 0.8 mm) than NM tumors (median 3.0 mm; data not shown). Men had thicker and more-invasive tumors than women. In women, 56% of the tumors were in T-stage I and in men, the proportion was 47.6%. T-stage and Clark level were different for men and women. The proportion of Clark II tumors was 31.2% in women and 25.8% in men (). Increasing T-stage with increasing age at diagnosis was seen for both sexes ().
Presentation of ulceration was more frequent in men (30.8%) than in women (23%; ). Moreover, the presence of ulceration increased by age () and with increasing T-stage; ulceration was present in 73.8% of the T4 tumors (data not shown).
In total, 91.8% of the cases were diagnosed in a local stage, whereas 5% had regional metastases and 3.2% had distant metastases (). Advanced clinical stage at diagnosis was more common in men than in women.
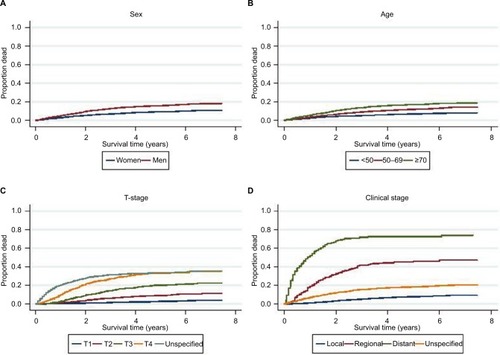
Median time of follow-up was 4.0 years (range 0.08–7.5 years), and during follow-up, 1015 deaths occurred due to CM, representing 52.8% of all deaths (n=1921) and 78.7% of all deaths before the age of 50 years. shows the cumulative incidences of CM-specific death and death due to other causes (2008–2015). After 3 years, the estimated risk of dying from CM was 9.7%, the risk of dying from other causes was 7.8%, and the odds of being alive were 82.5%. The corresponding numbers after 5 years were 12.5%, 12.1%, and 75.5%, respectively. Cumulative incidence plots showed higher risk of CM-specific death in men than in women, as well as an increasing risk of death with increasing age, T-stage, and clinical stage (). Low survival was observed for CMs with unspecified T-stage (). The NM type comprised the dominant proportion of fatal CM cases (55.3% in women and 64.8% in men), and 70.2% of patients were in T-stage T3 or T4 (data not shown).
Figure 2 Cumulative incidence of death due to CM and death from other causes in patients diagnosed in 2008–2012, based on the nonparametric method, taking competing events into account (n=8087).
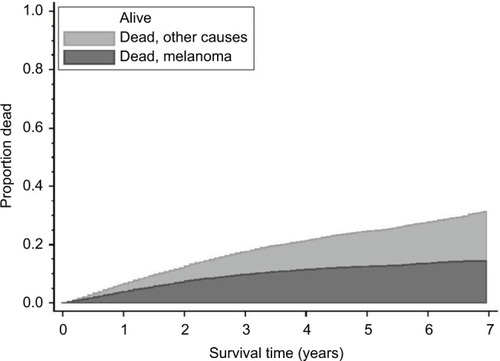
Figure 3 Cumulative incidence of death due to CM according to (A) sex, (B) age groups, (C) T-stage, and (D) clinical stage at diagnosis in patients diagnosed in 2008–2012 (n=8087), based on the nonparametric method, taking competing events into account.
In total, 5010 cases had complete data for the anatomic site, melanoma subtype, Breslow thickness, ulceration, and clinical stage. The percentage distribution per variable, in total and stratified by sex, was quite similar in the simulated data ().
Sex, age, anatomic site, T-stage, ulceration, clinical stage, and having a second primary CM were associated with CM-specific death, both in crude and multivariate analyses, whereas melanoma subtype was associated with CM-specific death in crude analysis only and not after adjustment for T-stage (). Complete-case analyses showed similar results (). The effect of sex was, however, slightly stronger in patients aged <70 years compared to those aged ≥70 years of age (HRs [95% CIs] were 1.41 [1.15–1.74] and 1.17 [0.95–1.45], respectively), but there was no interaction between age and sex.
Table 2 HRs for CM-specific death, with 95% CIs, by sex, age, anatomic site, melanoma subtype, T-stage, ulceration, clinical stage, and having a second primary CM from crude and multivariate analyses (n=8087)
Discussion
Our main findings are that male sex and advancing age are independent prognostic factors of CM-specific death and that higher T-stage, presence of ulceration, and advanced clinical stage are associated with male sex and high age, explaining the high mortality rate due to CM in Norwegian men. The dominant proportion of fatal CM cases was made up of the NM type. Analyses based on imputed missing data and the complete-case data gave similar results.
Few population-based CM registries with both clinical and histopathological data exist, and results from these registries cover different time periods and geographical areas.Citation7,Citation20–Citation25 Although this may complicate comparisons, the present results are discussed based on data from comparable registries.
We found that median age at first CM diagnosis was 64 years, and this is higher than that observed in Swedish (59.5 years, 1990–1999),Citation20 Australian (54 years, 1986–1996), and Central European databases (54 years, 1986–1996),Citation21 but similar to that reported in the most recent Swedish data (64 years, 2007–2011).Citation7 Our finding of increasing risk of CM-specific death by increasing age, even after adjustment for established prognostic factors, is in line with previous studies.Citation20,Citation26 Furthermore, we found a weaker effect of sex in CM patients ≥70 years of age, in line with Lasithiotakis et al,Citation26 but contrary to the more recent study by Khosrotehrani et al,Citation18 suggesting a survival benefit for patients >60 years of age (women only).
The anatomic site distribution of CM was in line with previous reports from NorwayCitation9,Citation27 and Sweden,Citation20 with the trunk as the main site (48.0%), although more prevalent in men (60.4%) than in women (35.8%). In agreement with other studies,Citation28,Citation29 trunk CM had a less favorable prognosis than CM at other anatomic sites, which may explain some of the difference in prognosis between the sexes.
SSM was, as expected, the dominant melanoma subtype. However, NM was more prevalent in Norway in the period 2008–2012 (22.8% in women and 28.6% in males) than reported in previous studies: Sweden (21.0%, 1990–1999),Citation20 Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (12.6%, 1989–2009),Citation25 and Queensland, Australia (15.2%, 1984–1996).Citation21 The median tumor thickness in Norway was 1.0 mm (1.1 mm in men), thicker than that reported in other registries.Citation20,Citation21,Citation25 Compared to other studies, we also found a higher proportion of cases with T4 tumorsCitation20,Citation21,Citation23–Citation25 and fewer with T1.Citation7,Citation20,Citation23,Citation25 In the SEERCitation22,Citation25 and in Australia,Citation23 the reported proportion of T1 tumors was close to 70%, compared to 51.8% in the current study. Hence, thicker tumors with higher levels of infiltration may explain the higher mortality rate in Norway. In the first 3 years after diagnosis, the highest risk of CM-specific death was seen for cases with unspecified T-stage (), and the HR for CM-specific death was at the same level as found for T3 (). This is in line with recent findings from the USA.Citation30
Ulceration is a strong prognostic factor.Citation3–Citation5 The proportion of missing data on ulceration in the current study was high, similar to that found in Swedish studies.Citation20,Citation31 In Norway, pathologists tend to report on ulceration only when it is present, not when it is absent. When collapsing the groups “unspecified” and “not present”, the proportion of ulceration was 14.8%, slightly lower than the proportion of ulceration found in Sweden (16.7%). Ulceration was more frequent in men, and the occurrence increased with age, further explaining the higher mortality rates in old men.
Clinical stage at diagnosis has also been established as a strong prognostic factor.Citation3–Citation5 In our population, 91.6% of the patients were diagnosed with local disease in 2008–2012 (92.8% including unspecified stage; ), a lower proportion than that reported from Sweden in 1990–1999 (95.3%)Citation20 and Central Europe in 1984–1996 (96.2%)Citation21 and comparable to that in the SEER cohort in 1989–2009 (92.4%).Citation25 Thus, Norwegian CM patients seem to be diagnosed in a more-advanced disease stage, explaining the lower survival relative to CM patients in other registries. Local disease was also less common in men (89.6%) than in women (93.7%), contributing to higher mortality rates among men.
The high proportions of T3 and T4 tumors, as well as NM, among the fatal CM cases are of special interest. In data from SEERCitation22 and Queensland, Australia,Citation32 the proportions of T1 tumors among CM patients with fatal outcome were 27% and 23%, respectively, ie, higher than that in the present study (12.1%). The NM/SSM ratio was 0.79 among the fatal CM cases in SEER in 1978–2007Citation33 and 1.45 in Victoria, Australia, in 1989–2004.Citation34 In Sweden, conflicting ratios for fatal CM have been published for local disease: 1.38 for 1990–1999Citation20 and 0.5 for 1989–2003.Citation35 We found an NM/SSM ratio of 1.4 for fatal CM and 1.1 for CM with local disease at diagnosis. Hence, the proportion of NM compared to SSM, both in incident and fatal cases, seems to be high in Norway compared to most other population-based registries. In SEER, the contribution of NM to fatal CM (excluding unspecified) was reported to be stable between 1978 and 2007 (37%) and SSM was the largest contributor to CM-specific death (46%).Citation33 In our study and in the study from Victoria, Australia,Citation34 NM was the main contributor to CM-specific death, 55% and 50%, respectively. Symmetrical NM is more easily misdiagnosed compared to SSM,Citation36 and a low diagnostic sensitivity for NM compared to SSM is documented.Citation37 Tumors of the NM type are escaping the established asymmetry, border, color, diameter, evolving (ABCD[E]) diagnostic guideline,Citation38 which affects awareness and thus prognosis. We found an association between melanoma subtype and CM-specific death in crude analysis only, in line with the literature, which states that the prognostic significance of melanoma subtype is due to tumor thickness.Citation4 CMs with high growth rate are associated with male sex, age >70 years, as well as symmetry and elevation of the lesion,Citation39 which together with low awareness, may explain the high mortality rate in Norway.
Individuals with a history of CM are found to have an increased risk of a second CM diagnosis,Citation40 and this may have an effect on survival in CM. We found that multiple primary CMs during the study period are a prognostic factor for CM-specific death, in accordance with a study by Rowe et al.Citation41
The main limitation of this study is the proportion of cases with missing clinical and histopathological data. We found that cases with unspecified data on T-stage had elevated risk of CM-specific death, indicating more advanced disease in these patients. Lack of information may result from incomplete diagnostic procedures in cases with thick tumors, distant metastases, or an unknown primary tumor. We had complete data on the anatomic site, melanoma subtype, Breslow thickness, ulceration, and clinical stage for 61.7% of the patients, a proportion in line with the Swedish data from 1990–1999 (65.1%).Citation20 According to the Norwegian guidelines for diagnosis, treatment, and follow-up of CM in the period 2008–2015,Citation14 no modifications have been done with regard to reporting and registration of clinical stage, Clark, subtype, or Breslow thickness during the study period. We used multiple imputation to impute missing data, and importantly, results from the Cox regression analyses of the prognostic factors were similar for the imputed and the complete-case data. Unfortunately, information on comorbidity was not available. This may be of particular importance for diagnostic procedures and mortality prediction in elderly patients. Information on education and ethnicity was not available. Educational level may influence sun exposure habits, health concerns, and doctor delay. Ethnicity may be of less relevance as Norway has a public health care system that aims to provide equal access to health care. Furthermore, except for breast cancer, no stage-based differences at diagnosis were observed between immigrant groups and Norwegians in a recent study.Citation42
During the final part of the study period, immunotherapies and kinase inhibitors have been implemented in the treatment of advanced CM in Norway. Thus, future comparisons of survival and mortality data must take secular trends into account, as new treatments for distant metastases are emerging.
Conclusion
The high mortality rates among Norwegian CM patients may be explained by the more-advanced disease stage at diagnosis compared with the rates among CM patients in other countries. The high proportion of NM may contribute to high mortality rates, and the occurrence of more-advanced disease in men can explain the difference seen between the sexes. The results indicate an impact of patient delay because the highest incidence rates, the worst prognostic factors, and the highest risk of CM-specific death are found among older patients, especially among men; hence, efforts should be made to improve secondary prevention of CM in these patients. The clinical characteristics of NM must be communicated to both health care professionals and the public for increased awareness.
Availability of data and material
The dataset analyzed during the current study is available at the Cancer Registry of Norway on reasonable request.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank Siri Larønningen and Hilde Hedemann Brenn of the Cancer Registry of Norway (CRN), as well as the Norwegian Melanoma Group, for supply of data.
Supplementary materials
Table S1 Distribution of the imputed variables
Table S2 HRs for CM death, with 95% CIs, by sex, age, anatomic site, melanoma subtype, T-stage, ulceration, clinical stage, and presence of a second primary CM from crude and multivariate analyses of complete-case data (n=5010)
Disclosure
The authors report no conflicts of interest in this work.
References
- ForseaAMDel MarmolVde VriesEBaileyEEGellerACMelanoma incidence and mortality in Europe: new estimates, persistent disparitiesBr J Dermatol201216751124113022759278
- Cancer Registry of NorwayCancer in Norway 2016 – Cancer Incidence, Mortality, Survival and Prevalence in NorwayOsloCancer Registry of Norway2017
- BalchCMGershenwaldJESoongSJFinal version of 2009 AJCC melanoma staging and classificationJ Clin Oncol200927366199620619917835
- ScolyerRAJudgeMJEvansAInternational Collaboration on Cancer ReportingData set for pathology reporting of cutaneous invasive melanoma: recommendations from the international collaboration on cancer reporting (ICCR)Am J Surg Pathol201337121797181424061524
- AminMBGreeneFLEdgeSBThe eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer stagingCA Cancer J Clin2017672939928094848
- TryggvadóttirLGislumMHakulinenTTrends in the survival of patients diagnosed with malignant melanoma of the skin in the Nordic countries 1964–2003 followed up to the end of 2006Acta Oncol201049566567220491525
- LythJErikssonHHanssonJTrends in cutaneous malignant melanoma in Sweden 1997–2011: thinner tumours and improved survival among menBr J Dermatol2015172370070625323770
- BayCKejsAMStormHHEngholmGIncidence and survival in patients with cutaneous melanoma by morphology, anatomical site and TNM stage: a Danish Population-Based Register study 1989–2011Cancer Epidemiol20153911725468643
- RobsahmTEBergvaGHestvikUEMollerBSex differences in rising trends of cutaneous malignant melanoma in Norway, 1954–2008Melanoma Res2013231707823222548
- AutierPKoechlinABoniolMThe forthcoming inexorable decline of cutaneous melanoma mortality in light-skinned populationsEur J Cancer201551786987825771950
- WeyersWThe ’epidemic’ of melanoma between under- and overdiagnosisJ Cutan Pathol201239191622211330
- LarsenIKSmastuenMJohannesenTBData quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timelinessEur J Cancer20094571218123119091545
- World Health OrganizationInternational Classification of Diseases for OncologyThird Edition First Revision edGenevaWorld Health Organization2013
- The National Guidelines for Diagnosis, Treatment and Follow-Up of Malignant MelanomasNorwegian: Directorate of Health Available from: https://helsedirektoratet.no/retningslinjer/nasjonalt-handlings-program-med-retningslinjer-for-diagnostikk-behandling-og-oppfol-ging-av-maligne-melanomerAccessed September 1, 2017
- AndersenPKGeskusRBde WitteTPutterHCompeting risks in epidemiology: possibilities and pitfallsInt J Epidemiol201241386187022253319
- SatagopanJMBen-PoratLBerwickMRobsonMKutlerDAuerbachADA note on competing risks in survival data analysisBr J Cancer20049171229123515305188
- PintilieMAn introduction to competing risks analysisRev Esp Cardiol201164759960521621892
- KhosrotehraniKDasguptaPByromLYouldenDRBaadePDGreenACMelanoma survival is superior in females across all tumour stages but is influenced by ageArch Dermatol Res20153071873174026103951
- WhiteIRRoystonPWoodAMMultiple imputation using chained equations: issues and guidance for practiceStat Med201130437739921225900
- LindholmCAnderssonRDufmatsMSwedish Melanoma Study GroupInvasive cutaneous malignant melanoma in Sweden, 1990–1999. A prospective, population-based study of survival and prognostic factorsCancer200410192067207815372475
- GarbeCMcLeodGRBuettnerPGTime trends of cutaneous melanoma in Queensland, Australia and Central EuropeCancer20008961269127811002222
- CriscioneVDWeinstockMAMelanoma thickness trends in the United States, 1988–2006J Invest Dermatol2010130379379719829301
- BaadePMengXYouldenDAitkenJYoulPTime trends and latitudinal differences in melanoma thickness distribution in Australia, 1990–2006Int J Cancer2012130117017821344376
- KruijffSBastiaannetEFranckenABSchaapveldMvan der AaMHoekstraHJBreslow thickness in the Netherlands: a population-based study of 40 880 patients comparing young and elderly patientsBr J Cancer2012107357057422713665
- ShaikhWRDuszaSWWeinstockMAOliveriaSAGellerACHalpernACMelanoma thickness and survival trends in the United States, 1989 to 2009J Natl Cancer Inst20151081djv29426563354
- LasithiotakisKLeiterUMeierFAge and gender are significant independent predictors of survival in primary cutaneous melanomaCancer200811281795180418306371
- MagnusKThe Nordic profile of skin cancer incidence. A comparative epidemiological study of the three main types of skin cancerInt J Cancer199147112191985867
- BalchCMSoongSRossMILong-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Inter-group Melanoma Surgical TrialAnn Surg Oncol200072879710761786
- JimenezREPanageasKBusamKJBradyMSPrognostic implications of multiple lymphatic basin drainage in patients with truncal melanomaJ Clin Oncol200523351852415659497
- ShaikhWRWeinstockMAHalpernACOliveriaSAGellerACDuszaSWThe characterization and potential impact of melanoma cases with unknown thickness in the United States’ Surveillance, Epidemiology, and End Results Program, 1989–2008Cancer Epidemiol2013371647022995853
- Månsson-BrahmeEJohanssonHSingnomklaoTLarsonORutquistLERingborgUTime Trends in Survival in Cutaneous Malignant Melanoma; a Population-Based Study in SwedenCutaneous Malignant Melanoma. Aspects on Prognostical Factors and Time-Trends in a Swedish Population [thesis]. Paper ll128StockholmKarolinska University Press2002
- WhitemanDCBaadePDOlsenCMMore people die from thin melanomas (1 mm) than from thick melanomas (>4 mm) in Queensland, AustraliaJ Invest Dermatol201513541190119325330295
- ShaikhWRXiongMWeinstockMAThe contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007Arch Dermatol20121481303621931016
- MarVRobertsHWolfeREnglishDRKellyJWNodular melanoma: a distinct clinical entity and the largest contributor to melanoma deaths in Victoria, AustraliaJ Am Acad Dermatol201368456857523182058
- ErikssonHFrohm-NilssonMJarasJPrognostic factors in localized invasive primary cutaneous malignant melanoma: results of a large population-based studyBr J Dermatol2015172117518624910143
- CicchielloMLinMJPanYMcLeanCKellyJWAn assessment of clinical pathways and missed opportunities for the diagnosis of nodular melanoma versus superficial spreading melanomaAustralas J Dermatol20165729710126563931
- LinMJMarVMcLeanCWolfeRKellyJWDiagnostic accuracy of malignant melanoma according to subtypeAustralas J Dermatol2014551354224283461
- McGovernTWLitakerMSClinical predictors of malignant pigmented lesions. A comparison of the Glasgow seven-point checklist and the American Cancer Society’s ABCDs of pigmented lesionsJ Dermatol Surg Oncol199218122261740563
- LiuWDowlingJPMurrayWKRate of growth in melanomas: characteristics and associations of rapidly growing melanomasArch Dermatol2006142121551155817178980
- RobsahmTEKaragasMRReesJRSyseANew malignancies after squamous cell carcinoma and melanomas: a population-based study from NorwayBMC Cancer20141421024645632
- RoweCJLawMHPalmerJMMacGregorSHaywardNKKhosrotehraniKSurvival outcomes in patients with multiple primary melanomasJ Eur Acad Dermatol Venereol201529112120212725864459
- ThøgersenHMøllerBRobsahmTEAaserudSBabigumiraRLarsenIKComparison of cancer stage distribution in the immigrant and host populations of Norway, 1990–2014Int J Cancer20171411526128369751
