Abstract
Introduction
The optimal revascularization strategy for patients with ST-segment elevation myocardial infarction and multivessel disease is unclear. In this study, we performed a meta-analysis to determine the optimal revascularization strategy for treating these patients.
Methods
Searches of PubMed, the Cochrane Library, clinicaltrial.gov, and the reference lists of relevant papers were performed covering the period between the year 2000 and March 20, 2017. A pairwise analysis and a Bayesian network meta-analysis were performed to compare the effectiveness of early complete revascularization (CR) during the index hospitalization, delayed CR, and culprit only revascularization (COR). The primary endpoint was the incidence of major adverse cardiac events (MACE), which were defined as the composite of recurrent myocardial infarction (MI), repeat revascularization, and all-cause mortality. The secondary endpoints were the rates of all-cause mortality, recurrent MI, and repeat revascularization. This study is registered at PROSPERO under registration number CRD42017059980.
Results
Eleven randomized controlled trials including a total of 3,170 patients were identified. A pairwise meta-analysis showed that compared with COR, early CR was associated with significantly decreased risks of MACE (relative risk [RR] 0.47, 95% CI 0.39–0.56), MI (RR 0.55, 95% CI 0.37–0.83), and repeat revascularization (RR 0.35, 95% CI 0.27–0.46) but not of all-cause mortality (RR 0.78, 95% CI 0.52–1.16). These results were confirmed by trial sequential analysis. The network meta-analysis showed that early CR had the highest probability of being the first treatment option during MACE (89.2%), MI (83.3%), and repeat revascularization (80.4%).
Conclusion
Early CR during the index hospitalization was markedly superior to COR with respect to reducing the risk of MACE, as CR significantly decreased the risks of MI and repeat revascularization compared with COR. However, further study is warranted to determine whether CR during the index hospitalization can improve survival in patients with concurrent ST-segment elevation myocardial infarction and multivessel disease. The optimal timing of CR remains inconclusive considering the small number of studies and patients included in the analysis comparing early and delayed CR.
Introduction
Primary percutaneous coronary intervention (PPCI) is the recommended treatment for patients with ST-segment elevation myocardial infarction (STEMI). Approximately 50% of patients with STEMI present with multivessel coronary disease at the time of PPCI,Citation1 and these patients tend to experience worse mortality and morbidity than patients with single-vessel disease.Citation1,Citation2 Therefore, effective treatments for multivessel disease in patients with STEMI are urgently needed.
However, only a limited amount of evidence is available regarding the treatment of STEMI and multivessel disease. The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines include a class IIb recommendation, ie, they recommend complete revascu-larization (CR) at the time of PPCI or staged revascularization as a second planned procedure for the treatment of patients with STEMI.Citation3 The European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines include a class IIa recommendation stating that CR should be considered before hospital discharge.Citation4 Therefore, the optimal treatment for the disease and the optimal timing of additional revascularization procedures intended to treat the disease remain unclear.
Therefore, we performed a pairwise analysis and a network meta-analysis to compare the effectiveness of current revascularization strategies for STEMI with multivessel disease, namely, early CR during the index hospitalization, delayed CR of nonculprit vessels, and culprit only revascularization (COR). Early CR was defined as the performance of percutaneous coronary intervention (PCI) involving culprit vessel lesions and nonculprit vessel lesions during the same procedure or hospitalization (mean or median number of days after PPCI ≤7). Delayed CR was defined as the performance of PCI involving only culprit vessel lesions during the index, followed by PCI involving nonculprit vessel lesions during a separate procedure (>7 days after PPCI). COR was defined as the performance of PCI involving only culprit vessel lesions.
Methods
Data sources and search strategy
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement for network meta-analysis.Citation5 The PRISMA checklist is provided in the Supplementary materials. Searches of PubMed, the Cochrane library, clinicaltrial.gov, and the reference lists of relevant papers were performed between the year 2000 and March 20, 2017. The following keywords and MeSH terms were used in the searches: “myocardial infarction”, “multivessel disease”, and “percutaneous coronary intervention”. This study is registered at PROSPERO under registration number CRD42017059980.
Study selection and eligibility criteria
The following studies were included in the analysis: 1) studies including patients with STEMI with multivessel disease; 2) studies whose intervention or control groups received the treatments of interest (CR during the index hospitalization, delayed CR of nonculprit lesions, and COR); 3) studies reporting the outcomes of major adverse cardiac events (MACE), recurrent myocardial infarction (MI), repeat revascularization or all-cause mortality; and 4) randomized controlled trials (RCTs).
Data extraction, outcomes, and quality assessment
We developed a data extraction sheet that was subsequently used by two of our authors (W-QG and QS) who independently extracted the following data from the studies included in the analysis: year of publication, treatment regimen, mean follow-up time, definition of MACE, and timing of the staged procedures. Disagreements were resolved via joint reviews of the literature. In cases in which we noted different results in the same trial, we extracted the data pertaining to the most recent results for analysis. The primary endpoint was the incidence of MACE (defined as the composite of recurrent MI, repeat revascularization, and all-cause mortality) over the longest available follow-up time, and the secondary endpoints were the rates of all-cause mortality, recurrent MI, and repeat revascularization over the longest available follow-up time. All endpoints were defined according to the definitions used in each trial. The intention-to-treat sample size was used when available, and the Cochrane risk-of-bias tool was used to assess the risks of bias.Citation6
Statistical analysis
Pairwise meta-analysis and trial sequential analysis
In the pairwise meta-analysis, we assessed all clinical outcomes by calculating pooled relative risks (RRs) and corresponding 95% CIs. To account for unexplained heterogeneity, we performed the meta-analysis using a random-effects model (DerSimonian–Laird method).Citation7 The Cochrane Q test and the inconsistency index (I2 test) were used to assess statistical heterogeneity.Citation8 I2 values <25% were indicative of low heterogeneity, whereas values between 25% and 50% were indicative of moderate heterogeneity and values >50% were indicative of high heterogeneity.Citation8 The funnel plot method and Egger’s regression asymmetry test were used to determine if publication bias was present.Citation9 A pairwise meta-analysis was performed using STATA software, version 12.0 (StataCorp, College Station, TX, USA). In a single trial, interim analysis may have increased the risk of type I errors. Trial sequential analysis (TSA) is regarded as an interim analysis in which monitoring boundaries are used to determine whether a trial may be terminated early due to the presence of a sufficiently small P-value.Citation10 Therefore, to determine whether the meta-analysis was less rigorous than a good single RCT, we performed TSA to assess the reliability and conclusiveness of the present evidence regarding the effectiveness of CR during the index hospitalization. The optimal information size was based on the assumption that a reduction in the relative risk (RR) was observed in the trials with a low risk of bias (adequate allocation concealment).Citation11 In addition, we used a default type I error of 5% (α=5%) and a default type II error of 20% (1−β=80%).Citation10 TSA was conducted by TSA software, version 0.9 beta (Copenhagen Trial Unit, Copenhagen, Denmark).
Network meta-analysis
The network meta-analysis was performed using a Bayes-ian random-effects model to fully preserve the randomized therapy comparisons within the trials and to account for unexplained between-trial heterogeneity.Citation12,Citation13 All outcomes are expressed as the RR and corresponding 95% credible interval (CrI). The model fit was based on the residual deviance,Citation14 and the analyses were performed using the Markov Chain Monte Carlo method.Citation13 We used noninformative uniform distributions to generate the posterior distributions of the model parameters and fitted four chains, yielding 400,000 iterations (100,000 per chain). Convergence was assessed by the Brooks–Gelman–Rubin diagnostic,Citation15 and global heterogeneity was assessed by the I2 statistic.Citation16 The “node-splitting” approach was used to evaluate inconsistency.Citation17 A Bayesian P-value <0.5 was suggestive of the presence of inconsistency between the direct and indirect evidence. In addition, performing a network meta-analysis afforded us the advantage of being able to calculate the probability that each intervention was the optimal intervention.Citation18 To assess the robustness of the results of the network meta-analysis, we performed the following sensitivity analyses: 1) analyses excluding unpublished studies, 2) analyses excluding studies that did not include repeat revascularization in their definition of MACE, 3) analyses excluding studies in which information regarding the mean or median timing of the second procedure was not available, 4) analyses excluding studies in which all outcomes were expressed with odds ratios (ORs) and corresponding 95% CrIs, and 5) analyses combining the indicated summary statistics (ie, hazard ratios [HRs] and ORs) into a single Bayesian analysis using the method devised by Woods et alCitation19 in cases in which HRs were reported. To explore the association between the log-risk of the primary outcome (MACE) and followup time, we performed a meta-regression by the Bayesian approach.Citation20 Moreover, we conducted a subgroup analysis of the data (angiography-guided PCI vs fractional flow reserve-guided PCI) regarding the primary outcome by calculating the interaction term β.Citation20 To detect dominant publication bias, we plotted a comparison-adjusted funnel plot with STATA version 12.0. The Bayesian frame network meta-analysis was conducted using gemtc and rjags in R software, version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Search results and characteristics of the included studies
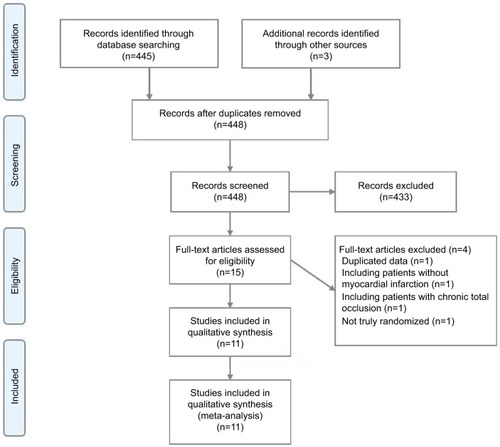
A flow diagram of the literature search and study selection process is shown in . We initially determined that 448 studies were eligible for the analysis based on their titles and abstracts. After screening, 11 RCTs involving 3,170 patients were included in the analysis.Citation21–Citation31 We excluded a study by Maamoun et al because it was a pilot cohort study,Citation32 a study by Ijsselmuiden et al because it included patients without STEMI,Citation33 a study by Dambrink et al because it was a short-term report that included data from the same trial reported in the publication by Ghani et al,Citation34 and also the EXPLORE trial because it assessed the efficacy of staged chronic total occlusion (CTO)-PCI.Citation35 The network plot of the comparisons of the revascularization strategies is shown in . Among these 11 RCTs, six compared early CR during the index hos-pitalization with COR,Citation21–Citation25,Citation31 two compared delayed CR with COR,Citation26,Citation27 two compared early CR with delayed CR,Citation28,Citation29 and one 3-arm trial compared early CR during the index hospi-talization, delayed CR, and COR.Citation30 The characteristics of the studies included in the analysis and their populations are listed in . All the studies included patients with STEMI with multivessel disease. Three trials performed fractional flow reserve (FFR) of the nonculprit lesions.Citation25,Citation26,Citation31 The follow-up time ranged from 6 to 38 months. The results of one of the trials were reported only in conference presentations.Citation27 The complications of revascularization are shown in Table S1. An assessment of the risk of bias of the included studies is presented in Figure S1. Overall, the included studies had a low to intermediate risk of bias.
Figure 2 Network plot for the Bayesian network meta-analysis.
Abbreviations: CR, complete revascularization; COR, culprit-only revascularization; MACE, major adverse cardiac events; MI, myocardial infarction; PPCI, primary percutaneous coronary intervention.
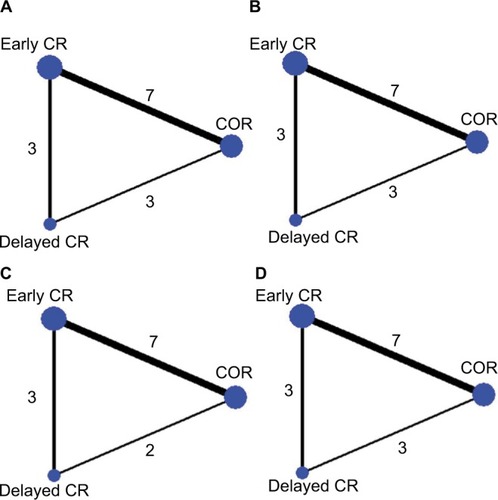
Table 1 Characteristics of the included randomized controlled trials
Results of the pairwise meta-analysis and TSA
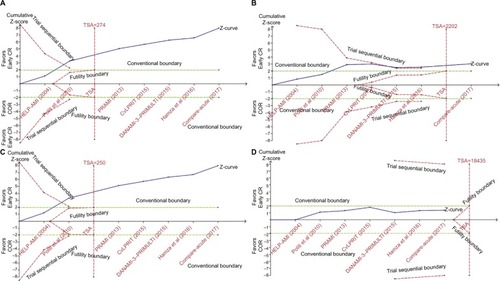
The results of the pairwise meta-analyses are shown in . Early CR was associated with a lower risk of MACE (RR 0.47, 95% CI 0.39–0.56; I2=0%) than COR, as early CR was associated with reduced risks of MI (RR 0.55, 95% CI 0.37–0.83; I2=0%) and repeat revascularization (RR 0.35, 95% CI 0.27–0.46; I2=0%) compared with COR. The risk of all-cause mortality was similar in the two groups (RR 0.78, 95% CI 0.52–1.16; I2=0%). The risks of MACE (RR 0.77, 95% CI 0.39–1.49; I2=76.4%), MI (RR 1.52, 95% CI 0.46–5.00; I2=55.4%), repeat revascularization (RR 0.62, 95% CI 0.23–1.64; I2=79.3%), and all-cause mortality (RR 0.72, 95% CI 0.28–1.85; I2=30.6%) were similar in the delayed CR and COR groups. Additionally, the risks of MACE, MI, repeat revascularization, and all-cause mortality were similar in the early CR and delayed CR groups. The TSA results are shown in . Regarding MACE, the TSA comparing early CR with COR showed that the cumulative z-curve crossed the conventional boundary (P=0.05) and the trial sequential boundary, indicating that the evidence showing early CR that reduced the risk of MACE by 53% (results from the meta-analysis with low-risk evidence, ) compared with COR was firm (). Regarding MI and repeat revas-cularization, the evidence of the TSA results indicated that early CR reduced the risks of MI and repeat revascularization by 44% and 65%, respectively, compared with COR (). However, regarding all-cause mortality, the TSA comparing early CR and COR showed that the cumulative z-curve failed to cross the conventional boundary (P=0.05) and the trial sequential boundary, indicating that early CR did not reduce the risk of all-cause mortality ().
Figure 3 Results of the pairwise meta-analysis of the overall rates of the clinical outcome.
Abbreviations: COR, culprit-only revascularization; CR, complete revascularization; MACE, major adverse cardiac events; MI, myocardial infarction; PPCI, primary percutaneous coronary intervention; RR, relative risk.
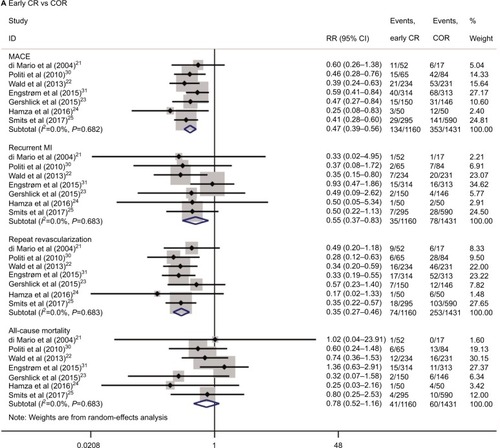
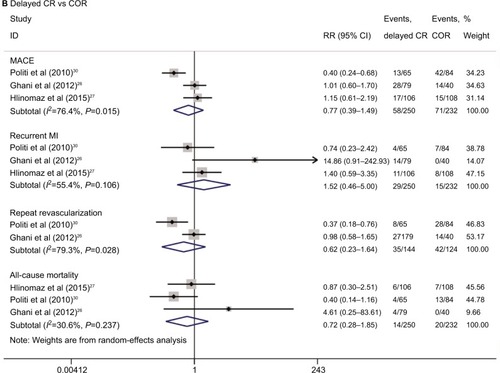
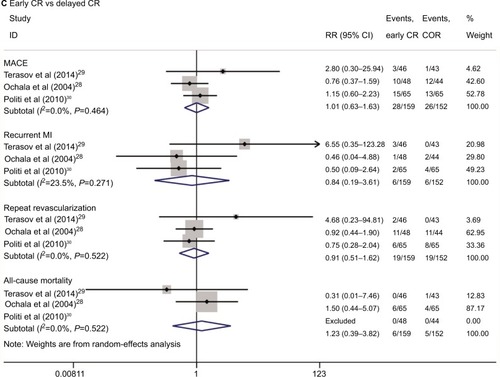
Figure 4 Results of the TSA of the risks of clinical outcomes.
Abbreviations: COR, culprit-only revascularization; CR, complete revascularization; MACE, major adverse cardiac events; MI, myocardial infarction; PPCI, primary percutaneous coronary intervention; TSA, trial sequential analysis.
Results of the network meta-analysis
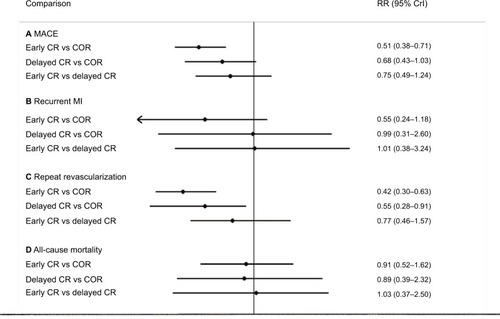
The results of the network meta-analysis are shown in . The risk of MACE was significantly lower in the early CR group than in the COR group (RR 0.51; 95% CrI 0.38, 0.71). There was no significant difference between the delayed CR group and the COR group (RR 0.68; 95% CrI 0.43, 1.03). Additionally, the risk of repeat revascularization was significantly lower in the early CR group than in the COR group (RR 0.42; 95% CrI 0.30, 0.63) and was significantly lower in the delayed CR group than in the COR group (RR 0.55; 95% CrI 0.28, 0.91). The risk of MI was not signifi-cantly different between the early CR and COR groups (RR 0.55; 95% CrI 0.24, 1.18). A comparison of early CR and delayed CR showed that the risk of overall clinical outcomes was similar between the two groups. There was no difference among the three approaches with respect to the risk of all-cause mortality.
Figure 5 Results of the network meta-analysis of the risks of clinical outcomes.
Abbreviations: COR, culprit-only revascularization; CR, complete revascularization; CrI, credible interval; MACE, major adverse cardiac events; MI, myocardial infarction; PPCI, primary percutaneous coronary intervention; RR, relative risk.
Ranking the probabilities of the overall clinical outcomes
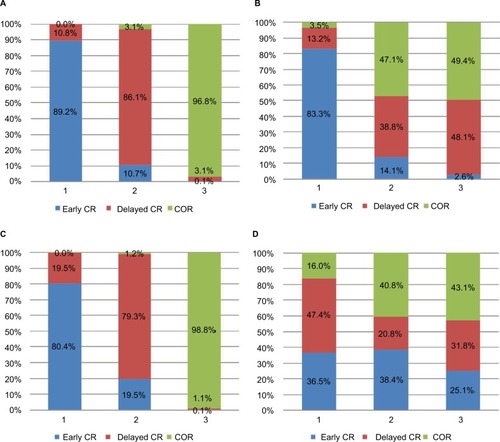
A graph of the probability rankings for each treatment is shown in . Early CR showed the highest probability of being the first treatment option for MACE (89.2%), followed by delayed CR (10.8%) and COR (0%). Similarly, early CR showed the highest probability of being the first treatment option for MI (83.3%) and repeat revascularization (80.4%). Delayed CR showed the highest probability of being the second treatment option for MACE (10.8%), MI (13.2%), and repeat revascularization (19.5%) but showed the highest probability of being the first treatment option for all-cause mortality (47.4%).
Figure 6 Graph of the probability rankings of each treatment for clinical outcomes.
Abbreviations: COR, culprit-only revascularization; CR, complete revascularization; MACE, major adverse cardiac events; MI, myocardial infarction; PPCI, primary percutaneous coronary intervention.
Additional analyses
In the pairwise meta-analysis, the funnel plot and Egger’s regression asymmetry test results suggested that no publication bias was present (Figure S3). In the network meta-analysis, the global heterogeneities were 35.2% for MACE, 13.9% for MI, 20.3% for repeat revascularization, and 0% for all-cause mortality. The results of the inconsistency analysis showed that some inconsistencies were present (). The results of the sensitivity analysis are shown in . The results of this analysis were similar to those of the main analysis. The Bayesian meta-regression results showed that follow-up time had no significant effect on the incidence of MACE (interaction term β=−0.08; CrI −0.86, 0.72, the graph of Bayesian meta-regression is shown in Figure S4), and the subgroup analysis showed that the type of guidance had no significant effect on the incidence of MACE (interaction term β=0.14; CrI −0.45, 0.71). The comparison-adjusted funnel plots suggested that the clinical outcomes of the study were not affected by publication bias (Figure S5).
Table 2 Assessment of inconsistency between the direct and indirect evidence
Table 3 The results of sensitivity analysis for the primary outcome
Discussion
The results of the pairwise meta-analysis and TSA clearly demonstrated that CR during the index hospitalization was superior to COR with respect to preventing MACE, MI, and repeat revascularization, and the network meta-analysis showed that early CR had the highest probability of being the first treatment option for MACE, MI, and repeat revascularization.
The performance of preventive PCI in noninfarcted coronary arteries during the index hospitalization was associated with a reduced risk of adverse cardiovascular events compared with the performance of COR.Citation22 However, the results of the PRAGUE 13 trial demonstrated that staged multives-sel PCI was not superior to COR with respect to reducing the risk of MACE.Citation27 Differences in the results of these trials may be due to significant differences in the times at which revascularization of nonculprit vessels was performed in each trial.Citation36 Recent observational studies uncovered evidence regarding the optimal timing of staged PCI in which early CR was superior to delayed CR and COR in reducing the risk of MACE.Citation37,Citation38 Therefore, in this analysis, we organized patients who underwent CR into groups comprising patients who underwent CR during the index hospitalization and patients who underwent delayed CR during a separate procedure. The probabilities that CR during the index hospitalization and delayed CR were the first treatment option for MACE were 89.2% and 10.8%, respectively. These results imply that the timing of the second procedure plays an important role in determining the prognoses of patients with STEMI with multivessel disease. Moreover, CR during the index hospi-talization was associated with a lower risk of MACE than COR. Therefore, CR during the index hospitalization reduced the risk of MACE in patients with STEMI and multivessel disease, which supports the present ESC guidelines. However, the number of studies comparing early CR and delayed CR was limited, and the results were inconclusive. Therefore, the optimal timing of CR is still not clear. Additionally, in our study, patients with CTOs and cardiac shock were excluded. Accordingly, the results of our analysis should be interpreted with caution. However, significant evidence can be yielded from the CULPRIT-SHOCK trial, which was published in the 2017 TCT and was the only RCT surrounding the issue of complete strategy for patients with STEMI and multives-sel disease present with cardiogenic shock. In this study, 706 patients with STEMI and multivessel disease present with cardiogenic shock were included and randomly divided into the group of multivessel CR and COR. The primary endpoint was death from any cause or renal replacement therapy. COR was associated with lower all-cause mortality or renal replacement therapy compared with multives-sel CR (45.9% and 55.4%, respectively, RR 0.83, 95% CI 0.71–0.96; P=0.01). When the primary endpoint was divided individually, the RR of all-cause mortality in the COR group, compared with the multivessel CR group, was 0.84 (95% CI 0.72–0.98; P=0.03), and the RR of renal replacement therapy was 0.71 (95% CI 0.49–1.03; P=0.07).Citation39
Interestingly, the results of the present analysis indicate that CR during the index hospitalization has satisfactory effects on the risk of MI, which may be due to the early revas-cularization of nonculprit vessels. Patients with STEMI have large plaque burdens in noninfarcted arteries. The incidence of MACE attributable to recurrence at the site of a nonculprit lesion was equal to the incidence of MACE attributable to recurrence at the site of a culprit lesion.Citation40 Of the nonculprit lesions associated with recurrence, the majority were thin-cap fibroatheromas or characterized by a large plaque burden and featured a small luminal area.Citation40 Therefore, the effectiveness of CR during the index hospitalization may be related to the pacification of vulnerable plaques in nonculprit lesions. Of note, the MI events included periprocedural and spontaneous MI in most analyzed studies. It is difficult to identify the type of MI (periprocedural or spontaneous MI) among STEMI patients undergoing immediate CR or very early CR (<3 days after the index procedure). Therefore, further study with MI classification is warranted to resolve this debate. In this study, approximately 43% of patients in the CR group (including early CR and delayed CR) were treated with FFR-guided CR. To identify an association between the risk of MACE and PCI guidance, we performed a subgroup analysis by dividing the CR group into FFR- and angiography-guided PCI. The type of guidance had no significant effect on the incidence of MACE. Indeed, the role of FFR in the treatment of STEMI and multivessel disease is debatable. In the study by Dambrink et al, nearly 40% of nonculprit lesions in the CR group were not hemodynamically significant (FFR>0.75).Citation34 FFR-guided PCI was not associated with a lower risk of MACE compared with conservative COR.Citation26 Additionally, in our study, we noted no difference in mortality between the early CR and COR groups. This finding may be attributable to the small patient populations of the included trials; thus, the ongoing COMPLETE trial (NCT01740479) and FULL REVASC trial (NCT02862119) will be helpful to determine whether early CR has a beneficial effect on patient survival.
Reliability and conclusiveness of our analyses
The conclusiveness of our analyses should be evaluated on the basis of several issues. The first is whether heterogeneity between studies was noted in each comparison. In the pairwise meta-analysis, no heterogeneity was observed in comparison between early CR and COR regardless of the clinical outcome. The second issue is the threshold of statistical significance. We performed TSA to quantify the statistical reliability of our data. Early CR significantly reduced the risk of MACE – as it reduced the risks of MI and repeat revascularization – but did not appear to reduce the risk of death compared with COR. The third issue is the precision of our estimates. The network meta-analysis integrated our direct and indirect evidence to increase the precision of our results.Citation41 The results of the pairwise meta-analysis showed that the risk of MACE was similar in the delayed CR and COR groups. The corresponding CI ranged from 0.39 to 1.49. The estimate from the network meta-analysis was more precise than that from the pairwise meta-analysis, as the former analysis yielded a lower RR (0.77 in the pairwise meta-analysis vs 0.68 in the network meta-analysis) and a smaller CrI, which ranged from 0.43 to 1.03, than the latter analysis. The fourth issue is the consistency between the direct and indirect estimates in the network meta-analysis. Unfortunately, certain inconsistencies were noted in the network analysis (eg, inconsistency was noted in the results of comparison of the effects of early CR and COR on the risk of MI). These inconsistencies may have been caused by limitations in the amount of data comparing early CR and delayed CR. In the pairwise meta-analysis, the MI outcome was significantly better with early CR than with COR (RR 0.55, 95% CI 0.37–0.83). In the Bayesian network meta-analysis, the RR of MI risk in the early CR group, compared with the delayed CR group, was 1.60 (95% CrI 0.41, 7.39), and the corresponding CrI was wide, whereas the RR of MI risk in the delayed CR group, compared with the COR group, was 1.55 (95% CrI 0.52, 5.47). Therefore, early CR was indirectly compared with COR via a common comparator, namely, delayed COR, and the results of the comparison showed that the RR of MI risk in the early CR group, compared with the COR group, was 1.25 (95% CrI 0.44, 4.48). However, the results of the direct comparison of early CR and COR showed that the risk of MI was lower in the early CR group than in the COR group (RR 0.38; 95% CrI 0.27, 0.54). These results were inconsistent with those of the indirect estimate. Additionally, when combining the direct and indirect evidence, the network evidence showed that the MI risk was similar in the early CR and COR groups (RR 0.55; 95% CrI 0.24, 1.18). Thus, the small number of studies comparing the effectiveness of early CR with that of delayed CR may be the main source of inconsistency in the network meta-analysis.Citation42 Therefore, the results of our network meta-analysis should be interpreted with caution. Finally, in this study, we used probability ranking to determine the probability that each therapy is the best among the revascularization strategies. The number of events for the survival outcomes was low and the corresponding CrI was wide, suggesting that the estimates of RR were imprecise. Because of the low event rates of all-cause mortality, a sample of >18,000 patients is needed to provide the trial power to establish cardiovascular safety. However, performing such a large trial is difficult, considering the limited amount of funding available. Additionally, statistically robust evidence of a beneficial effect was not evident, as no survival benefit was observed for the evaluated strategy.Citation43 Therefore, we performed probability ranking as an alternative way to display data that would be more readily interpretable in this situation.Citation18
Comparison with previous studies
Some updated pairwise and network meta-analyses evaluating the efficacy and safety of multivessel and CR were recently published. Elgendy et al performed a study in which they divided the revascularization strategies analyzed therein into four groups (immediate CR, CR during the index hospitalization, CR after discharge, and COR) and thus increased the uncertainty caused by the small sample size.Citation44 The effectiveness of immediate PCI seemed to be similar to that of multivessel PCI during hospitalization, indicating that immediate multivessel PCI and CR during the index hospitalization can be combined into one group. The revascularization strategies assessed in our study were organized into three groups. We noted no heterogeneity between CR during the index hospitalization and COR with respect to clinical outcomes, a finding that supports the above hypothesis. Additionally, Elgendy et al mainly reported the results of a pairwise meta-analysis comparing CR with COR, but the methodology of the network meta-analysis was flawed. In our study, we clearly reported the methodology of the network meta-analysis and performed a consistency analysis and sensitivity analysis, which were important to assess the quality of evidence. A similar analysis was conducted by Tarantini et al, who suggested that staged CR be performed in patients with concurrent STEMI and multivessel disease because it is associated with lower long- and short-term mortality.Citation45 Notably, the superior efficacy of staged CR noted in the above study was driven mainly by the outcomes of non-RCTs, which had selective bias due to the nature of the study (observational). The evidence in our study based on data from RCTs is more convincing. Moreover, our study included data from several recent trials whose results have not been used in previous meta-analyses.Citation25
Study limitations
However, our study was not without limitations. First, the PRAGUE 13 trial has not yet been published. To obtain conclusive evidence, we performed a sensitivity analysis from which this study was excluded. The results of the sensitivity analysis were largely similar to those of the main analysis. Second, variations existed in the designs of the trials included in the analysis. For example, three trials included in this analysis performed FFR of nonculprit lesions to guide decision making regarding the performance of PCI. However, we performed a subgroup analysis by calculating the interaction term β. FFR-guided PCI did not influence the study outcomes. Third, the definition of MACE differed between studies. Most definitions of MACE included death, MI, and revascularization. To make the evidence conclusive, we performed a sensitivity analysis by excluding studies that did not include revascularization in their definition of MACE. The results from the sensitivity analysis were consistent with the primary analysis. Fourth, this study demonstrated a benefit of CR during the index hospitalization driven mainly by a lower rate of repeat revas-cularization and MI but not the hard endpoint of mortality. Thus, most included studies were open-label RCTs. The endpoint of repeat revascularization should be regarded as less stringent, and its “true” benefit in the setting of an open-label study should be questioned. Finally, undertreatment of the COR group cannot be ruled out in certain studies, which strongly discouraged testing for ischemia. Overall, there is increasing evidence that a CR strategy is safe and probably beneficial in STEMI patients.
Conclusion
CR during the index hospitalization was markedly superior to COR with respect to reducing the risk of MACE, as CR reduced the risks of MI and repeat revascularization compared with COR. Further studies are warranted to determine whether CR during the index hospitalization can improve survival in patients with STEMI with multivessel disease. The optimal timing of CR remains inconclusive considering the small number of studies and patients included in the analysis comparing early and delayed CR.
Acknowledgments
We thank American Journal Experts (AJE) for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
- JensenLOTerkelsenCJHorváth-PuhóEInfluence of multives-sel disease with or without additional revascularization on mortality in patients with ST-segment elevation myocardial infarctionAm Heart J20151701707826093866
- LiuKLLinSMChangCHChenYCChuPHPlasma angiopoietin-1 level, left ventricular ejection fraction, and multivessel disease predict development of 1-year major adverse cardiovascular events in patients with acute ST elevation myocardial infarction - a pilot studyInt J Cardiol201518215516025577775
- LevineGNBatesERBlankenshipJC2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and InterventionsCirculation2016133111135114726490017
- IbanezBJamesSAgewallS2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC)Eur Heart J201839211917728886621
- HuttonBSalantiGCaldwellDMThe PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanationsAnn Intern Med20151621177778426030634
- HigginsJPAltmanDGGøtzschePCThe Cochrane Collaboration’s tool for assessing risk of bias in randomised trialsBMJ2011343d592822008217
- DersimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- ThorlundKEngstrmJWetterslevJBrokJImbergerGGluudCUser manual for Trial Sequential Analysis (TSA) Available from: http://www.ctu.dk/tsa/files/tsa_manual.pdfAccessed April 24, 2017
- WetterslevJThorlundKBrokJGluudCTrial sequential analysis may establish when firm evidence is reached in cumulative meta-analysisJ Clin Epidemiol2008611647518083463
- MillsEJThorlundKIoannidisJPDemystifying trial networks and network meta-analysisBMJ2013346f291423674332
- DiasSSuttonAJAdesAEWeltonNJEvidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trialsMed Decis Making201333560761723104435
- CooperNJSuttonAJLuGKhuntiKMixed comparison of stroke prevention treatments in individuals with nonrheumatic atrial fibrillationArch Intern Med2006166121269127516801509
- BrooksSPGelmanAGeneral methods for monitoring convergence of iterative simulationsJ Comput Graph Stat19987434455
- van ValkenhoefGLuGde BrockBHillegeHAdesAEWel-tonNJAutomating network meta-analysisRes Synth Methods20123428529926053422
- DiasSWeltonNJCaldwellDMAdesAEChecking consistency in mixed treatment comparison meta-analysisStat Med2010297–893294420213715
- SalantiGAdesAEIoannidisJPGraphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorialJ Clin Epidemiol201164216317120688472
- WoodsBSHawkinsNScottDANetwork meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorialBMC Med Res Methodol2010105420537177
- DiasSSuttonAJWeltonNJAdesAEEvidence synthesis for decision making 3: heterogeneity--subgroups, meta-regression, bias, and bias-adjustmentMed Decis Making201333561864023804507
- di MarioCMaraSFlavioASingle vs multivessel treatment during primary angioplasty: results of the multicentre randomised HEpa-coat for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELP AMI) StudyInt J Cardiovasc Intervent200463–412813316146905
- WaldDSMorrisJKWaldNJRandomized trial of preventive angioplasty in myocardial infarctionN Engl J Med2013369121115112323991625
- GershlickAHKhanJNKellyDJRandomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trialJ Am Coll Cardiol2015651096397225766941
- HamzaMMahmoudNElgendyIYA randomized trial of complete versus culprit-only revascularization during primary percutaneous coronary intervention in diabetic patients with acute ST elevation myocardial infarction and multivessel diseaseJ Interv Cardiol201629324124727245121
- SmitsPCAbdel-WahabMNeumannFJFractional flow reserve-guided multivessel angioplasty in myocardial infarctionN Engl J Med2017376131234124428317428
- GhaniADambrinkJHvan ‘t HofAWOttervangerJPGosselinkATHoorntjeJCTreatment of non-culprit lesions detected during primary PCI: long-term follow-up of a randomised clinical trialNeth Heart J201220934735322622701
- HlinomazOGrochLPolokovaKPRAGUE 13: Multivessel PCI Failed to Best Culprit-Vessel PCI After STEMIParisEuroPCR2015
- OchalaASmolkaGAWojakowskiWThe function of the left ventricle after complete multivessel one-stage percutaneous coronary intervention in patients with acute myocardial infarctionJ Invasive Cardiol2004161269970215596873
- RomanSTVladimirIGAlexeyVPOlgaLBLeonidSBSix month results of randomized clinical trial: multivessel stenting in primary percutaneous coronary intervention and staged revascularization for ST-elevation myocardial infarction patients with second generation drug eluting stentsClin Med Res201435125129
- PolitiLSguraFRossiRA randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: major adverse cardiac events during long-term follow-upHeart201096966266719778920
- EngstrømTKelbækHHelqvistSComplete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3 – PRIMULTI): an open-label, randomised controlled trialLancet2015386999466567126347918
- MaamounWElkhaeatNElarasyRSafety and feasibility of complete simultaneous revascularization during primary PCI in patients with STEMI and multi-vessel diseaseEur Heart J20116313943
- IjsselmuidenAJEzechielsJWestendorpICComplete versus culprit vessel percutaneous coronary intervention in multives-sel disease: a randomized comparisonAm Heart J2004148346747415389234
- DambrinkJHDebrauwereJPvan ‘t HofAWNon-culprit lesions detected during primary PCI: treat invasively or follow the guidelines?EuroIntervention20105896897520542783
- HenriquesJPHoebersLPRåmunddalTPercutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trialJ Am Coll Cardiol201668151622163227712774
- GuoWQLiLSuQMeta-analysis of strategies for patients with multivessel disease undergoing percutaneous coronary intervention: does the timing of staged procedures matter?JACC Cardiovasc Interv201710111180118128595887
- KimIKimMCJeongHCOptimal timing of percutaneous coronary intervention for nonculprit vessel in patients with ST-segment elevation myocardial infarction and multivessel diseaseKorean Circ J2017471364328154589
- IqbalMBNadraIJDingLCulprit vessel versus multivessel versus in-hospital staged intervention for patients with ST-segment elevation myocardial infarction and Multivessel disease: stratified analyses in high-risk patient groups and anatomic subsets of nonculprit diseaseJACC Cardiovasc Interv2017101112328057282
- ThieleHAkinISandriMPCI strategies in patients with acute myocardial infarction and cardiogenic shockN Engl J Med2017377252419243229083953
- StoneGWMaeharaALanskyAJA prospective natural-history study of coronary atherosclerosisN Engl J Med2011364322623521247313
- TrelleSReichenbachSWandelSCardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysisBMJ2011342c708621224324
- ParkCHJungYSNamEEunCSParkDIHanDSComparison of efficacy of prophylactic endoscopic therapies for postpolypectomy bleeding in the colorectum: a systematic review and network meta-analysisAm J Gastroenterol201611191230124327402502
- AltmanDGBlandJMAbsence of evidence is not evidence of absenceBMJ199531170034857647644
- ElgendyIYMahmoudANKumbhaniDJBhattDLBavryAAComplete or culprit-only revascularization for patients with multivessel coronary artery disease undergoing percutaneous coronary intervention: a pairwise and network meta-analysis of randomized trialsJACC Cardiovasc Interv201710431532428231899
- TarantiniGD’AmicoGBrenerSJSurvival after varying revas-cularization strategies in patients with ST-segment elevation myocardial infarction and multivessel coronary artery disease: a pairwise and network meta-analysisJACC Cardiovasc Interv20169171765177627609250
- MoherDLiberatiATetzlaffJThe PRISMA Group (2009)Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA StatementPLoS Med200967e100009719621072