Abstract
Objective
We investigated the burden of chronic kidney disease (CKD) among patients with severe mental illness (SMI).
Methods
We identified patients with SMI among all those aged 25–74 registered in the UK Clinical Practice Research Datalink as on March 31, 2014. We compared the prevalence of CKD (two measurements of estimated glomerular filtration rate <60 mL/min/1.73 m2 for ≥3 months) and renal replacement therapy between patients with and without SMI. For patients with and without a history of lithium prescription separately, we used logistic regression to examine the association between SMI and CKD, adjusting for demographics, lifestyle characteristics, and known CKD risk factors.
Results
The CKD prevalence was 14.6% among patients with SMI and a history of lithium prescription (n = 4,295), 3.3% among patients with SMI and no history of lithium prescription (n = 24,101), and 2.1% among patients without SMI (n = 2,387,988; P < 0.001). The prevalence of renal replacement therapy was 0.23%, 0.15%, and 0.11%, respectively (P = 0.012). Compared to patients without SMI, the fully adjusted odds ratio for CKD was 6.49 (95% CI 5.84–7.21) for patients with SMI and a history of lithium prescription and 1.45 (95% CI 1.34–1.58) for patients with SMI and no history of lithium prescription. The higher prevalence of CKD in patients with SMI may, in part, be explained by more frequent blood testing as compared to the general population.
Conclusion
CKD is identified more commonly among patients with SMI than in the general population.
Introduction
People with severe mental illness (SMI), including schizophrenia, bipolar disorder, and other nonorganic psychotic illnesses, are known to have shorter life expectancy, by ~10–20 years, than the general population.Citation1–Citation6 Their premature death is attributed not only to suicide and accident but also to physical illnesses – especially cardiovascular diseases such as coronary heart disease and stroke.Citation5–Citation9 The high rate of cardiovascular deaths in patients with SMI has been attributed to the high prevalence of lifestyle-related conditions (eg, smoking and obesity),Citation10–Citation13 suboptimal screening/assessment of cardiovascular risks,Citation14,Citation15 and suboptimal management of underlying diseases (eg, hypertension, diabetes, dyslipidemia).Citation16–Citation18
Chronic kidney disease (CKD) – the continuous presence of kidney damage and/or decreased level of kidney functionCitation19 – is independently associated with increased risk of death, cardiovascular events, and all-cause hospitalization.Citation20 CKD can progress to end-stage renal disease (ESRD) requiring renal replacement therapy (RRT), a substantial burden for both – quality of life of patients and health budgets. The UK National Institute for Health and Care Excellence guidance for CKD recommends early identification and appropriate management of CKD.Citation21
People with SMI have an increased prevalence of several risk factors for CKD, including smoking and diabetes.Citation13,Citation16,Citation22 Moreover, patients with SMI may be treated with lithium, which is associated with the development of CKD.Citation23,Citation24 However, there has been limited research investigating the prevalence of, and risk factors for, CKD in people with SMI.Citation13,Citation25,Citation26 In the UK, primary care plays an important role in the management of physical health conditions in people with SMI. As part of the Quality and Outcomes Framework (QOF), there have been recent financial incentives for general practitioners (GPs) to undertake physical health checks in people with SMI.Citation27 However, it is unclear whether CKD in patients with SMI is appropriately recognized and managed by GPs.
Therefore, we used a UK primary care database to: 1) compare the prevalence of CKD and RRT among patients with SMI (with and without a history of lithium use) and those without SMI; 2) investigate whether there is an independent association between SMI and CKD after adjusting for known CKD risk factors; and 3) compare the recognition and management of CKD between CKD patients with and without SMI.
Materials and methods
Data source
The Clinical Practice Research Datalink (CPRD) is an observational data and interventional research service provided by the National Health Service. Around 98% of the UK population are registered with a primary care practice. Currently, >650 general practices contribute data conforming to quality control standards to the CPRD, including ~ 7% of the UK population. The included practices are representative of the UK general population in terms of age and sex.Citation28 The database includes the following data: patient demographics, diagnoses based on Read codes (a hierarchical coding system), prescriptions based on British National Formulary codes, laboratory test results, and referrals made by GPs. Ethical approval for this study was obtained from the Independent Scientific Advisory Committee of CPRD (protocol no. 16_055). Informed consent was waived because data are anonymized for research purposes.
Study population
Our study population included all people, aged 25–74 years, registered in the CPRD for at least 1 year as on March 31, 2014 (ie, the end of a financial or QOF year). We selected the 25–74 age range because our preliminary analysis suggested that CKD and/or SMI were rare in people outside these parameters, thereby limiting the power for comparative prevalence analyses. To ensure that we had reliable measures of morbidity (to allow time for the recording of past medical history in newly registered patients), we required that all participants had at least 1 year of continuous registration in the CPRD.
Disease definition
We identified SMI using Read morbidity codes that have been validated in UK primary care data, with high sensitivity (91%), specificity (99.9%), and positive predictive value (91%).Citation29 Patients with SMI were further classified as those with and without any record of lithium prescription in the period between the CPRD registration and March 31, 2014. We excluded patients who had been prescribed lithium without recorded SMI from the general population comparison group because the indication for treatment was not known.
Our definition of CKD was based on two measurements of estimated glomerular filtration rate <60 mL/min/1.73 m2 separated by 3 months or longer,Citation19 calculated from serum creatinine records in the CPRD in the past 5 years, using the Chronic Kidney Disease Epidemiology Collaboration equation.Citation30 RRT was characterized on the basis of diagnosis codes suggesting hemodialysis, peritoneal dialysis, or kidney transplantation. Previous research has shown that the prevalence of estimated glomerular filtration rate <60 mL/min/1.73 m2 and RRT in the CPRD is similar to that estimated in a nationally representative population survey (Health Survey for England) and disease registry (UK Renal Registry), suggesting that most cases of CKD and RRT are captured in the CPRD.Citation31
Covariate definition
We defined baseline characteristics of patients with and without SMI: age, sex, ethnicity, socioeconomic status (SES), country of the UK (ie, England, Northern Ireland, Scotland, and Wales) body mass index (BMI), smoking status, chronic diseases that are associated with CKDCitation21 – diabetes, hypertension, cardiovascular disease (ie, myocardial infarction, chronic heart failure, peripheral arterial disease, and stroke), urological disease (ie, vesicoureteral reflux, renal tract stone, and prostatic hypertrophy), systematic lupus erythematosus, and polycystic kidney disease – based on relevant diagnosis codes. Based on previous studies using UK primary care data,Citation32,Citation33 we grouped patients with no record of ethnicity into those of white ethnicity. SES was assigned at the general practice level using quintiles of the Index of Multiple Deprivation in each country of the UK.Citation34 BMI and smoking status were based on the most recent records prior to the date of study inclusion (ie, March 31, 2014). For chronic diseases, if a diagnosis code was recorded for a patient during the period between CPRD registration and the study date, then that disease was regarded as being present.
Data analysis
We compared baseline characteristics and overall prevalence of CKD (including patients on RRT) among the three groups: patients with SMI with and without a history of lithium prescription as well as those without SMI, using chi-squared tests. We stratified CKD prevalence by age (using 10-year age bands) and sex. We estimated the prevalence of RRT among the three groups and compared it overall by chi-squared tests. Moreover, we compared the distribution of the most recent category of RRT modality (hemodialysis, peritoneal dialysis, or kidney transplantation) between patients with and without SMI using chi-squared tests.
Next, we carried out logistic regression analyses to examine the association between SMI (with and without a history of lithium use) and CKD. To understand which factors are more likely to explain the association between SMI and CKD, we adjusted step-by-step for age and sex, ethnicity, SES, BMI, smoking status, and chronic diseases associated with decline in kidney function (ie, diabetes, hypertension, cardiovascular disease, urological disease, systemic lupus erythematosus, and polycystic kidney disease).Citation21 Patients with missing data of BMI and smoking status were excluded from the analysis. We took into account variations in coding and testing practices by different general practices through clustering by general practice in the logistic regression models.
Finally, we restricted analysis to patients with CKD who were not on RRT and compared the recognition and management of CKD between patients with and without SMI. We used the QOF CKD indicators (QOF version 2013/14) as markers of the recognition and management of CKD in the UK primary care.Citation27 We determined the proportion of CKD patients with and without SMI who had: 1) a record of a diagnostic code for CKD, using CKD codes listed in the QOF version 2013/14;Citation35 2) the most recent blood pressure measure ≤140/90 mmHg (recorded in the year before the date of the study); 3) urine test for proteinuria/albuminuria (including dipstick test) in the year before the date of the study; and 4) for those with hypertension and proteinuria (recorded any time between CPRD registration and study date), at least one prescription for a renin–angiotensin system antagonist in the past 3 months. In addition, because the National Institute for Health and Care Excellence guidance for cardiovascular disease recommends statins as primary prevention for all CKD patients regardless of serum cholesterol levels,Citation36 we compared the proportion of CKD patients with and without SMI who had at least one prescription for a statin in the past 3 months.
All statistical analyses were conducted using Stata 14 (Stata Corp, College Junction, TX, USA).
Results
Of the 2,418,730 people aged 25–74 years who were registered in the CPRD for >1 year on March 31, 2014, we identified 28,396 (1.17%) patients with SMI, including 4,295 patients with a history of lithium prescription in the CPRD and 24,101 without (). In patients with SMI (both with and without a previous lithium prescription) compared to those without, the SES was generally lower and the proportions of black ethnicity, obesity (BMI ≥30 kg/m2), and current smokers were higher. Diabetes, hypertension, and cardiovascular diseases were more common in patients with SMI, whereas the prevalence of other diseases (urological disease, systematic lupus erythematosus, and polycystic kidney disease) was similar between patients with and without SMI ().
Figure 1 Flow chart showing identification of study participants.
Table 1 Characteristics of patients by SMI diagnosis and history of lithium use
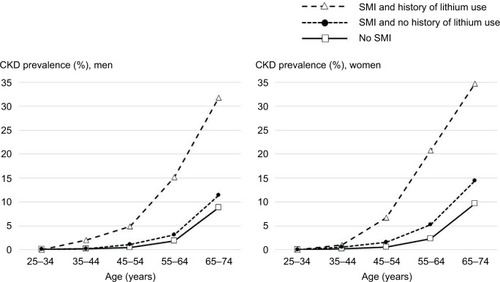
Overall, the prevalence of CKD was 14.64% (629/4,295) in patients with SMI and history of lithium prescription, 3.34% (805/24,101) in patients with SMI and no history of lithium prescription, and 2.09% (49,870/2,387,988) in patients without SMI (P < 0.001). The absolute difference in CKD prevalence among the three groups increased with age in both men and women (). Moreover, there was evidence (P = 0.012) that the overall prevalence of RRT was higher in patients with SMI (0.23% [10/4, 295] and 0.15% [36/24, 101] in those with and without a history of lithium prescription, respectively) than those without SMI (0.11% [2,645/2,387,988]). There was strong evidence that the distribution of RRT modalities was different between patients with and without SMI (P = 0.001). In patients with SMI, 50% (23/46) were receiving hemodialysis and 2% (1/46) peritoneal dialysis, and 48% (22/46) underwent kidney transplantation. However, in patients without SMI, the corresponding figures were 26% (700/2,645), 7% (194/2,645), and 66% (1,751/2,645).
Figure 2 Prevalence of CKD in patients with and without SMI by age in men and women.
The age–sex-adjusted odds ratio (OR) for CKD was 7.13 (95% confidence interval [CI] 6.47–7.85) for patients with SMI and history of lithium use, and 1.69 (1.56–1.83) for those with SMI and no history of lithium use, compared to those without SMI. After adjusting for SES, BMI, smoking, and chronic diseases associated with CKD, the adjusted ORs decreased to 6.49 (5.84–7.21) and 1.45 (1.34–1.58) but were still significant in patients with SMI with and without previous lithium prescription, respectively ().
Table 2 Adjusted logistic regression analyses of the association between SMI and chronic kidney disease
Among patients with biochemically defined CKD (not on RRT), a higher proportion of people with SMI had a recorded diagnosis of CKD (SMI 70.3%, without SMI 65.5%) and a recorded blood pressure ≤140/90 mmHg (SMI 80.1%, without SMI 75.6%; ). However, a lower proportion of patients with SMI had recently been prescribed statins (SMI 50.5%, without SMI 59.0%).
Table 3 Recognition and management of CKD in CKD patients with and without SMI
Discussion
Main findings
We found that, in UK primary care, patients with SMI had a greater prevalence of CKD compared to the general population. This was pronounced (6.5-fold increase) in patients with a history of lithium prescription, but there was a 1.5-fold increase in odds of CKD even among patients never known to be prescribed lithium and after adjustment for differences in known risk factors for CKD. In addition, patients with SMI had an increased prevalence of RRT and were more likely to be receiving hemodialysis as their modality than patients without SMI on RRT.
Strengths and limitations
There are several strengths of our study. There is likely to be a good recording of the diagnoses examined because GPs manage patients with SMI for their physical and mental health – with or without the support of psychiatrists in secondary care. In addition, the QOF – the reward and incentive program for GPs – included both SMI and CKD during this time period.Citation27 Moreover, the diagnosis of SMI has been validated at the individual level,Citation29 and the prevalence estimates of CKD and RRT have been validated at the population level.Citation31
However, we need to acknowledge several limitations. First, this is a cross-sectional study, in which temporal relationships cannot always be clarified. The majority of patients with SMI have developed symptoms by early adulthood,Citation37 whereas the prevalence of CKD starts to increase after age 55.Citation31 Therefore, it is unlikely that CKD precedes the onset of SMI. However, the interpretation of potential risk factors in the association between SMI and CKD requires caution. For example, cessation of smoking and lithium may be as a result of the development of CKD. Therefore, we did not differentiate previous and current users in the regression models. Second, a greater prevalence of CKD among patients with SMI may, in part, be influenced by surveillance or ascertainment bias. Patients with SMI take medications, such as lithium and other psychotropic drugs, which need regular monitoring. In addition, in 2013–2014, GPs were incentivized to monitor blood glucose and cholesterol levels for people with SMI; therefore, many patients would have had concurrent testing of renal function.Citation38 In contrast, in the general population, creatinine testing in primary care is not universal: in 2013–2014, this was recommended and incentivized only for people with known CKD risk factors.Citation21,Citation27 However, we have previously demonstrated that the prevalence of CKD identified in the CPRD was very similar to that seen in the Health Survey for England, a nationally representative survey of the general population.Citation31 This suggests that most patients with CKD are captured by the current testing strategy in primary care, and the proportion of patients with unmeasured CKD in CPRD is small. Therefore, underascertainment of CKD in people without SMI is unlikely to be a substantial contributor to our results. Third, RRT is a rare outcome: the incidence of RRT in the UK is ~100 per million population.Citation39 Combined with the cross-sectional design, this meant that, despite the large sample size, the number of patients with SMI and RRT was limited (n = 46). Although there was a statistically significant difference in the prevalence of RRT between patients with and without SMI, more detailed comparison (eg, stratification by age and sex) or analysis of risk factors for RRT was not possible with these numbers. Finally, our adjusted analyses suggested that differences in the prevalence of risk factors between patients with and without SMI did not completely explain the association between SMI and CKD. This finding may be due to additional unconsidered risk factors, differences in patient management, or residual confounding. For example, antipsychotics used for SMI have been suggested to cause acute kidney injury,Citation40,Citation41 which is a known risk factor for subsequent CKD.Citation21 Furthermore, insufficient control – or later initiation – of treatment for the CKD risk factors (eg, high blood pressure, diabetes, and obesity) may lead to higher incidence of CKD in the SMI population; however, this information could not be adequately captured in this cross-sectional study. Using recorded CPRD diagnoses, moreover, means that the misclassification or underidentification of disease status (eg, diabetes and heart failure) is possible. We did not have any information on the use of lithium before the CPRD registration and, therefore, some patients may have been wrongly classified as not having used lithium. This also meant that we were unable to examine the association between the length or cumulative dose of lithium prescription and CKD.
Comparison with other studies
Few studies have examined the prevalence of CKD in the population with SMI. A small cross-sectional study in London, UK, found a similar relative difference in CKD prevalence between those with and without SMI despite a less accurate CKD definition than in the present study.Citation13 A Taiwanese cohort study showed that people with schizophrenia are more likely to develop CKD,Citation25 whereas a cross-sectional analysis of Scottish primary care demonstrated that people with bipolar disorder had a higher prevalence of CKD than people without the condition.Citation18 Several studies have focused on the prevalence or incidence of CKD in patients using lithium. A Swedish cohort study showed that the prevalence of CKD (defined as serum creatinine level >150 μmol/L) and ESRD requiring RRT was higher in people ever exposed to lithium than in the general population,Citation42 whereas a Danish cohort study also showed that lithium prescription was associated with an increased rate of CKD diagnosis.Citation26
The relative risk of lithium use for the prevalence of CKD in our study (the fully adjusted OR of 6.5) was larger than those estimated in previous studies: the adjusted hazard ratio by lithium for CKD was nearly 2 in a cohort study in Oxford, UK,Citation43 and around 3 in the Danish cohort study.Citation26 These differences can be explained by the different nature of the study population in each study. We compared people with lithium prescription for SMI and those without SMI in the general population, whereas the UK cohort study compared those with and without lithium use among people with at least two blood samplings in hospitals in Oxford,Citation43 and the Danish study estimated the risk of lithium prescription in the population with a single manic episode or bipolar disorder.Citation26 Therefore, our comparison group is healthier than in the other studies, although again, ascertainment bias may play a role to some extent in our study.
The management of CKD includes prevention of cardiovascular events. Several previous studies have focused on inequalities in medication use for cardiovascular risk between patients with and without SMI. Studies from the UK showed that statin prescribing is lower in patients with schizophrenia and bipolar disorder than in the general population, although their cardiovascular risks are higher.Citation16,Citation18 A US study suggests that prescribing of renin–angiotensin system antagonists and statins is suboptimal in patients with SMI and type 2 diabetes.Citation17 Our finding of lower prescribing of statins among patients with SMI and CKD, compared to the general population, are consistent with these earlier studies.
Explanation of findings and clinical relevance
CKD is strongly and independently associated with mortality and cardiovascular risk.Citation20 Therefore, the higher prevalence of CKD we have established may contribute to the known shorter life expectancy in people with SMI. Moreover, we have shown that the difference in CKD prevalence between patients with and without SMI increased with age. It is possible that progressive accumulation and biological effect of CKD risk factors in patients with SMI (eg, obesity, smoking, and diabetes) leads to an increased incidence of CKD at an older age.
We have confirmed a higher burden of CKD risk factors among patients with SMI, and adjustment for these partially explained the association between SMI and CKD. However, many questions about the cause of the higher prevalence of CKD remain unanswered. Our snapshot of GP’s management of patients with SMI suggests that CKD is more commonly coded as a diagnosis than in the general population. The extent to which this is driven by incentivized management schemes and regular testing of renal function for patients with SMI – particularly those prescribed lithium and other psychotropic medications – is unknown. Whereas blood pressure appeared better controlled among SMI patients, this may reflect different underlying renal pathologies associated with lower rates of hypertension (eg, interstitial nephritis related to lithium). Other aspects of management – proteinuria testing as well as prescription of statins and renin–angiotensin system antagonists – were lower among patients with SMI.
Of greatest concern is our finding of the substantial increase in the prevalence of RRT among patients with SMI. The development of ESRD results in markedly reduced quality of life and psychological stress. Renal transplantation is associated with better quality of life and, possibly, longer survival.Citation44 However, our results showed that the proportion of patients receiving kidney transplantation was substantially lower in patients with SMI than those without. It is important to understand why this difference arises and ensure that there are no inappropriate barriers to the consideration for renal transplantation for people with SMI.
Conclusion
We found that the prevalence of CKD and RRT was substantially higher in patients with SMI as compared to the general population. There was a greater burden of risk factors among patients with SMI, but these did not fully explain the increased prevalence of CKD. Further information about the management of CKD in SMI patients such as referral to specialist care and management of comorbidities is needed to identify opportunities for prevention of CKD and its progression.
Acknowledgments
Masao Iwagami is supported by the Honjo International Scholarship Foundation. Laurie A Tomlinson is funded by a Wellcome Intermediate Clinical Fellowship (WT101143MA). Liam Smeeth is supported by a Wellcome Senior Research Fellowship in Clinical Science (grant number 098504/Z/12/Z). David PJ Osborn is supported by the UCLH NIHR Biomedical Research Centre and he was also in part supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Bart’s Health NHS Trust. The funders had no role in the execution of this study or in the interpretation of results.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChangCKHayesRDPereraGLife expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in LondonPLoS One201165e1959021611123
- DemblingBPChenDTVachonLLife expectancy and causes of death in a population treated for serious mental illnessPsychiatr Serv19995081036104210445651
- HannerzHBorgåPBorritzMLife expectancies for individuals with psychiatric diagnosesPublic Health2001115532833711593442
- Roshanaei-MoghaddamBKatonWPremature mortality from general medical illnesses among persons with bipolar disorder: a reviewPsychiatr Serv200960214715619176408
- CrumpCWinklebyMASundquistKSundquistJComorbidities and mortality in persons with schizophrenia: a Swedish national cohort studyAm J Psychiatry2013170332433323318474
- BrownSKimMMitchellCInskipHTwenty-five year mortality of a community cohort with schizophreniaBr J Psychiatry2010196211612120118455
- OsbornDPLevyGNazarethIPetersenIIslamAKingMBRelative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Rsearch DatabaseArch Gen Psychiatry200764224224917283292
- OsbyUCorreiaNBrandtLEkbomASparénPMortality and causes of death in schizophrenia in Stockholm county, SwedenSchizophr Res2000451–2212810978869
- OsbyUBrandtLCorreiaNEkbomASparénPExcess mortality in bipolar and unipolar disorder in SwedenArch Gen Psychiatry200158984485011545667
- OsbornDPNazarethIKingMBRisk for coronary heart disease in people with severe mental illness: cross-sectional comparative study in primary careBr J Psychiatry200618827127716507970
- DaumitGLClarkJMSteinwachsDMGrahamCMLehmanAFordDEPrevalence and correlates of obesity in a community sample of individuals with severe and persistent mental illnessJ Nerv Ment Dis20031911279980514671456
- McEvoyJPMeyerJMGoffDCPrevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES IIISchizophr Res2005801193216137860
- WoodheadCAshworthMSchofieldPHendersonMPatterns of physical co-/multi-morbidity among patients with serious mental illness: a London borough-based cross-sectional studyBMC Fam Pract20141511724919453
- OsbornDPBaioGWaltersKInequalities in the provision of cardiovascular screening to people with severe mental illnesses in primary care: cohort study in the United Kingdom THIN Primary Care Database 2000–2007Schizophr Res20111292–310411021550783
- SmithDJLanganJMcLeanGGuthrieBMercerSWSchizophrenia is associated with excess multiple physical-health comorbidities but low levels of recorded cardiovascular disease in primary care: cross-sectional studyBMJ Open201334e002808
- Hippisley-CoxJParkerCCouplandCVinogradovaYInequalities in the primary care of patients with coronary heart disease and serious mental health problems: a cross-sectional studyHeart200793101256126217344333
- KreyenbuhlJMedoffDRSeligerSLDixonLBUse of medications to reduce cardiovascular risk among individuals with psychotic disorders and Type 2 diabetesSchizophr Res20081011–325626518353616
- SmithDJMartinDMcLeanGLanganJGuthrieBMercerSWMultimorbidity in bipolar disorder and undertreatment of cardiovascular disease: a cross sectional studyBMC Med20131126324359325
- National Kidney FoundationK/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratificationAm J Kidney Dis2002392 Suppl 1S1S26611904577
- GoASChertowGMFanDMcCullochCEHsuCYChronic kidney disease and the risks of death, cardiovascular events, and hospitalizationN Engl J Med2004351131296130515385656
- National Institute for Health and Care ExcellenceChronic kidney disease in adults: assessment and management Available from: https://www.nice.org.uk/guidance/cg182Accessed October 22, 2017
- DixonLWeidenPDelahantyJPrevalence and correlates of diabetes in national schizophrenia samplesSchizophr Bull200026490391211087022
- MarkowitzGSRadhakrishnanJKambhamNValeriAMHinesWHD’AgatiVDLithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathyJ Am Soc Nephrol20001181439144810906157
- HayesJFMarstonLWaltersKGeddesJRKingMOsbornDPAdverse renal, endocrine, hepatic, and metabolic events during maintenance mood stabilizer treatment for bipolar disorder: a population-based cohort studyPLoS Med2016138e100205827483368
- TzengNSHsuYHHoSYIs schizophrenia associated with an increased risk of chronic kidney disease? A nationwide matched-cohort studyBMJ Open201551e006777
- KessingLVGerdsTAFeldt-RasmussenBAndersenPKLichtRWUse of lithium and anticonvulsants and the rate of chronic kidney disease: a nationwide population-based studyJAMA Psychiatry201572121182119126535805
- Health and Social Care Information CentreQuality and outcomes framework Available from: www.Hscic.Gov.Uk/qofAccessed October 22, 2017
- HerrettEGallagherAMBhaskaranKData Resource Profile: Clinical Practice Research Datalink (CPRD)Int J Epidemiol201544382783626050254
- NazarethIKingMHainesARangelLMyersSAccuracy of diagnosis of psychosis on general practice computer systemBMJ1993307689532348343670
- LeveyASStevensLASchmidCHCKD-EPI (Chronic Kidney Disease Epidemiology Collaboration)A new equation to estimate glomerular filtration rateAnn Intern Med2009150960461219414839
- IwagamiMTomlinsonLAMansfieldKEValidity of estimated prevalence of decreased kidney function and renal replacement therapy from primary care electronic health records compared with national survey and registry data in the United KingdomNephrol Dial Transplant201732Suppl 2ii142ii15028201668
- Hippisley-CoxJCouplandCDerivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort studyBMJ2012344e342722619194
- Hippisley-CoxJCouplandCPredicting risk of upper gastrointestinal bleed and intracranial bleed with anticoagulants: cohort study to derive and validate the QBleed scoresBMJ2014349g460625069704
- Department for Communities and Local GovernmentEnglish indices of deprivation Available from: www.Gov.Uk/government/collections/english-indices-of-deprivationAccessed October 22, 2017
- NHS Staffordshire Commissioning Support UnitRecommended read codes for quality & outcomes framework Available from: https://www.pcc-cic.org.uk/article/qof-read-codes-v28Accessed October 22, 2017
- National Institute for Health and Care ExcellenceCardiovascular disease: risk assessment and reduction, including lipid modification Available from: https://www.nice.org.uk/guidance/cg181Accessed October 22, 2017
- OchoaSUsallJCoboJLabadXKulkarniJGender differences in schizophrenia and first-episode psychosis: a comprehensive literature reviewSchizophr Res Treatment2012201291619822966451
- Quality and Outcomes Framework guidance for GMS contract 2013/14 Available from: https://www.bma.org.uk/-/media/files/pdfs/practical%20advice%20at%20work/contracts/gpqofguidance20132014.pdfAccessed October 22, 2017
- RaoACasulaACastledineCUK Renal Registry 17th Annual Report: Chapter 2 UK Renal Replacement Therapy Prevalence in 2013: National and Centre-specific AnalysesNephron2015129Suppl 1315625695806
- JiangYMcCombsJSParkSHA retrospective cohort study of acute kidney injury risk associated with antipsychoticsCNS Drugs201731431932628290080
- HwangYJDixonSNReissJPAtypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort studyAnn Intern Med2014161424224825133360
- BendzHSchönSAttmanPOAurellMRenal failure occurs in chronic lithium treatment but is uncommonKidney Int201077321922419940841
- ShineBMcKnightRFLeaverLGeddesJRLong-term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory dataLancet2015386999246146826003379
- ThiruchelvamPTWillicombeMHakimNTaubeDPapaloisVRenal transplantationBMJ2011343d730022084316
