Abstract
Purposes
To assess whether the positive predictive value (PPV) of first-time rheumatoid arthritis (RA) diagnosis registration in the Danish National Patient Registry increases when data are linked to the RA treatment codes and to assess the PPV of first-time RA diagnoses according to RA serological subtypes.
Methods
Participants from the Danish Diet, Cancer, and Health cohort with at least one RA diagnosis, registered at one of the Central Denmark Region hospitals in the Danish National Patient Registry during the period 1977–2016, were identified. Register-based RA diagnoses were verified by scrutinizing medical records against RA classification criteria or clinical case RA. PPVs for overall RA, seropositive RA, and other RA were calculated for two models: first-time RA diagnosis registration ever in the Danish National Patient Registry and first-time RA diagnosis registration ever where subsequently a prescription had been redeemed for a synthetic disease-modifying antirheumatic drug.
Results
Overall, 205 of 311 first-time register-based RA diagnoses were verified (PPV: 61.9%; 95% CI: 56.9–67.0). Regarding RA serological subtypes, 93 of 150 register-based seropositive RA (PPV: 62.0; 95% CI: 53.9–69.5) and 36 of 144 other RA (PPV: 25.0; 95% CI: 18.5–32.8) were confirmed. When register-based RA diagnosis codes were linked to RA treatment codes, the PPVs increased substantially: the PPV for overall RA was 87.7% (95% CI: 82.5–91.5), the PPV for seropositive RA was 80.2% (95% CI: 71.6–86.7), and the PPV for other RA was 41.1% (95% CI: 30.2–52.9).
Conclusion
The first-time RA diagnoses in the Danish National Patient Registry should be used with caution in epidemiology research. However, linking registry-based RA diagnoses to the subsequent RA treatment codes increases the probability of identifying true RA diagnoses, especially overall RA and seropositive RA.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune inflammatory disease characterized by inflammation of synovial joints; it leads to irreversible joint damage, deformity, and functional impairment. Furthermore, RA patients are at higher risk of comorbidities and have a higher mortality rate than the general population.Citation1,Citation2
Large-scale registries have been widely used in research, contributing to knowledge regarding epidemiology, comorbidities, and mortality rates of RA patients.Citation1–Citation6
The Danish National Patient Registry is a Danish key health registry.Citation7 It was established for administrative purposes in 1977 as an extension of county-based hospital registration systems. For many decades, the Danish National Patient Registry has been extensively used for epidemiological and clinical research. The registry contains comprehensive data regarding hospital contacts, including International Classification of Disease (ICD) codes.Citation8 However, the validity of both in- and outpatient diagnostic data is open to discussion. Pedersen et alCitation9 investigated the validity of 217 RA diagnoses registered in the Danish National Patient Registry during the period 1977–2001. They found that the positive predictive value (PPV) of RA diagnoses was 59%. Ibfelt et alCitation10 validated incident RA diagnoses recorded ≥2 times within 90 days in rheumatology departments during 2011. The PPV of RA diagnoses in their study was 79%. A study from the USA showed that the PPV of administrative registry-based RA diagnoses increased from 66% to 81% when data were linked to RA treatment codes.Citation11
It is well known that seropositive and seronegative RA differ with respect to their phenotype, susceptibility genes, and the impact of smoking and obesity as risk factors.Citation12–Citation18 Therefore, it is important to study these two serological subtypes separately.
The aims of our study were, first, to assess whether the PPV of first-time RA diagnosis registration in the Danish National Patient Registry increases when data are linked to the RA treatment codes and, second, to assess the PPV of first-time RA diagnoses according to serological subtypes.
Methods
Setting
The study explored the PPV of RA diagnoses in the Danish National Patient Registry during the period 1977–2016 among participants in the Danish Diet, Cancer, and Health cohort.Citation19
Data sources
The Danish Diet, Cancer, and Health cohort study
The cohort was recruited during the period 1993–1997. Overall, 80,996 men and 79,729 women were invited, and a total of 57,053 accepted the invitation. The eligibility criteria were the following: age 50–64 years, born in Denmark, living in the Copenhagen or Aarhus counties, and no cancer diagnosis registered in the Danish Cancer Registry.Citation20 A detailed description of the cohort has been published previously.Citation19
All participants of the Danish Diet, Cancer, and Health cohort were linked to the Danish National Patient RegistryCitation7 in February 2017 and subsequently linked to the Danish National Prescription Registry using the unique Civil Personal Registration number. This number is used in all Danish registries, enabling data linkage at the individual level.
The Danish National Patient Registry
The registry contains data on all admissions to somatic hospitals since 1977 and on all visits to outpatient clinics since 1995,Citation7 including hospital information, dates of hospital admissions, ward types, referral and discharge diagnoses, dates of each attendance at outpatient clinics, type of clinics, and diagnoses recorded at each attendance. The diagnoses were classified in accordance with the Danish version of the ICD-8 until the end of 1993 and thereafter in accordance with the updated version, ICD-10.Citation8
The Danish National Prescription Registry
The Danish National Prescription Registry is a redemption database that was established in 1994.Citation21 Every pharmacy in Denmark is equipped with an electronic accounting system to secure reimbursement from the National Health Service, which funds a variable proportion of the cost of prescribed medicine for every Danish citizen. Data are transferred to the Danish National Prescription Registry, which thus covers all reimbursed drugs at the level of the individual user.
The registry does not include information about drugs dispensed by hospital pharmacies directly to inpatients in relation to their hospital stays or to outpatients during their outpatient clinic attendances.
Validation of registry-based RA diagnoses
Identification of RA diagnoses
We included participants from the Danish Diet, Cancer, and Health cohort with at least one RA diagnosis, registered at one of the Central Denmark RegionCitation22 hospitals in the Danish National Patient Registry during the period 1977–2016.
The following ICD codes regarding RA diagnoses were retrieved from the Danish National Patient Registry: 712.39 (arthritis rheumatoides alia et non specificata), M05 (seropositive RA), and M06 (other RA) though not including M06.1 (adult-onset Still disease). Only RA diagnoses registered as the main or the secondary diagnoses were included in the analyses, and referral diagnoses were not taken into account.
Verification of RA diagnoses
RA diagnoses identified in the Danish National Patient Registry were verified by scrutinizing medical records as follows: first-time RA diagnosis registrations were retrieved through the Danish National Patient Registry as described previously; then, relevant medical records from public hospitals and out-patient clinics were retrieved, and RA diagnoses were verified against the American College of Rheumatology (ACR) RA classification 1958 criteria,Citation23 the ACR 1987 criteria,Citation24 or the ACR/European League Against Rheumatism (ACR/EULAR) 2010 criteria.Citation25
If no RA classification criteria were met, verification was undertaken against clinical case RA. Clinical case RA was defined as arthritis where an assessment of RA was based on a rheumatologist’s expert opinion. If the RA diagnosis could not be confirmed, the likely alternative diagnosis was registered.
Register-based RA diagnoses with insufficient medical record information regarding satisfaction of the classification criteria or clinical case RA definition were categorized as nonverified RA diagnoses.
Validation of registry-based RA diagnoses according to serological subtypes
Identification of RA diagnoses according to serological subtypes
Considering that only the ICD-10 diagnosis codes distinguish between serological subtypes of RA,Citation8 verification of serological subtypes was exclusively carried out among first-time ICD-10 code registrations.
Diagnoses registered with one of the “M05” codes as the first registration in the Danish National Patient Registry were defined as registry-based seropositive RA, and diagnoses registered with one of “M06” codes were registered as other RA.
Only RA diagnoses registered as the main or the secondary diagnoses were included in the analyses, and referral diagnoses were not taken into account.
Patients with a previous ICD-8 RA code registration were not included in the analysis.
Verification of RA diagnoses according to serological subtypes
Register-based RA diagnoses were verified against the RA classification criteria or clinical case definition by reviewing medical records, as described in the previous section. The criterion standard of RA seropositivity was defined as a positive blood test for IgM rheumatoid factor (RF) and/or anti-citrullinated protein antibody (ACPA) before, at or within 1 month after the first-time registration in the Danish National Patient Registry. The criterion standard of other RA was defined as a negative blood test for IgM RF and, if an ACPA test had been undertaken, a negative blood test for ACPA before, at or within 1 month after the first-time registration in the Danish National Patient Registry.
Register-based RA diagnoses whose medical record information was insufficient in relation to satisfying the classification criteria, clinical case RA definition, or IgM RF/ACPA blood test results were categorized as nonverified RA diagnoses.
Statistical analyses
Verification of the registry-based RA diagnoses
Every first-time RA diagnosis in the Danish National Patient Registry was classified as a true positive RA diagnosis or a nonverified RA diagnosis using the RA classification criteria or clinical case definition based on medical records, as described earlier.
The PPV of registry-based RA diagnosis was estimated for two different models:
Model 1: first-time RA diagnosis registration ever in the Danish National Patient Registry;
Model 2: first-time RA diagnosis registration ever in the Danish National Patient Registry and where subsequently, at least once, a prescription had been redeemed for a synthetic disease-modifying antirheumatic drug (sDMARD). Information about prescribed drugs was available only from 1995. Therefore, subanalyses for RA diagnoses registered in the Danish National Patient Registry before and after 1995 were carried out using the second model. The following Anatomical Therapeutic Chemical (ATC) codesCitation26 from the Danish National Prescription Registry were retrieved: methotrexate (ATC: L01BA01, L04AX03), sulfasalazine (ATC: A07EC01), azathioprine (ATC: L04AX01), hydroxychloroquine (ATC: P01BA02), and leflunomide (ATC: L04AA13).
In Model 1, the PPV was determined as the fraction of true positive RA diagnoses among all first-time RA diagnoses recorded in the registry. In Model 2, the PPV was determined as the fraction of true positive RA among all first-time RA diagnoses recorded in the registry for cases, where subsequently at least once a prescription had been redeemed for a sDMARD. PPVs were presented with 95% CIs.
The PPV of RA diagnoses was subsequently explored according to patients’ gender, age group at the time of diagnosis (<50, 50–59, 60–69, >70 years), type of department (rheumatology, internal medicine, or other), type of patient (inpatient, outpatient, or other), type of RA diagnosis (main or secondary), calendar periods, based on the development of RA classification criteria (1977–1987, 1988–2010, 2011–2016),Citation23–Citation25 and ICD codes.Citation8
Verification of the registry-based RA diagnoses according to serological subtypes
Every first-time ICD-10 “M05” RA diagnosis in the Danish National Patient Registry was classified as a true positive seropositive RA or nonverified seropositive RA using the classification criteria or clinical case definition for RA and blood test results, as described earlier.
In the same manner, every first-time ICD-10 “M06” RA diagnosis in the registry was classified as a true positive other RA or nonverified other RA.
The PPVs of registry-based RA diagnoses, including serological subtypes, were estimated for two different models:
Model 1: first-time RA diagnosis registration ever in the Danish National Patient Registry with one of the ICD-10 RA codes;
Model 2: first-time RA diagnosis registration ever in the Danish National Patient Registry with one of ICD-10 RA codes and where subsequently, at least once, a prescription had been redeemed for a sDMARD.
For seropositive RA in Model 1, the PPV was calculated as the fraction of true positive seropositive RA diagnoses among all first-time seropositive RA diagnoses (ICD-10 “M05”) recorded in the Danish National Patient Registry. In Model 2, the PPV was ascertained as the fraction of true positive seropositive RA among all first-time seropositive RA diagnoses recorded in the registry and where subsequently, at least once, a prescription had been redeemed for a sDMARD.
The PPV of registry-based other RA diagnosis in Model 1 was ascertained as the fraction of true positive other RA diagnoses among all first-time other RA diagnoses (ICD-10 “M06”) recorded in the Danish National Patient Registry. In Model 2, the PPV was ascertained as the fraction of true positive other RA among all first-time other RA diagnoses recorded in the registry and, where subsequently, at least once, a prescription had been redeemed for a sDMARD.
Analyses were performed using Stata, version 14.2 (StataCorp LP, College Station, TX, USA).
Results
A total of 331 participants in the Danish Diet, Cancer, and Health cohort were recorded as having RA at one of the Central Denmark Region hospitals in the period 1977–2016.
The characteristics of the registry-based RA diagnoses are presented in . As expected, the majority of persons recorded as having RA were women (70%). Most of the first-time RA diagnoses were recorded at a rheumatology or internal medicine department (78%) as the main diagnosis.
Table 1 Characteristics of first-time rheumatoid arthritis diagnoses recorded in the Danish National Patient Registry among participants in the Danish Diet, Cancer, and Health cohort and derived from the Central Denmark Region hospitals
Verification of first-time RA diagnoses
For a total of 7% (23/331) of the diagnoses, no sufficient medical record information was available, and these diagnoses were therefore defined as nonverified RA.
shows the PPVs of the first-time RA diagnoses registered in the Danish National Patient Registry. Using the Model 1 approach, RA diagnoses were confirmed for 62% (205/331) of the registry-based diagnoses (95% CI: 56.6–67.0). The majority of the confirmed diagnoses satisfied ACR or ACR/EULAR classification criteria (n=202), while others (n=3) met only the clinical case RA definition. ICD-8 coded diagnoses had the highest PPV (PPV: 78%; 95% CI: 61.5–89.2). Furthermore, a large proportion of the diagnoses was verified in the medical records if they were registered at a rheumatology department (PPV: 72%; 95% CI: 65.0–77.8), recorded as a main diagnosis (PPV: 68%; 95% CI: 62.1–73.5) or as a seropositive RA (PPV: 72%; 95% CI: 64.2–78.7).
Table 2 The positive predictive values of first-time overall rheumatoid arthritis diagnoses recorded in the Danish National Patient Registry in the period 1977–2016 among the participants in the Danish Diet, Cancer, and Health cohort and derived from the Central Denmark Region hospitals
When registry-based diagnosis codes were linked to the RA treatment codes in the Danish National Prescription Registry (Model 2), the PPV of RA diagnosis was substantially higher (). Overall, 88% of RA diagnoses were confirmed (95% CI: 82.5–91.5). The PPV was higher for all categories compared with the PPV calculated using Model 1. The results were not different when stratified by periods (ie, before and after 1994 [establishment of the Danish National Prescription Registry], the PPV was 90% and 87%, respectively).
Verification of first-time RA diagnoses according to serological subtypes
A total of 89% of the RA diagnoses (294/331) were registered with one of the ICD-10 RA codes, and these were almost equally distributed between “M05” (n=150) and “M06” (n=144) codes. Stratification by patient age at the diagnosis showed preponderance of seropositive RA diagnostic codes “M05” in younger patients (). Overall, for 8.7% (13/150) of the “M05” and 25% (36/144) of the “M06” diagnoses, no sufficient medical record information was available, including IgM RF and/or ACPA test results, and these cases were thus defined as nonverified RA.
shows the PPVs of first-time RA diagnoses according to serological status, registered in the Danish National Patient Registry with one of the ICD-10 RA codes. Using the Model 1 approach, 62% of registry-based, seropositive RA diagnoses were confirmed (95% CI: 53.9–69.5). The PPV of other RA diagnoses was 25% (95% CI: 18.5–32.8). Stratification by age group at the time of diagnosis registration revealed the highest PPVs in the youngest age group (<50 years) for both seropositive (PPV: 100%) and other (PPV: 50%) RA. However, there were only a few RA diagnosis registrations in both groups (). After linking the data to the RA treatment codes (Model 2), the PPV increased for both seropositive (PPV: 80%; 95% CI: 71.6–86.7) and other RA (PPV: 41%; 95% CI: 30.2–52.9) diagnoses. The highest PPV for seropositive RA diagnosis was in the youngest age group (PPV: 100%), while the highest PPV for other RA diagnosis was in the oldest age group (PPV: 60%; 95% CI: 40.9–76.5) ().
Table 3 Positive predictive values of first-time rheumatoid arthritis diagnoses according to serological subtypes registered in the Danish National Patient Registry in the period 1977–2016 among the participants in the Danish Diet, Cancer, and Health cohort and derived from the Central Denmark Region hospitals, overall and by age groups at the time of rheumatoid arthritis diagnosis
Calculating the PPVs by gender showed that the PPVs for seropositive RA diagnoses were slightly higher for women than for men (using Model 1 – 63.4% vs 59.2%; Model 2 – 81.6% vs 77.1%), whereas the PPVs for other RA diagnoses were higher for men than for women (using Model 1 – 28.2% vs 23.8%; Model 2 – 45.0% vs 39.6%) ().
Table 4 Positive predictive values of first-time rheumatoid arthritis diagnoses according to serological subtypes registered in the Danish National Patient Registry in the period 1977–2016 among the participants in the Danish Diet, Cancer, and Health cohort and derived from the Central Denmark Region hospitals by gender
shows the PPVs calculated stratifying by calendar periods. The PPVs for seropositive RA diagnoses were similar for both periods. Though, the PPVs for other RA diagnoses after 2010 were higher than in the period before (using Model 1 – 40.0% vs 18.2%; Model 2 – 73.9% vs 26.0%).
Table 5 Positive predictive values of first-time rheumatoid arthritis diagnoses according to serological subtypes registered in the Danish National Patient Registry in the period 1977–2016 among the participants in the Danish Diet, Cancer, and Health cohort and derived from the Central Denmark Region hospitals by calendar periods
and and show available IgM RF and ACPA blood test results and their distribution among confirmed RA diagnoses. In almost 70% of verified RA diagnoses, ACPA test results were not available at the time of RA diagnosis registration.
The most likely other diagnosis among nonverified RA diagnoses was osteoarthritis. shows the distribution of the most likely alternative diagnoses. Post hoc analyses were performed taking some of the most likely alternative diagnoses into account. The following additional criterion in determining RA cases was used: patients with their first RA diagnosis in the Danish National Patient Registry who had subsequently, at least once, redeemed a prescription for a sDMARD and who had no ICD code for psoriatic or enteropathic arthropathies (ICD-8: 696.0; ICD-10: M07), systemic connective tissue disorders (ICD-8: 446, 716, 734; ICD-10: M30–M35), inflammatory bowel diseases (ICD-8: 563; ICD-10: K50–K51), or sarcoidosis (ICD-8: 135.99; ICD-10: D86) in the previous or following years in the Danish National Patient Registry. Using this additional criterion, 96% of register-based overall RA diagnoses were confirmed (95% CI: 91.7–98.1); 85% of seropositive RA diagnoses (95% CI: 76.0–90.6) and 48% of other RA diagnoses (95% CI: 34.2–62.1) were confirmed.
Table 6 The most likely alternative diagnoses among patients, first-time registered with rheumatoid arthritis diagnosis in the Danish National Patient Registry during 1977–2016, but not confirmed by reviewing medical records
Discussion
We found that, based on the ACR, ACR/EULAR classification criteria, or clinical case definition, only 62% of registry-based first-time RA diagnoses could be verified. Verification of RA diagnoses according to serological status was as low as 62% for seropositive RA and only 25% for other RA.
These findings are in line with those reported in previous Danish studies. Pedersen et alCitation9 also validated RA diagnoses in the Danish National Patient Registry, using data from two large population-based cohorts. They identified 217 RA diagnoses with available medical records registered at least once in the Danish National Patient Registry during 1977–2001. A total of 59% of RA diagnoses were verified using the clinical case RA definition as a reference, and only 46% of RA diagnoses were verified using the ACR 1987 classification criteria as a reference. Another Danish study validated first-time RA diagnoses recorded in the Danish National Patient Registry during 2011.Citation10 A total of 1,468 RA diagnoses were identified at a rheumatology department with one of the ICD-10 codes M05.9, M06.0, M06.8, or M06.9, and having an additional visit due to RA within 90 days. They verified 79% register-based RA diagnoses, which is in line with our findings of 72% verified RA diagnoses registered at a rheumatology department.
We included all register-based RA diagnoses in the analysis, even if medical records or IgM RF and ACPA blood test results were unavailable or inaccessible. Therefore, we estimated the lowest possible PPVs within the study population.
Our findings of relatively low overall PPVs may have several explanations. First of all, referral RA diagnoses may be carried forward as tentative diagnoses until conclusive determination of diagnoses can be made. Second, physicians are expected to record as many relevant diagnoses as possible during daily clinical practice; however, no routine validation takes place, and self-reported arthritis may be registered as RA. We found that the most likely alternative diagnoses were osteoarthritis, polymyalgia rheumatica, crystal arthropathy, psoriatic arthritis, and connective tissue diseases, a finding that supports these misclassifications pitfalls. Finally, the registry may contain self-reported RA as a secondary diagnosis if patients were treated in private rheumatology clinics, and these are not captured by the Danish National Patient Registry. Private practice medical records were not accessible for this study. It is, however, unlikely that this would have had a substantial impact on the overall results since the number of diagnoses with no sufficient medical records was low (7%).
In our study, register-based RA diagnoses were almost equally distributed among seropositive and other RA. Several inception cohorts report that majority of RA patients are seropositive.Citation27–Citation29 The discrepancy may have several explanations. Our study showed that register-based other RA diagnoses are misclassified more frequently than seropositive RA diagnoses. Moreover, in our study, both register-based and confirmed other RA diagnoses were observed more frequently among older patients (>60 years) than younger patients (<60 years). Thus, the mean age at the time of RA diagnosis in our study was in favor of other RA. Furthermore, incident RA cohorts may not precisely reflect the real RA population due to their inclusion and exclusion criteria. Lower PPVs were estimated for the other RA codes than the seropositive RA codes. Given that RA is a slowly progressing disease with clinical similarities to other joint diseases, a tendency to record other RA rather than seropositive RA may appear if the RA diagnosis is uncertain. This misclassification may also be explained by physicians’ lack of awareness when selecting “M05” or “M06” RA codes in clinical practice.
The mean age at the time of RA diagnosis in our data was 65 years, which is higher than expected for the disease onset as indicated by inception cohorts.Citation27,Citation28 This may partly be attributed to the eligibility criteria for the Danish Diet, Cancer, and Health cohort.Citation19 Furthermore, reduced functional status due to long-standing RA and concurrent comorbid conditions may have prevented potential attendees from participating in the Danish Diet, Cancer, and Health cohort.
We have observed that PPVs of other RA diagnoses registered during 2011–2016 were substantially higher than for diagnoses registered before 2011. It could partly be explained by availability of medical records, including IgM RF/ACPA test results.
PPVs increased substantially for both overall RA diagnoses and serological subtypes when diagnoses were linked to RA treatment codes. This is in line with the findings of an American study of RA diagnosis validation.Citation11 In that study, the PPV also increased substantially (from 66% to 81%) when the data were linked to RA treatment codes.
Our study has some limitations that merit discussion. First of all, we included RA diagnoses derived from only the Central Denmark Region hospitals, and therefore approximately 22% of the Danish population.Citation22 Although the Danish health care system is uniformly organized throughout all regions, including the diagnosis registration procedure, regional differences may appear, and hospitals may vary in their registration practices, with higher PPVs anticipated for more accurate registrations.
Furthermore, the density of private rheumatology clinics differs between the regions,Citation30 and the PPVs would be expected to be lower in regions with a higher density of private practice rheumatologists due to inaccessibility to medical records. Still, our overall findings are in line with those reported by two previous Danish studies,Citation9,Citation10 suggesting that such organizational differences had only a limited impact on the overall findings. It must, moreover, be taken into account that the structure of administrative registries differs between countries, and therefore our findings are not more broadly generalizable.
Unfortunately, we were unable to assess sensitivity, specificity, or negative predictive values due to the inaccessibility of medical records at private clinics. Furthermore, some RA patients may not seek medical advice, and thereby not be captured by any medical record or registry. These measures could have led to more precise assessment of the diagnostic accuracy of registry-based RA diagnoses.
Conclusion
Our findings show that data for first-time RA diagnoses in the Danish National Patient Registry must be used with caution due to misclassification. However, linking registry-based RA diagnoses to the subsequent RA treatment codes increases the probability of identifying true RA diagnoses. The relatively high PPV of RA diagnosis registered with one of ICD-10 “M05” codes indicates a reliable means of coding seropositivity.
Author contributions
KO, KSP, AT, and AL contributed to the conception and design of the study. KO and AL were involved in the acquisition of data. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank the Danish Cancer Society for the funding of the Danish Diet, Cancer, and Health cohort study; the Steering Committee of the cohort study, for providing the opportunity to conduct the present study; programmer Katja Boll, for preparation of the dataset; and the participants of the cohort study. The present study was funded by the Danish Rheumatism Association, the Danish Heart Foundation, North Denmark Regional Hospital, Central Denmark Region, and Scandinavian Rheumatology Research Foundation, Grant 2014. The funders were not involved in the study design, data collection, analysis, interpretation of the results, manuscript writing, or decision to submit the paper for publication.
Supplementary materials
Figure S1 Serological status among confirmed RA diagnoses with available blood test results on IgM RF and/or ACPA before, at or within 1 month after the first-time registration in the Danish National Patient Registry in the period 1977–2016 among the participants in the Danish Diet, Cancer, and Health cohort and derived from the Central Denmark Region hospitals.
Notes: aRegistered with the ICD-8 code “712.39” (Arthritis rheumatoides alia et non specificata) at their first registration in the Danish National Patient Registry. bRegistered with one of ICD-10 “M05” (seropositive RA) codes at their first registration in the Danish National Patient Registry. cRegistered with one of ICD-10 “M06” (other RA) codes at their first registration in the Danish National Patient Registry.
Abbreviations: ACPA, anti-citrullinated protein antibody; ICD, International Classification of Diseases; IgM RF, immunoglobulin M rheumatoid factor; RA, rheumatoid arthritis.
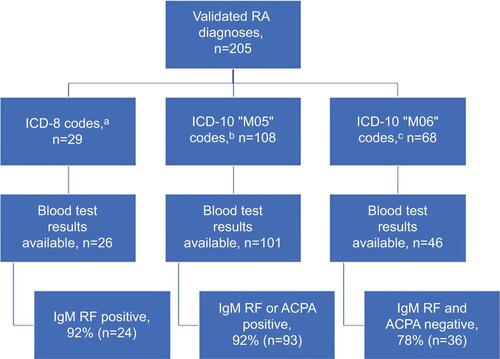
Table S1 The blood test results of IgM RF and ACPA among confirmed ICD-10 M05 (seropositive) RA diagnoses in the Danish National Patient Registry in the period 1977–2016 among the participants in the Danish Diet, Cancer, and Health cohort and derived from the Central Denmark Region hospitals
Table S2 The blood test results of IgM RF and ACPA among confirmed ICD-10 M06 (other) RA diagnoses in the Danish National Patient Registry in the period 1977–2016 among the participants in the Danish Diet, Cancer, and Health cohort and derived from the Central Denmark Region hospitals
Disclosure
The authors report no conflicts of interest in this work.
References
- DougadosMSoubrierMAntunezAPrevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA)Ann Rheum Dis2014731626824095940
- MyasoedovaEDavisJMCrowsonCSGabrielSEEpidemiology of rheumatoid arthritis: rheumatoid arthritis and mortalityCurr Rheumatol Rep201012537938520645137
- NeoviusMSimardJFAsklingJARTIS Study GroupNationwide prevalence of rheumatoid arthritis and penetration of disease-modifying drugs in SwedenAnn Rheum Dis201170462462921149495
- LindhardsenJAhlehoffOGislasonGHThe risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort studyAnn Rheum Dis201170692993421389043
- LindhardsenJAhlehoffOGislasonGHRisk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort studyBMJ2012344e125722403267
- HyldgaardCHilbergOPedersenABA population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortalityAnn Rheum Dis201776101700170628611082
- LyngeESandegaardJLReboljMThe Danish National Patient RegisterScand J Public Health2011397 Suppl303321775347
- World Health OrganizationClassificationsGenevaWorld Health Organization Available from: http://www.who.int/classifications/icd/en/Accessed October 15, 2018
- PedersenMKlarlundMJacobsenSSvendsenAJFrischMValidity of rheumatoid arthritis diagnoses in the Danish National Patient RegistryEur J Epidemiol200419121097110315678789
- IbfeltEHSørensenJJensenDVValidity and completeness of rheumatoid arthritis diagnoses in the nationwide DANBIO clinical register and the Danish National Patient RegistryClin Epidemiol20179962763229238225
- SinghJAHolmgrenARNoorbaloochiSAccuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritisArthritis Rheum200451695295715593102
- WeyandCMMcCarthyTGGoronzyJJCorrelation between disease phenotype and genetic heterogeneity in rheumatoid arthritisJ Clin Invest1995955212021267738179
- ChibnikLBKeenanBTCuiJGenetic risk score predicting risk of rheumatoid arthritis phenotypes and age of symptom onsetPLoS One201169e243802438721931699
- LahiriMMorganCSymmonsDPBruceINModifiable risk factors for RA: prevention, better than cure?Rheumatology201251349951222120459
- AjeganovaSHuizingaTWRheumatoid arthritis: seronegative and seropositive RA: alike but different?Nat Rev Rheumatol20151118925403158
- PedersenMJacobsenSKlarlundMEnvironmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptidesArthritis Res Ther200684R13316872514
- WesleyABengtssonCElkanACAssociation between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: results from a population-based case-control studyArthritis Care Res2013651107112
- LahiriMLubenRNMorganCUsing lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register – the EPIC-2-NOAR Study)Ann Rheum Dis201473121922623505230
- TjønnelandAOlsenABollKStudy design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in DenmarkScand J Public Health200735443244117786808
- GjerstorffMLThe Danish Cancer RegistryScand J Public Health2011397 Suppl424521775350
- KildemoesHWSørensenHTHallasJThe Danish National Prescription RegistryScand J Public Health2011397 Suppl384121775349
- Danske RegionerRegional DenmarkKøbenhavnDanske Regioner Available from: http://www.regioner.dk/services/in-english/regional-denmarkAccessed June 18, 2018
- RopesMWBennettGACobbSJacoxRJessarRA1958 Revision of diagnostic criteria for rheumatoid arthritisBull Rheum Dis19589417517613596783
- ArnettFCEdworthySMBlochDAThe American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritisArthritis Rheum19883133153243358796
- AletahaDNeogiTSilmanAJ2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiativeArthritis Rheum20106292569258120872595
- WHO Collaborating Centre for Drug Statistics MethodologyATC. Structure and PrinciplesOslo, NorwayWHO Collaborating Centre for Drug Statistics Methodology Available from: https://www.whocc.no/atc/structure_and_principles/Accessed June 18, 2018
- HetlandMLStengaard-PedersenKJunkerPCombination treatment with methotrexate, cyclosporine, and intraarticular betamethasone compared with methotrexate and intraarticular betamethasone in early active rheumatoid arthritis: an investigator-initiated, multicenter, randomized, double-blind, parallel-group, placebo-controlled studyArthritis Rheum20065451401140916645967
- Hørslev-PetersenKHetlandMLJunkerPAdalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcino-lone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trialAnn Rheum Dis201473465466123434570
- SandbergMECBengtssonCKällbergHOverweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritisAnn Rheum Dis201473112029203324818635
- sundhed.dkKøbenhavnDenmarksundhed.dk Available from: https://www.sundhed.dk/borger/guides/find-behandler/?Informationskategori=Speciallæge&InformationsUnderkategori=Reumatolog(gigtlæge)AccessedJune 18, 2018
