Abstract
Purpose
A post hoc analysis of a recent trial on direct oral anticoagulants versus vitamin K antagonists showed that amongst patients with mildly decreased kidney function, use of vitamin K antagonists was associated with a greater decline in renal function than use of direct oral anticoagulants. Whether these vitamin K antagonist effects are the same in pre-dialysis patients is unknown. Therefore, the aim of this study was to investigate the association between vitamin K antagonist use and the rate of renal function decline and time until start of dialysis in incident pre-dialysis patients.
Methods
Data from 984 patients from the PREdialysis PAtient REcord study, a multicenter follow-up study of patients with chronic kidney disease who started pre-dialysis care in the Netherlands (1999–2011), were analyzed. Of these patients, 101 used a vitamin K antagonist. Linear mixed models were used to compare renal function decline between vitamin K antagonist users and non-users. Cox proportional hazards models were used to estimate the HR with 95% CI for starting dialysis.
Results
Vitamin K antagonist use was associated with an extra change in renal function of −0.09 (95% CI −1.32 to 1.13) mL/min/1.73 m2 per year after adjustment for confounding. The adjusted HR for the start of dialysis was 1.20 (95% CI 0.85 to 1.69) in vitamin K antagonist users, compared to non-users.
Conclusion
In incident pre-dialysis patients, the use of vitamin K antagonists was not associated with an accelerated kidney function decline or an earlier start of dialysis compared to non-use. The lack of knowledge on the indication for vitamin K antagonist use could lead to confounding by indication.
Introduction
Vitamin K antagonists are used to prevent and treat thrombotic complications. Several clinical trials performed in patients with thrombotic complications and a normal kidney function have shown that the benefits of vitamin K antagonists outweigh the side effects such as bleeding.Citation1–Citation4
Recent studies have debated whether the benefits of vitamin K antagonists also outweigh the risks in patients with early stage chronic kidney disease or in dialysis patients.Citation5–Citation11 A post hoc analysis of a recent trial comparing direct oral anticoagulants with vitamin K antagonists showed that patients with a mildly decreased kidney function receiving vitamin K antagonists exhibited a greater decline in renal function than patients receiving direct oral anticoagulants.Citation12 Furthermore, most studies in dialysis patients have failed to show a protective effect of vitamin K antagonists on stroke risk or all-cause mortality,Citation5–Citation9,Citation13 with the exception of two studies that showed a decreased risk of stroke and a survival benefit for vitamin K antagonists in dialysis patients.Citation10,Citation14 The proposed hypotheses of these negative effects due to vitamin K antagonists are that the medication could either cause damage to the kidneys by vascular calcifications due to the inhibition of matrix Gla protein or cause damage by glomerular hemorrhage which could lead to tubular obstruction.Citation11,Citation15–Citation19
As patients with chronic kidney disease are often prescribed vitamin K antagonists, an adequate insight into the benefits and risks of vitamin K antagonist use is crucial.Citation20 To our knowledge, there are no existing studies that investigated the association between vitamin K antagonist use and decline of glomerular filtration rate or time until start of dialysis in pre-dialysis patients with stage 4–5 chronic kidney disease. From a clinical point of view, withholding medication that could lead to kidney function decline is very important to postpone or prevent dialysis in pre-dialysis patients.
Therefore, the aim of this study was to investigate the association between vitamin K antagonist use and the rate of kidney function decline and time until start of dialysis in incident pre-dialysis patients.
Methods
Study design and population
The PREdialysis PAtient REcord (PREPARE) studyCitation29,Citation30 is a multicenter follow-up study of incident patients starting specialized pre-dialysis care (age ≥18 years) in the Netherlands. At inclusion, these patients had an estimated glomerular filtration rate (eGFR) between 20 and 30 mL/min per 1.73 m2 (chronic kidney disease [CKD] stages 4–5). The PREPARE study consists of a retrospective and a prospective cohort. In the retrospective cohort (PREPARE-I), incident pre-dialysis patients who had their first pre-dialysis visit between 1999 and 2001 were included from eight outpatient clinics. In the prospective cohort (PREPARE-II),Citation31 incident pre-dialysis patients who started pre-dialysis care in one of the 25 participating outpatient clinics between 2004 and 2011, and understood the Dutch language were included. In PREPARE-I, patients who experienced kidney failure from a kidney transplant were excluded. In PREPARE-II, patients who experienced kidney failure from a kidney transplant that was received within a year before referral to pre-dialysis care were excluded. The study was approved by the medical ethics committee or the institutional review board of the participating centers (Supplementary materials). Patients included in PREPARE-II gave written informed consent before study inclusion.
Demographic and clinical data
Data were collected from medical records and extracted from the hospital information systems. Data on demography, primary kidney disease, comorbidities, medication use including vitamin K antagonist use (phenprocoumon or acenocoumarol), and laboratory values were collected at baseline and during routine visits to the pre-dialysis outpatient clinics. These visits took place at the start of specialized pre-dialysis care and when one of the study endpoints was reached. In PREPARE-II, data were also collected every 6 months at follow-up visits. The closest laboratory measurement performed within 90 days before or after the date of a visit was appointed to that visit. Patients were categorized as non-users or users of vitamin K antagonists based on medication use at baseline. We had no information about the indication for vitamin K antagonist use. The eGFR was calculated using the CKD-EPI (CKD Epidemiology Collaboration) formula from 2009, taking age, sex, race, and serum creatinine into account.Citation21 Hypertension was defined as a history of hypertension, a systolic blood pressure ≥140 mmHg, or a diastolic blood pressure ≥90 mmHg at baseline.Citation22 Cardiovascular disease (CVD) was defined as angina pectoris, myocardial infarction, heart failure, ischemic stroke, or claudication. Primary kidney disease was classified according to the codes of the European Renal Association-European Dialysis and Transplantation Association.Citation23 Patients were grouped into four classes of primary kidney disease: glomerulonephritis, diabetes mellitus, renal vascular disease, and other kidney diseases.
Outcomes
In the PREPARE study, patients were followed until the start of renal replacement therapy (defined as dialysis or renal transplantation), death, loss to follow-up, refusal to further participate in the study (PREPARE-II), recovery of renal function, or the end of the study period (January 2008 for PREPARE-I and October 2016 for PREPARE-II), whichever came first. The main outcomes were change in the rate of kidney function decline and the start of dialysis within 2 years. For the current study, the follow-up time was restricted to 2 years; after this period, the number of patients at risk became too small.Citation24,Citation25 The date of dialysis initiation was collected from medical records. To calculate the kidney function decline rate, all available eGFR measurements from 3 months prior to inclusion until 2 years of follow-up were used.
Statistical analyses
Baseline characteristics are presented stratified for vitamin K antagonist use. Continuous variables are described by their median and interquartile range (IQR), and categorical variables are presented as percentages.
Follow-up time was defined as the time between baseline and the start of dialysis, other renal replacement therapy, death, or withdrawal or end of follow-up (2 years). To estimate the change of renal function decline rate in vitamin K antagonist users, compared to non-users, a linear mixed model (LMM) was used. This model takes the correlation between eGFR measurements within each individual patient into account. Multivariable analysis was used to adjust for age, sex, race, diabetes mellitus, hypertension, cardiovascular disease, malignancy, gastro-intestinal problems, antiplatelet drug use, primary kidney disease, and hemoglobin levels.
The proportional hazard assumption was tested using a log minus log plot. Incidence rates of dialysis initiation within 2 years of follow-up were calculated for both vitamin K antagonist users and non-users. Survival curves for the start of dialysis were determined with the Kaplan–Meier method, stratifying for vitamin K antagonist use. We conducted Cox proportional hazards regression analyses, obtaining HR with 95% CI to estimate the effect of vitamin K antagonist use on the start of dialysis. Analyses were adjusted for potential confounders, including age, sex, race, diabetes mellitus, hypertension, cardiovascular disease, malignancy, gastrointestinal problems, antiplatelet drug use, primary kidney disease, hemoglobin levels, and eGFR levels.
Multiple imputation was used to impute missing potential confounders at baseline. To test the robustness of the results, several sensitivity analyses were performed. Analyses were repeated with stratification by PREPARE-I and PREPARE-II. Furthermore, we repeated the analyses without correcting for cardiovascular disease, since cardiovascular disease could be both a confounder and part of the causal pathway. Next, we added angiotensin converting enzyme inhibitor (ACEi), angiotensin II receptor blocker (ARB), systolic blood pressure, and diastolic blood pressure to our linear mixed model. These variables could be both a confounder or part of the causal pathway. For the same reason, C-reactive protein (CRP), body mass index (BMI), albumin, and proteinuria were added as confounders in a sensitivity analysis.
Next we restricted our analysis to patients who were persistent users or non-users of vitamin K antagonists during the entire study period, since changes in therapy during the follow-up period might dilute treatment effects. Then, we restricted our analyses to patients with cardiovascular disease. This could give an indication of the effects of confounding by indication. Finally, we performed our analyses with a follow-up period of 5 years. All analyses were performed using SPSS version 23.0 for Windows.
Results
Baseline characteristics
Of the 1,049 patients in PREPARE, 547 patients originate from PREPARE-I and 502 patients originate from PREPARE-II. Vitamin K antagonist use was known for all PREPARE-I patients and for 437 PREPARE-II patients, resulting in 984 patients. Of these patients, 101 used a vitamin K antagonist and 883 did not use a vitamin K antagonist. The baseline characteristics of these 984 patients are shown in . Vitamin K antagonist users were older, were more often male, had more cardiovascular disease, and used antiplatelet drugs less often than vitamin K antagonist non-users. In vitamin K antagonist users, 59% had missing values for the variable malignancy, 62% for gastro-intestinal disease, 20% for eGFR, and 22% for hemoglobin. In the vitamin K antagonist nonusers, this was 43% for malignancy, 43% for gastro-intestinal disease, 11% for eGFR, and 10% for hemoglobin. A total of 846 (96%) patients were persistent vitamin K antagonist non-users, and 86 (85%) patients were persistent vitamin K antagonist users during the entire study period.
Table 1 Baseline characteristics of vitamin K antagonist users and vitamin K antagonist non-users (N=984)
During follow-up, the occurrence of vascular cerebral complications was recorded. A total of six brain infarctions occurred, all in vitamin K antagonist non-users. A cerebral hematoma was diagnosed in five vitamin K antagonist nonusers and in one vitamin K antagonist user.
Vitamin K antagonist and decline of kidney function
During the first 2 years of follow-up, patients had on average 1.5 (SD 1.2) serum creatinine measurements. The mean change in kidney function was −1.45 (95% CI −1.80 to −1.10) mL/min/1.73 m2/year for the total group. shows the difference in kidney function change between vitamin K antagonist users and non-users. The difference in kidney function change was −0.09 (95% CI −1.32 to 1.13) mL/min/1.73 m2/year after adjustment (the minus indicates an extra change of 0.09 units in the vitamin K antagonist users). In absolute numbers, this means the change in vitamin K antagonist non-users was −3.23 mL/min/1.73 m2/year, and the change in vitamin K antagonist users −3.32 mL/min/1.73 m2/year.
Table 2 Vitamin K antagonist use and renal function decline
Vitamin K antagonists and start of dialysis within 2 years
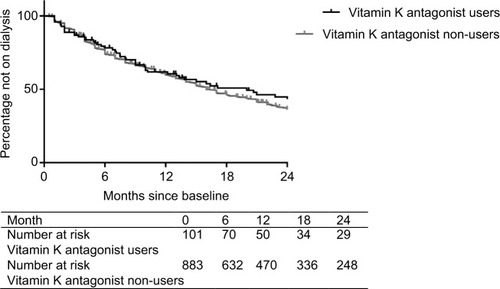
Incidence rates for start of dialysis were 47/100 person-years for vitamin K antagonist users and 46/100 person-years for non-users. During the 2 years of follow-up, 48 (48%) vitamin K antagonist users started dialysis, and 520 (59%) non-users started dialysis. shows the Kaplan–Meier for start of dialysis for vitamin K antagonist users and non-users. The crude and adjusted HRs for start of dialysis are presented in . As compared with no vitamin K antagonist use (reference category), pre-dialysis patients who used vitamin K antagonists did not have an increased risk of start of dialysis (HR 1.20; 95% CI 0.85 to 1.69), adjusted for age, sex, race, diabetes mellitus, hypertension, cardiovascular disease, malignancy, gastro-intestinal problems, antiplatelet drug use, primary kidney disease, hemoglobin levels, and eGFR at baseline.
Table 3 Vitamin K antagonist use and HRs for start of dialysis
Sensitivity analyses
After stratification for study cohort, the extra change in kidney function in vitamin K antagonist users was 1.11 (−0.86 to 3.07) mL/min/1.73 m2/year in PREPARE-I and −0.40 (−1.89 to 1.09) mL/min/1.73 m2/year in PREPARE-II (Table S1). Vitamin K antagonist users in PREPARE-I had an HR for start of dialysis of 1.38 (95% CI 0.87 to 2.18) as compared with non-users, and in PREPARE-II, this was 1.09 (95% CI 0.63 to 1.86) (Table S2). Performing the analyses without correction for cardiovascular disease resulted in an extra change in kidney function of −0.12 (95% CI −1.30 to 1.05) in vitamin K antagonist users and an HR for start of dialysis of 1.23 (0.88 to 1.73) as compared with non-users (Tables S3 and S4). Adding ACEi, ARB, systolic blood pressure, and diastolic blood pressure to the linear mixed model changed the change in kidney function to −0.17 (95% CI −1.42 to 1.08) (Table S3). The addition of CRP, BMI, albumin, and proteinuria changed the change in kidney function to 0.23 (95% CI −1.74 to 2.20) (Table S3). The HR for start of dialysis changed to 0.80 (95% CI 0.37 to 1.74) (Table S4). Restricting the analyses to persistent users and non-users changed the adjusted change in kidney function to 0.15 (95% CI −1.17 to 1.47), in absolute numbers this means the change in vitamin K antagonist non-users was –2.41 mL/min/1.73 m2/year, and the change in vitamin K antagonist users −2.26 mL/min/1.73 m2/year (Table S5). The adjusted HR for start of dialysis changed to 1.36 (95% CI 0.96 to 1.93) in vitamin K antagonist users (Table S6). Restricting the analyses to patients with cardiovascular disease (417 non-users and 72 users) resulted in an adjusted change in kidney function of 0.07 (95% CI −1.50 to 1.64), which is −1.25 mL/min/1.73 m2/year in vitamin K antagonist non-users and −1.18 mL/min/1.73 m2/year in vitamin K antagonist users (Table S7). The adjusted HR for start of dialysis was 1.04 (0.66 to 1.65) in vitamin K antagonist users (Table S8). Using a follow-up period of 5 years changed the adjusted change in kidney function to 0.09 (95% CI −0.97 to 1.15), which means the absolute change in vitamin K antagonist non-users was −0.55 mL/min/1.73 m2/year; in vitamin K antagonist users, this was −0.46 mL/min/1.73 m2/year (Table S9). The adjusted HR for start of dialysis was 0.99 (95% CI 0.98 to 0.99) for vitamin K antagonist users (Table S10). Figure S1 shows the Kaplan–Meier for start of dialysis stratified for vitamin K antagonist use for 5 years of follow-up.
Discussion
Key findings
In this cohort of 984 incident pre-dialysis patients with a follow-up of 2 years, we found no difference in annual kidney function decline between vitamin K antagonist users and nonusers. Furthermore, vitamin K antagonist use as compared with non-use was not associated with an increased risk of start of dialysis within 2 years of follow-up. Restricting the population to patients with cardiovascular disease shows the same lack of an association for kidney function decline and risk of start of dialysis. All results should be viewed with the possible presence of confounding by indication in mind.
Previous studies on the association between vitamin K antagonist use and kidney function decline and start of dialysis
To our knowledge, there are no previous studies that investigated the association between vitamin K antagonist use and kidney function decline in pre-dialysis patients. However, a post hoc analysis of the RE-LY trial (warfarin versus dabigatran), in which patients with an eGFR <30 mL/min were excluded, showed that warfarin users had more kidney function decline over the first 30 months (−3.68 mL/min/1.73m2) than dabigatran users (−2.57 mL/min/1.73m2 with 110 µg and −2.46 mL/min/1.73m2 with 150 µg).Citation12 The long-term effects of vitamin K antagonists on kidney function have not yet been investigated.
Vitamin K antagonists and start of dialysis
Although we did not find an association between vitamin K antagonist and start of dialysis within 2 years of follow-up, we did find a 1.2-fold increased risk with a wide confidence interval. It could be that vitamin K antagonist use, which is a marker for cardiovascular disease, is associated with dialysis initiation through pathways other than kidney function decline, including fluid overload.
Pathophysiological effects of vitamin K antagonists on kidney
There are several pathophysiological mechanisms through which vitamin K antagonists could influence kidney function. First, vitamin K antagonists are associated with increased arterial calcifications and accelerate preexisting vascular calcifications due to the inhibition of matrix Gla protein. This could potentially lead to an acceleration of kidney dysfunction decline.Citation17–Citation19 Furthermore, vitamin K antagonist use can cause glomerular hemorrhage, with dysmorphic red blood cells and tubular red blood cell casts causing tubular obstruction and thereby kidney damage.Citation11,Citation15,Citation16 However, in our pre-dialysis patients, we did not find an association between vitamin K antagonist use and kidney function decline.
Clinical implications
Our study results suggest that the effect of vitamin K antagonist use on kidney function decline and start of dialysis is probably limited. Therefore, based on our study, we do not recommend withholding vitamin K antagonists to slow kidney function decline or postpone dialysis. However, the possibility of confounding by indication should be taken into account when interpreting these results. Other benefits and risks of vitamin K antagonist use in pre-dialysis patients are not known, since existing trials have excluded these patients due to their high bleeding risk. Guidelines mention this knowledge gap concerning risks and benefits of anticoagulation with vitamin K antagonists for stroke prevention, especially in dialysis patients.Citation26 A recent meta-analysis showed that treatment with vitamin K antagonists was associated with a non-significant 26% risk reduction of ischemic stroke, a 21% increase in total bleeding risk, and no effect on mortality.Citation27
Strengths and limitations of this study
The main strength of this study is the well-defined cohort of incident pre-dialysis patients who received standardized treatments and check-ups by nephrologists. A wide range of incident pre-dialysis patients was included, and all patient information was used to perform the analyses, making the results generalizable to the clinical practice of pre-dialysis care. The main limitation of this study is the possibility of confounding by indication when comparing vitamin K antagonist users with non-users in an observational study design. We cannot exclude the possibility that doctors anticipate renal effects when prescribing or withholding vitamin K antagonists. In that case, patients with a worse kidney function would receive vitamin K antagonists less often, leading to a possible underestimation of negative effects of vitamin K antagonists. We tried to minimize this problem by correcting for multiple confounders, but cannot exclude residual confounding. Another limitation is the lack of information on the indication for vitamin K antagonist use in our population. Therefore, it could be that vitamin K antagonists were used for other indications than atrial fibrillation. However, since atrial fibrillation has a 2–3 times higher prevalence in CKD patients, compared to the general population, it is likely that the indication for vitamin K antagonist use in most of our population was atrial fibrillation.Citation28 Another limitation is the possible non-adherence of the vitamin K antagonist users. Medication adherence often is not 100%, which is very likely to be the case in this study too. Non-adherence can lead to an underestimation of the true effects of vitamin K antagonists. However, the adherence in this study is a representation of adherence in clinical practice and therefore gives an estimate of the expected effects in clinical practice. In addition, we had no information on the number of patients that experienced kidney failure after receiving a kidney transplant. A final limitation is the inclusion of prevalent vitamin K antagonist users, which could have led to an underestimation of the negative effects on kidney function and start of dialysis in vitamin K antagonist users.
Conclusion
In conclusion, there was no association between vitamin K antagonist use and rate of renal function decline in our pre-dialysis population. Furthermore, this study showed no association between vitamin K antagonist use and time until start of dialysis in incident pre-dialysis patients. This study should be viewed with the possibility of confounding by indication in mind, which emphasizes the need for randomized controlled trials comparing vitamin K antagonists with placebo or direct oral anticoagulants in pre-dialysis patients to investigate their effect on kidney function decline. This would provide better insight into the adverse effects of vitamin K antagonists and more personalized prescription of anticoagulant drugs in pre-dialysis patients.
Author contributions
PWMV, FWD, and GO contributed to the conception or design of the work. PWMV, MBR, MCV, WJWB, and MvD contributed to the acquisition, analysis, or interpretation of data for the work. PWMV and GO drafted the manuscript. FWD, MBR, MCV, WJWB, and MvD critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgments
The authors gratefully thank all patients who participated in the PREPARE-II study. The nursing staff members of the participating centers, as well as the trial nurses and data managers from the Hans Mak Institute, are gratefully acknowledged for collecting the clinical data. This study was supported by the Dutch Kidney Foundation (grant number SB 110) and previously by unrestricted grants from Amgen and Baxter. None of the sponsors were involved in study design, collection of data, statistical analyses, interpretation of data, writing of the manuscript, or in the decision to submit the paper for publication. The results presented in this paper have not been published previously in whole or part, except in abstract format. The abstract was published in Nephrology Dialysis Transplantation, Volume 32, Issue supplement 3, May 1, 2017.
Disclosure
The authors report no conflicts of interest in this work.
References
- HartRGPearceLAAguilarMIMeta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillationAnn Intern Med20071461285786717577005
- LoewenPDahriKRisk of bleeding with oral anticoagulants: an updated systematic review and performance analysis of clinical prediction rulesAnn Hematol201190101191120021670974
- KirchhofPBenussiSKotechaD2016ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTSEuropace201618111609167827567465
- JanuaryCTWannLSAlpertJS2014AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm SocietyCirculation2014130232071210424682348
- ChanKELazarusJMThadhaniRHakimRMWarfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillationJ Am Soc Nephrol200920102223223319713308
- ShahMAvgil TsadokMJackeviciusCAWarfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysisCirculation2014129111196120324452752
- ShenJIMontez-RathMELenihanCRTurakhiaMPChangTIWinkelmayerWCOutcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillationAm J Kidney Dis201566467768826162653
- WakasugiMKazamaJJTokumotoAAssociation between warfarin use and incidence of ischemic stroke in Japanese hemodialysis patients with chronic sustained atrial fibrillation: a prospective cohort studyClin Exp Nephrol201418466266924113782
- WinkelmayerWCLiuJSetoguchiSChoudhryNKEffectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillationClin J Am Soc Nephrol20116112662266821959598
- OlesenJBLipGYKamperALStroke and bleeding in atrial fibrillation with chronic kidney diseaseN Engl J Med2012367762563522894575
- BrodskySVNadasdyTRovinBHWarfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rateKidney Int201180218118921389969
- BöhmMEzekowitzMDConnollySJChanges in renal function in patients with atrial fibrillation: an analysis from the RE-LY trialJ Am Coll Cardiol201565232481249326065986
- WizemannVTongLSatayathumSAtrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapyKidney Int201077121098110620054291
- AbbottKCTrespalaciosFCTaylorAJAgodoaLYAtrial fibrillation in chronic dialysis patients in the United States: risk factors for hospitalization and mortalityBMC Nephrol20034112546711
- BrodskySVSatoskarAChenJAcute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 casesAm J Kidney Dis20095461121112619577348
- HolbrookAMPereiraJALabirisRSystematic overview of warfarin and its drug and food interactionsArch Intern Med2005165101095110615911722
- WheelerDSGiuglianoRPRangaswamiJAnticoagulation-related nephropathyJ Thromb Haemost201614346146726670286
- ChatrouMLWinckersKHackengTMReutelingspergerCPSchurgersLJVascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonistsBlood Rev201226415516622520397
- SchurgersLJJoosenIALauferEMVitamin K-antagonists accelerate atherosclerotic calcification and induce a vulnerable plaque phenotypePLoS One201278e4322922952653
- LimdiNABeasleyTMBairdMFKidney function influences warfarin responsiveness and hemorrhagic complicationsJ Am Soc Nephrol200920491292119225037
- LeveyASStevensLASchmidCHA new equation to estimate glomerular filtration rateAnn Intern Med2009150960461219414839
- JamesPAOparilSCarterBL2014Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8)JAMA2014311550752024352797
- ERA/EDTA Registry(ERA/EDTA) Registry Annual Report 2009Amsterdam, the NetherlandsAcademic Medical Center, Department of Medical Informatics2011
- van den BeukelTOde GoeijMCDekkerFWSiegertCEHalbesmaNPREPARE Study GroupDifferences in progression to ESRD between black and white patients receiving predialysis care in a universal health care systemClin J Am Soc Nephrol2013891540154723846464
- JagerKJvan DijkPCZoccaliCDekkerFWThe analysis of survival data: the Kaplan-Meier methodKidney Int200874556056518596735
- HerzogCAAsingerRWBergerAKCardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO)Kidney Int201180657258621750584
- Van Der MeerschHDe BacquerDDe VrieseASVitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: A systematic review and meta-analysisAm Heart J2017184374627892885
- SolimanEZPrineasRJGoASChronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC)Am Heart J201015961102110720569726
- VoormolenNNoordzijMGrootendorstDCHigh plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patientsNephrol Dial Transplant200722102909291617517792
- de GoeijMCMeulemanYvan DijkSHaemoglobin levels and health-related quality of life in young and elderly patients on specialized predialysis careNephrol Dial Transplant20142713911398
- VoormolenNNoordzijMGrootendorstDCHigh plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patientsNephrol Dial Transplant200722102909291617517792