Abstract
Purpose
Substantial heterogeneity exists in reported kidney function decline in pre-dialysis chronic kidney disease (CKD). By design, kidney function decline can be studied in CKD 3–5 cohorts or dialysis-based studies. In the latter, patients are selected based on the fact that they initiated dialysis, possibly leading to an overestimation of the true underlying kidney function decline in the pre-dialysis period. We performed a systematic review and meta-analysis to compare the kidney function decline during pre-dialysis in CKD stage 3–5 patients, in these two different study types.
Patients and methods
We searched PubMed, EMBASE, Web of Science and Cochrane to identify eligible studies reporting an estimated glomerular filtration rate (eGFR) decline (mL/min/1.73 m2) in adult pre-dialysis CKD patients. Random-effects meta-analysis was performed to obtain weighted mean annual eGFR decline.
Results
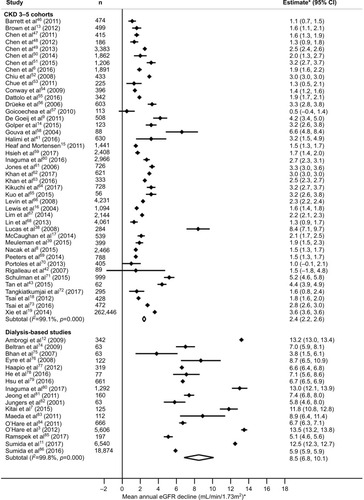
We included 60 studies (43 CKD 3–5 cohorts and 17 dialysis-based studies). The meta-analysis yielded a weighted annual mean (95% CI) eGFR decline during pre-dialysis of 2.4 (95% CI: 2.2, 2.6) mL/min/1.73 m2 in CKD 3–5 cohorts compared to 8.5 (95% CI: 6.8, 10.1) in dialysis-based studies (difference 6.0 [95% CI: 4.8, 7.2]).
Conclusion
To conclude, dialysis-based studies report faster mean annual eGFR decline during pre-dialysis than CKD 3–5 cohorts. Thus, eGFR decline data from CKD 3–5 cohorts should be used to guide clinical decision making in CKD patients and for power calculations in randomized controlled trials with CKD progression during pre-dialysis as the outcome.
Introduction
Chronic kidney disease (CKD) is a major public health problem worldwide with poor clinical outcomes.Citation1 Prevalence and incidence of CKD are increasing rapidly, and the demand for pre-dialysis care is growing.Citation2 Pre-dialysis care aims to slow down decline in kidney function and to prepare patients for their potential start of renal replacement therapy (RRT; dialysis and kidney transplantation). Detailed knowledge of the rate of kidney function decline in moderate to advanced CKD patients before the start of RRT could guide clinical decision making and anticipate treatment choices and priorities.Citation3–Citation5
Studies among CKD patients point to substantial heterogeneity in kidney function decline during the pre-dialysis period.Citation3,Citation6–Citation12 The estimated glomerular filtration rate (eGFR) is commonly used as a measure for renal insufficiency in CKD patients during the pre-dialysis period. Kidney function decline during the pre-dialysis trajectory can be studied in CKD 3–5 cohorts, or in a subgroup of patients who initiated dialysis at some point, dialysis-based studies (Figure S1).Citation3,Citation11–Citation19 These populations differ with regard to patient selection. In CKD 3–5 cohorts, patients are followed from a certain point in the pre-dialysis phase and an overall eGFR decline is reported, while not all patients end up on RRT. When patients on dialysis are selected (dialysis-based studies), eGFR decline is determined in a specified period prior to this dialysis initiation. As a consequence, we hypothesize that decline rates obtained from dialysis-based studies overestimate the true underlying kidney function decline in the overall pre-dialysis CKD population (see Supplementary material 1 for a more detailed theoretical explanation).
A comprehensive characterization of the actual magnitude of annual kidney function decline during the pre-dialysis period is essential for clinical decision making in the management of CKD patients, including the anticipation of dialysis onset. It is also important for power calculations of randomized controlled trials aimed to study kidney disease progression. Therefore, we aimed to perform a systematic review and meta-(regression) analysis to assess and compare kidney function decline during the pre-dialysis trajectory between CKD 3–5 cohorts and dialysis-based studies.
Patients and methods
Eligibility criteria
We searched for studies reporting kidney function decline in the pre-dialysis period (CKD stage 3–5 [eGFR <60 mL/min/1.73 m2]) in adult populations. The following inclusion criteria were applied: studies which defined and reported kidney function decline as eGFR or creatinine clearance were eligible, comprising a four-variable modification of diet in renal disease (MDRD), Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation or Cockcroft–Gault formula.Citation20–Citation24 In case of multiple studies describing the same study population and study outcome, the study with the most complete data was selected. Only studies comprising a population of 50 patients or more were included. Meeting abstracts, case reports, editorials and animal studies were excluded. Also, articles in languages other than English, French, German, Dutch or Spanish were not eligible.
Search strategy
We searched in PubMed, EMBASE, Web of Science and the Cochrane Database for eligible literature published between January 2000 and December 2016 (both published and epubs published in advance, Supplementary material 2). Furthermore, references of key articles were searched to identify potentially relevant studies. The systematic review was conducted according to the PRISMA guidelines.Citation25
Data extraction
Studies retrieved from the search strategy were entered into reference manager software (EndNote X7) and were screened based on title and abstract. Potentially relevant studies were retrieved for detailed assessment. For eligible studies, data were independently extracted by two reviewers (CJJ and CCEH). Disagreements between reviewers were resolved by consensus, or by a third reviewer (OMD) in case of remaining doubt.
For all included studies, the following data were extracted and entered into an electronic database: first author and year of publication, number of participants and population studied, setting (e.g., referral center/name of study and country), mean age, proportion of male and diabetes, kidney function measure (e.g., MDRD, CKD-EPI, Cockcroft–Gault formula), duration of pre-dialysis period, mean baseline eGFR and unadjusted rates of estimated annual kidney function decline (mL/min/1.73 m2).
For CKD 3–5 cohorts, we extracted data on the number/proportion of patients lost to follow-up and the proportion/number of patients who started dialysis or died before the end of the study. When CKD 3–5 cohorts reported both an overall kidney function decline rate during the pre-dialysis period and a separate kidney function decline for patients starting dialysis, the overall decline of the CKD 3–5 cohort was extracted. In case no patient in the CKD 3–5 cohorts reached dialysis/RRT, these cohorts were excluded and the length of follow-up during the pre-dialysis period was considered to be too short.
For dialysis-based studies, we also extracted data on the value of kidney function at the moment of dialysis initiation. For these studies, loss to follow-up was not applicable. Noteworthy, the unit of eGFR values is reported as mL/min/1.73 m2, which is correct using the MDRD or CKD-EPI equation. However, the Cockcroft–Gault formula estimates the creatinine clearance and is expressed in mL/min, without correction for body surface area. The creatinine clearance exceeds the GFR because creatinine is also secreted by the proximal tubule as well as filtered by the glomerulus. For the sake of readability, we have chosen to report all eGFR and creatinine clearance values as mL/min/1.73 m2 for consistency, and because only a few studies reported the creatinine clearance values based on the Cockcroft–Gault formula.
Risk of bias assessment
Risk of bias assessment focused on design elements that could potentially bias the assessment of kidney function decline in CKD patients during the pre-dialysis period:
Adequacy of measurement of kidney function decline. The CKD-EPI and MDRD equation were considered adequate methods for measurement of eGFR. The Cockcroft–Gault formula was considered high risk of bias.Citation23,Citation26
A proportion of loss to follow-up <10% was considered low risk of bias (CKD 3–5 cohorts).
Selection of patients: Inclusion of consecutive CKD 3–5 or dialysis patients was considered adequate. As an alternative, a random sample of all CKD 3–5 or dialysis patients was also considered adequate.
Elements of risk of bias assessment and potential differences of these elements between studies were used to explore potential between-study heterogeneity. Studies with low risk of bias assessment for all elements were rated as low risk of bias overall. Because only two of these three elements applied to dialysis-based studies, risk of bias assessment was repeated for CKD 3–5 cohorts using only these two selection criteria.
Statistical analysis
The main outcome of this meta-analysis was the weighted annual eGFR decline. Results were presented separately for CKD 3–5 cohorts and dialysis-based studies. When a monthly kidney function decline was reported, the decline rate was multiplied by 12 to estimate the annual decline rate. For papers presenting results as median with interquartile range, we recalculated this to the accompanying mean with SD.Citation27,Citation28 Furthermore, in case a paper provided separate kidney function declines for subgroups and no decline rate for the whole study population, we calculated a weighted mean with a pooled SD in a fixed-effect model.Citation28 For the included studies reporting no kidney function decline, the kidney function values (including variance) at the start and end of follow-up/at dialysis initiation were used to estimate an annual mean decline rate with pooled SD.
Meta-analysis was performed using the DerSimonian and Laird method.Citation29 Given the expected clinical heterogeneity, a random-effects model was performed to take the between-study variation into account and no fixed-effects analysis was performed (unless less than five studies presented data for a specific outcome). Between-study heterogeneity was estimated using the I2 statistic.Citation28 For the risk of bias assessment, a meta-analysis was also performed for subgroups according to the risk of bias status for both CKD 3–5 cohorts and dialysis-based studies.
Several preplanned univariate random-effects meta-regression analyses were performed. First, the annual eGFR decline from CKD 3–5 cohorts and dialysis-based studies was compared. Sources of heterogeneity for different reported mean annual eGFR decline rates were identified in CKD 3–5 cohorts, as these studies better reflect an inception cohort (Supplementary material 1). We investigated the association between the mean eGFR decline and the proportion of patients with diabetes in the study population, as diabetes is known to increase kidney function decline.Citation30 Furthermore, we investigated the association between the mean eGFR decline and the proportion of males in the study population, given the existing paradox that CKD 3–5 is more prevalent among women, although women are less likely to start dialysis.Citation31 Another important source of heterogeneity might be the nonlinear kidney function decline over time.Citation3,Citation32–Citation34 To test whether the linearity assumption was violated, we performed univariate random-effects meta-regression analysis between the annual eGFR decline and two explanatory variables: duration of pre-dialysis period and mean baseline eGFR of the study population. If either of these associations was significant, this could be explained by a violation of the linearity assumption. To investigate the presence of potential publication bias, we assessed the association between the study size and the magnitude of reported eGFR decline by investigating the presence of funnel plot asymmetry, using Egger’s test.Citation35
Several sensitivity analyses were performed to validate the robustness of the results. Since random-effects models fitted by the DerSimonian and Laird method could negatively bias the between-study variance, meta-analysis was also fitted by restricted maximum likelihood.Citation29,Citation36,Citation37 Furthermore, in CKD 3–5 cohorts, a stratified meta-analysis according to CKD stages, based on the mean baseline eGFR of each cohort, was performed. We did not perform subgroup analyses to assess whether or not the slope of decline in eGFR and creatinine clearance was different between the three formulas (i.e., MDRD versus CKD-EPI, and Cockcroft–Gault versus MDRD and CKD-EPI) or primary kidney disease, due to small subgroups or lack of information. Statistical analyses were performed with Stata Statistical Software 14.0 (Stata-Corp LP, College Station, TX, USA).
Results
Search results
We identified 1231 unique publications by searching PubMed, EMBASE, Cochrane Database, Web of Science and by screening reference lists of included articles (n=60). After exclusion of 1,143 publications by screening of title and abstract, 88 publications were retrieved for detailed assessment, of which 60 fulfilled the inclusion criteria. To avoid multiple inclusions of the same study participants and the same study outcome, we excluded 10 publications originating from the same study populations (Supplementary material 3) and included the publication with the most complete data. Of the 60 included publications, 43 studies presented data based on CKD 3–5 cohorts and 17 studies presented data based on dialysis-based studies ().
Study characteristics
Study characteristics of the 60 included studies are summarized in . In most studies, the kidney function measure during the pre-dialysis period was based on an MDRD equation (31 CKD 3–5 cohorts and 10 dialysis-based studies). In total, only six studies used the CKD-EPI equation and three studies used the Cockcroft–Gault equation. In CKD 3–5 cohorts, the mean pre-dialysis follow-up period ranged from 0.4 to 8.2 years and the mean baseline eGFR was between 10 and 45 mL/min/1.73 m2. Individual study characteristics of the included studies are shown in Tables S1 and S2.
Table 1 Characteristics of included CKD 3–5 cohorts and dialysis-based studies
Risk of bias assessment
The risk of bias assessment is summarized in Table S3. Only three studies used the Cockcroft–Gault formula (two CKD 3–5 cohorts and one dialysis-based study). In CKD 3–5 cohorts, the percentage loss to follow-up ranged from 1% to 41%. Twelve studies had a loss to follow-up of <10% (low risk of bias), and nine studies had a loss to follow-up of >10%; in most studies, the percentage loss to follow-up was unclear. For 19 CKD 3–5 cohorts and 10 dialysis-based studies, consecutive or random patient sampling was applied. However, the sampling method was unclear for most studies.
Annual eGFR decline in CKD 3–5 versus dialysis-based studies
In a random-effects meta-analysis, the weighted mean annual eGFR decline was 2.4 (95% CI: 2.2, 2.6; I2 99.1%) and 8.5 (95% CI: 6.8, 10.1; I2 99.8%) mL/min/1.73 m2 in CKD 3–5 cohorts and dialysis-based studies, respectively ().
Figure 2 Random-effects meta-analyses of weighted annual eGFR decline during the pre-dialysis period based on CKD 3–5 cohorts or dialysis-based studies.
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Identification of sources of heterogeneity using meta-regression analysis
Univariate meta-regression analysis showed a large difference in kidney function decline between CKD 3–5 cohorts and dialysis-based studies: difference 5.99 mL/min/1.73 m2/year, (95% CI: 4.80, 7.19). It is important to identify which cohort characteristics are associated with a faster mean annual kidney function decline, such as the proportion of diabetes or males in the study population. The mean annual eGFR decline and the proportion of diabetes in CKD 3–5 cohorts were not significantly associated in meta-regression analysis (per 10%, β=0.06 mL/min/1.73 m2, 95% CI: −0.14, 0.27; Figure S2A). We should note here that there was one outlier with a reported mean annual kidney function decline of 8.4 (±11.1) mL/min/1.73 m2 and only 9.2% of the population had diabetes.Citation38 After exclusion of this outlier, the meta-regression analysis yielded a significant association between annual eGFR decline and the proportion of participants with diabetes in CKD 3–5 cohorts (β=0.18 mL/min/1.73 m2, 95% CI: 0.04, 0.33; Figure S2B). This equates to a 0.18 mL/min/1.73 m2 increase in weighted mean annual eGFR decline for every 10% increase in the proportion of participants with diabetes. The mean annual eGFR decline and the proportion of males in CKD 3–5 cohorts were not significantly associated in meta-regression (per 10%, β=0.12 mL/min/1.73 m2, 95% CI: −0.36, 0.60). Meta-regression analysis showed that the mean annual eGFR decline in the pre-dialysis period was not clearly associated with the duration of the pre-dialysis period (difference=0.19 mL/min/1.73 m2, 95% CI: −0.09, 0.48) or the mean baseline eGFR value (difference=0.01 mL/min/1.73 m2, 95% CI: −0.06, 0.05) in CKD 3–5 cohorts. We found an association between the study size and the magnitude of reported mean annual eGFR decline for CKD 3–5 cohorts (Egger’s test p value=0.002) and no clear association for dialysis-based studies (Egger’s test p value=0.11; see Figure S3 for funnel plots).
Sensitivity and subgroup analysis
For CKD 3–5 cohorts, 6 studies were assessed as low risk of bias and 37 as high risk of bias, with a weighted mean annual eGFR decline of 2.6 (95% CI 2.0, 3.2) and 2.4 (2.2, 2.7) mL/min/1.73 m2, respectively. For dialysis-based studies, 7 studies were assessed as low risk of bias and 10 as high risk of bias, with a weighted mean (95% CI) annual eGFR decline of 8.2 (6.5, 9.9) and 8.7 (6.8, 10.1) mL/min/1.73 m2, respectively. Risk of bias assessment was repeated for CKD 3–5 cohorts using the two selection criteria applied to dialysis-based studies. This yielded similar weighted mean annual eGFR decline rates of 2.6 (95% CI: 2.3, 3.0) and 2.4 (2.0, 2.6) mL/min/1.73 m2 for studies with low risk and high risk of bias, respectively. In the subgroup analysis for CKD stage 3a, 3b, 4 and 5, the decline rates were 1.7 (3 cohorts; 95% CI: 1.4, 2.1), 2.4 (17 cohorts; 95% CI: 2.0, 2.7), 2.5 (21 cohorts; 95% CI: 2.2, 2.8) and 3.0 (2 cohorts; 95% CI: 0.8, 5.3) mL/min/1.73 m2, respectively. In a random-effects meta-analysis using linear mixed models fitted with restricted maximum likelihood, similar results were obtained.
Discussion
This meta-analysis showed that the reported mean annual eGFR decline during the pre-dialysis period is larger in patients from dialysis-based studies compared to that in CKD 3–5 cohorts. We found that the weighted mean annual eGFR decline was 8.5 (95% CI: 6.8, 10.1) in dialysis-based studies compared to 2.4 (95% CI: 2.2, 2.6) mL/min/1.73 m2 in CKD 3–5 cohorts. Importantly, CKD 3–5 cohorts are more likely to represent the true eGFR decline prior to dialysis, given the way dialysis-based studies select their patients. These results underline that eGFR decline estimations from CKD 3–5 cohorts, as opposed to dialysis-based studies, should be used for clinical decision making in CKD 3–5 patients, such as in the context of anticipating treatment decisions and priorities, for instance, the moment to start dialysis. These eGFR decline estimations from CKD 3–5 cohorts should also be used for power calculations in randomized controlled trials with kidney disease progression in pre-dialysis CKD patients as a primary outcome.
To our knowledge, this is the first meta-analysis directly comparing the annual eGFR decline in CKD 3–5 cohorts and dialysis-based studies. A number of previous CKD 3–5 cohorts reported both an overall eGFR decline and an eGFR decline for patients who initiated dialysis, as in dialysis-based studies. In these studies, the reported annual eGFR decline for the whole CKD population ranged between 1.5 and 2.1 mL/min/1.73 m2, and for patients who initiated dialysis, it ranged between 3.9 and 7.3 mL/min/1.73 m2.Citation15,Citation17,Citation18 Previous literature is in line with our finding that the mean annual rates of kidney function decline in CKD 3–5 cohorts are much lower than in dialysis-based studies.
CKD 3–5 cohorts comply with the definition of an inception cohort, in which patients are included from a well-defined point in the course of kidney disease progression, irrespective of their outcome. However, in dialysis-based studies, patients are selected based on their outcome, that is, dialysis start, providing biased estimates of kidney function decline in CKD 3–5 patients (for more in-depth explanation of the inception cohort, see Supplementary material 1). An intuitive interpretation is that some patients in CKD 3–5 cohorts will only progress very slowly, or even stay stable for such a long period that they will never initiate dialysis. Such patients are not included in dialysis-based studies. This is also shown empirically in the Netherlands: during the first years on pre-dialysis care, 45%–64% of CKD 4–5 patients start dialysis therapy; 1%–8% of these patients receive a kidney transplant as the first form of RRT and 5%–7% die without receiving any form of RRT.Citation9,Citation10,Citation39,Citation40
We should acknowledge substantial study diversity was present in our meta-analysis. We used different methods to identify sources of heterogeneity, including differences in risk of bias, publication bias or heterogeneity due to study diversity. Risk of bias assessment showed that the mean annual eGFR decline did not materially differ between studies with a low risk of bias compared to those with a high risk of bias, for both CKD 3–5 cohorts and dialysis-based studies.
Surprisingly, we did not find a strong association between the proportion of diabetes and the mean annual eGFR decline in our meta-analysis. This could be due to one outlier, with only 9.2% of diabetics in the CKD population and a mean annual rate of kidney function decline of 8.4 (±SD 11.1) mL/min/1.73 m2.Citation38 This high annual eGFR decline could be explained by the fact that the study population comprised human immunodeficiency virus–positive patients and was mostly of African-American origin. Both human immunodeficiency virus and African-American descent are well-known risk factors for a greater annual eGFR decline.Citation30 After exclusion of this outlier, the association became significant, in line with our current understanding of the association between diabetes and kidney function. Of note, in our meta-analysis, three CKD 3–5 cohorts comprised only diabetic CKD 3–5 patients, showing mean annual eGFR decline rates of 1.5, 3.2 and 4.4 mL/min/1.73 m2, respectively.Citation41–Citation43 It should be emphasized that a meta-analysis with study-level data is not optimal to assess the association between variables such as diabetes and eGFR decline.Citation44
A major strength of this study is that the mean annual eGFR decline was investigated separately and compared for CKD 3–5 cohorts and dialysis-based studies. Also, a large number of studies were included (n=60), comprising 43 CKD 3–5 cohorts and 17 dialysis-based studies. Therefore, the weighted effect estimates were not influenced largely by random error and it was possible to examine sources of heterogeneity within the CKD 3–5 cohorts.
Our study has some potential limitations. First, the outcome kidney function decline was not always reported in the title or abstract, which made assessing eligibility cumbersome. Second, we assumed a linear decline in kidney function in our modeling, although it has been proposed in the nephrology literature that this is not necessarily the case.Citation3,Citation19,Citation45 However, meta-regression techniques are known to have difficulties with correct model specification. In our meta-regression, we could not show an association between either the mean duration of the pre-dialysis period or the mean baseline eGFR value and the reported annual eGFR decline in CKD 3–5 cohorts, which suggests that the linear assumption is not violated. In other words, the reported annual eGFR decline did not significantly differ for varying durations of the pre-dialysis period or mean baseline eGFR values reported in the included cohorts. Third, publication bias is an issue of concern in all meta-analyses. In our analysis, we aimed to study the decline in eGFR, and it is difficult to predict what role publication bias could play when assessing a descriptive outcome such as eGFR. We found an association between study size and reported eGFR magnitude for CKD 3–5 cohorts, implying that publication bias could be present. However, in the funnel plot, no clear pattern is visible that studies with a smaller sample size tend to report smaller or larger annual eGFR decline than studies with a larger sample size. Finally, we did not have individual patient data.
Conclusion
In summary, we showed that the reported mean annual eGFR decline during the pre-dialysis period is much larger in patients from dialysis-based studies compared to that in CKD 3–5 cohorts. Importantly, implications for clinical decision making with regard to the management of CKD patients during the pre-dialysis period and the active planning of RRT should be based on CKD 3–5 cohorts, due to the improper selection of the CKD population in dialysis-based studies.
Acknowledgments
The authors acknowledge Jan W Schoones (Walaeus Library, Leiden University Medical Center, Leiden, the Netherlands) for his help in the search strategy. Preliminary versions of these data were published in abstract communication and poster presentation at the European Dialysis and Transplant Association (EDTA) conference, June 3–6, 2017 in Madrid, Spain.
Disclosure
The authors report no conflicts of interest in this work.
References
- EckardtKUCoreshJDevuystOEvolving importance of kidney disease: from subspecialty to global health burdenLancet2013382988715816923727165
- LozanoRNaghaviMForemanKGlobal and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592095212823245604
- O’HareAMBattenABurrowsNRTrajectories of kidney function decline in the 2 years before initiation of long-term dialysisAm J Kidney Dis201259451352222305760
- RosanskySEarly dialysis initiation and renal function trajectoryJ Intern Med2011269327527721083856
- MurtaghFEMurphyESheerinNSIllness trajectories: an important concept in the management of kidney failureNephrol Dial Transplant200823123746374818809974
- ChenCHWuHYWangCLProteinuria as a therapeutic target in advanced chronic kidney disease: a Retrospective Multicenter Cohort StudySci Rep201662653927198863
- KitaiYDoiYOsakiKSugiokaSKoshikawaMSugawaraANephrotic range proteinuria as a strong risk factor for rapid renal function decline during pre-dialysis phase in type 2 diabetic patients with severely impaired renal functionClin Exp Nephrol20151961037104325680889
- NacakHvan DiepenMQureshiARUric acid is not associated with decline in renal function or time to renal replacement therapy initiation in a referred cohort of patients with Stage III, IV and V chronic kidney diseaseNephrol Dial Transplant201530122039204526185050
- de GoeijMCVoormolenNHalbesmaNPREPARE-1 study groupAssociation of blood pressure with decline in renal function and time until the start of renal replacement therapy in pre-dialysis patients: a cohort studyBMC Nephrol2011123821835038
- NacakHvan DiepenMde GoeijMCRotmansJIDekkerFWPREPARE-2 study group. Uric acid: association with rate of renal function decline and time until start of dialysis in incident pre-dialysis patientsBMC Nephrol2014159124939671
- SumidaKMolnarMZPotukuchiPKAssociation between vascular access creation and deceleration of estimated glomerular filtration rate decline in late-stage chronic kidney disease patients transitioning to end-stage renal diseaseNephrol Dial Transplant201732813301337
- AmbrogiVThillyNBoiniSPatterns and predictors of kidney function decline in the last year prior to dialysisNephron Clin Pract20091112c95c10119142021
- BrownRNMohsenAGreenDBody mass index has no effect on rate of progression of chronic kidney disease in non-diabetic subjectsNephrol Dial Transplant20122772776278022442391
- GolperTAHartlePMBianAArteriovenous fistula creation may slow estimated glomerular filtration rate trajectoryNephrol Dial Transplant201530122014201825888388
- HeafJGMortensenLSUraemia progression in chronic kidney disease stages 3–5 is not constantNephron Clin Pract20111184c367c37421325868
- LewisJGreeneTAppelLA comparison of iothalamate-GFR and serum creatinine-based outcomes: acceleration in the rate of GFR decline in the African American Study of Kidney Disease and HypertensionJ Am Soc Nephrol200415123175318315579521
- McCaughanJACourtneyAEMaxwellAPEstimated glomerular filtration rate decline as a predictor of dialysis in kidney transplant recipientsAm J Nephrol201439429730524732139
- TsaiYCChiuYWHungCCAssociation of symptoms of depression with progression of CKDAm J Kidney Dis2012601546122495469
- XieYBoweBXianHBalasubramanianSAl-AlyZEstimated GFR trajectories of people entering CKD stage 4 and subsequent kidney disease outcomes and mortalityAm J Kidney Dis201668221922826948835
- CockcroftDWGaultMHPrediction of creatinine clearance from serum creatinineNephron197616131411244564
- LeveyASBoschJPLewisJBGreeneTRogersNRothDA more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study GroupAnn Intern Med1999130646147010075613
- LeveyASCoreshJGreeneTChronic Kidney Disease Epidemiology CollaborationUsing standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rateAnn Intern Med2006145424725416908915
- LeveyASStevensLAEstimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictionsAm J Kidney Dis201055462262720338463
- LeveyASStevensLASchmidCHCKD-EPI (Chronic Kidney Disease Epidemiology Collaboration)A new equation to estimate glomerular filtration rateAnn Intern Med2009150960461219414839
- MoherDLiberatiATetzlaffJAltmanDGGroup PPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementBMJ2009339b253519622551
- WhiteSLPolkinghorneKRAtkinsRCChadbanSJComparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) StudyAm J Kidney Dis201055466067020138414
- HozoSPDjulbegovicBHozoIEstimating the mean and variance from the median, range, and the size of a sampleBMC Med Res Methodol200551315840177
- HigginsJPTGreenSCochrane Handbook for Systematic Reviews of InterventionsWest SussexJohn Wiley & Sons2008
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- KazanciogluRRisk factors for chronic kidney disease: an updateKidney Int Suppl (2011)20133436837125019021
- CoboGHeckingMPortFKSex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysisClin Sci (Lond)2016130141147116327252402
- Al-AlyZPrediction of renal end points in chronic kidney diseaseKidney Int201383218919123364584
- LiLAstorBCLewisJLongitudinal progression trajectory of GFR among patients with CKDAm J Kidney Dis201259450451222284441
- XieYBoweBXianHBalasubramanianSAl-AlyZRate of kidney function decline and risk of hospitalizations in stage 3A CKDClin J Am Soc Nephrol201510111946195526350437
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- BrockwellSEGordonIRA comparison of statistical methods for meta-analysisStat Med200120682584011252006
- SidikKJonkmanJNA comparison of heterogeneity variance estimators in combining results of studiesStat Med20072691964198116955539
- LucasGMLauBAttaMGFineDMKerulyJMooreRDChronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two racesJ Infect Dis2008197111548155718422458
- MeulemanYde GoeijMCHalbesmaNChilcotJDekkerFWvan DijkSPREPARE-2 Study Group. Illness Perceptions in Patients on Predialysis Care: associations with time until start of dialysis and decline of kidney functionPsychosom Med201577894695426230483
- VoormolenNNoordzijMGrootendorstDCPREPARE study groupHigh plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patientsNephrol Dial Transplant200722102909291617517792
- HalimiJMJolyDCombeCBlood pressure and proteinuria control remains a challenge in patients with type 2 diabetes mellitus and chronic kidney disease: experience from the prospective observational ALICE-PROTECT studyBMC Nephrol201617113527655374
- RigalleauVLasseurCRaffaitinCNormoalbuminuric renal-insufficient diabetic patients: a lower-risk groupDiabetes Care20073082034203917485574
- TanJManleyPGambleGLong-term effectiveness of a community-based model of care in Maori and Pacific patients with type 2 diabetes and chronic kidney disease: a 4-year follow up of the DElay Future End Stage Nephropathy due to Diabetes (DEFEND) studyIntern Med J201545884384925872126
- GreenlandSMorgensternHEcological bias, confounding, and effect modificationInt J Epidemiol19891812692742656561
- SkupienJWarramJHSmilesAMStantonRCKrolewskiASPatterns of estimated glomerular filtration rate decline leading to end-stage renal disease in type 1 diabetesDiabetes Care201639122262226927647852
- BarrettBJGargAXGoereeRA nurse-coordinated model of care versus usual care for stage 3/4 chronic kidney disease in the community: a randomized controlled trialClin J Am Soc Nephrol2011661241124721617090
- ChenSCSuHMHungCCEchocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney diseaseClin J Am Soc Nephrol20116122750275821980185
- ChenSCChangJMTsaiYCSuHMChenHCBrachial-ankle pulse wave velocity and brachial pre-ejection period to ejection time ratio with renal outcomes in chronic kidney diseaseHypertens Res201235121159116322855129
- ChenSCHungCCKuoMCAssociation of dyslipidemia with renal outcomes in chronic kidney diseasePLoS One201382e5564323390545
- ChenSCLinMYHuangTHVariability in estimated glomerular filtration rate by area under the curve predicts renal outcomes in chronic kidney diseaseScientificWorldJournal2014201480203725401155
- ChenPMLaiTSChenPYMultidisciplinary care program for advanced chronic kidney disease: reduces renal replacement and medical costsAm J Med20151281687625149427
- ChiuYLChienKLLinSLChenYMTsaiTJWuKDOutcomes of stage 3–5 chronic kidney disease before end-stage renal disease at a single center in TaiwanNephron Clin Pract20081093c109c11818663322
- ChueCDEdwardsNCDavisLJSteedsRPTownendJNFerroCJSerum phosphate but not pulse wave velocity predicts decline in renal function in patients with early chronic kidney diseaseNephrol Dial Transplant20112682576258221248296
- ConwayBWebsterARamsayGPredicting mortality and uptake of renal replacement therapy in patients with stage 4 chronic kidney diseaseNephrol Dial Transplant20092461930193719181760
- DattoloPCGalloPMichelassiSConservative management of chronic kidney disease stage 5: role of angiotensin converting enzyme inhibitorsJ Nephrol201629680981527015900
- DruekeTBLocatelliFClyneNNormalization of hemoglobin level in patients with chronic kidney disease and anemiaN Engl J Med2006355202071208417108342
- GoicoecheaMde VinuesaSGVerdallesUEffect of allopurinol in chronic kidney disease progression and cardiovascular riskClin J Am Soc Nephrol2010581388139320538833
- GouvaCNikolopoulosPIoannidisJPSiamopoulosKCTreating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trialKidney Int200466275376015253730
- HsiehYPChangCCYangYWenYKChiuPFLinCCThe role of uric acid in chronic kidney disease patientsNephrology (Carlton)201722644144826610276
- InagumaDImaiETakeuchiARisk factors for CKD progression in Japanese patients: findings from the Chronic Kidney Disease Japan Cohort (CKD-JAC) studyClin Exp Nephrol Epub2016713
- JonesCRoderickPHarrisSRogersonMDecline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney diseaseNephrol Dial Transplant20062182133214316644779
- KhanYHSarriffAAdnanASKhanAHMallhiTHJummaatFProgression and outcomes of non-dialysis dependent chronic kidney disease patients: A single center longitudinal follow-up studyNephrology (Carlton)2017221253426718476
- KhanYHSarriffAAdnanASKhanAHMallhiTHChronic Kidney Disease, Fluid Overload and Diuretics: A Complicated TrianglePLoS One2016117e015933527442587
- KikuchiHKandaEMandaiSCombination of low body mass index and serum albumin level is associated with chronic kidney disease progression: the chronic kidney disease-research of outcomes in treatment and epidemiology (CKD-ROUTE) studyClin Exp Nephrol2017211556226920126
- KuoTHYangDCLinWHCompliance Index, a Marker of Peripheral Arterial Stiffness, may Predict Renal Function Decline in Patients with Chronic Kidney DiseaseInt J Med Sci201512753053726180508
- LevinADjurdjevOBeaulieuMErLVariability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohortAm J Kidney Dis200852466167118805347
- LimLMKuoHTKuoMCLow serum calcium is associated with poor renal outcomes in chronic kidney disease stages 3–4 patientsBMC Nephrol20141518325412875
- LinCMYangMCHwangSJSungJMProgression of stages 3b–5 chronic kidney disease--preliminary results of Taiwan national pre-ESRD disease management program in Southern TaiwanJ Formos Med Assoc20131121277378224309170
- PeetersMJvan ZuilenADvan den BrandJANurse practitioner care improves renal outcome in patients with CKDJ Am Soc Nephrol201425239039824158983
- PortolesJGorrizJLRubioEThe development of anemia is associated to poor prognosis in NKF/KDOQI stage 3 chronic kidney diseaseBMC Nephrol201314223295149
- SchulmanGBerlTBeckGJRandomized Placebo-Controlled EPPIC Trials of AST-120 in CKDJ Am Soc Nephrol20152671732174625349205
- TangkiatkumjaiMWalkerDMPraditpornsilpaKBoardmanHAssociation between medication adherence and clinical outcomes in patients with chronic kidney disease: a prospective cohort studyClin Exp Nephrol201721350451227438073
- TsaiYCTsaiJCChenSCAssociation of fluid overload with kidney disease progression in advanced CKD: a prospective cohort studyAm J Kidney Dis2014631687523896483
- BeltranSGavelaEKanterJBeginning hemodialysis: do patients with a failed renal transplant start in worse condition?Transplant Proc20094162129213119715852
- BhanVSorokaSConstantineCKiberdBABarriers to access before initiation of hemodialysis: a single-center reviewHemodial Int200711334935317576301
- EyreSAttmanPOHaraldssonBPositive effects of protein restriction in patients with chronic kidney diseaseJ Ren Nutr200818326928018410883
- HaapioMHelveJKurimoPForslundTGronhagen-RiskaCFinnePDecline in glomerular filtration rate during pre-dialysis phase and survival on chronic renal replacement therapyNephrol Dial Transplant20122731157116321810761
- HeLLiuXLiZRate of Decline of Residual Kidney Function Before and After the Start of Peritoneal DialysisPerit Dial Int201636333433927044795
- HsuRKChaiBRoyJAAbrupt Decline in Kidney Function Before Initiating Hemodialysis and All-Cause Mortality: The Chronic Renal Insufficiency Cohort (CRIC) StudyAm J Kidney Dis201668219320226830447
- InagumaDMurataMTanakaAShinjoHRelationship between mortality and speed of eGFR decline in the 3 months prior to dialysis initiationClin Exp Nephrol201721115916827084516
- JeongJUKimHKChoYPKwonTWKimSBArteriovenous access creation defers chronic hemodialysis initiationClinical Nephrology201175211311921255540
- JungersPChoukrounGOualimZRobinoCNguyenA-TManNKBeneficial influence of recombinant human erythropoietin therapy on the rate of progression of chronic renal failure in predialysis patientsNephrology Dialysis Transplantation2001162307312
- MaedaKHamadaCHayashiTEfficacy of adsorbent in delaying dialysis initiation among chronic kidney disease patientsDialysis and Transplantation2011405212216
- O’HareAMChoiAIBoscardinWJTrends in timing of initiation of chronic dialysis in the United StatesArch Intern Med2011171181663166921987197
- RamspekCLNacakHvanDMPre-dialysis decline of measured glomerular filtration rate but not serum creatinine-based estimated glomerular filtration rate is a risk factor for mortality on dialysisNephrol Dial Transplant2017321899627312146
- SumidaKMolnarMZPotukuchiPKAssociation of Slopes of Estimated Glomerular Filtration Rate With Post-End-Stage Renal Disease Mortality in Patients With Advanced Chronic Kidney Disease Transitioning to DialysisMayo Clin Proc201691219620726848002