Abstract
Background
Infants with atopic eczema have an increased risk of impaired growth, but the origin of this impairment is unclear. The aim of this study was to examine fetal and infant growth in relation to infantile atopic eczema.
Methods
Within the UK Southampton Women’s Survey, 1,759 infants with known maternal menstrual data had anthropometric measurements at 11, 19, and 34 weeks’ gestation, birth, and ages 6 and 12 months, enabling derivation of growth velocity SD scores. Infantile atopic eczema at ages 6 and/or 12 months was ascertained using modified UK Working Party diagnostic criteria.
Results
Expressed per SD increase, higher femur length and abdominal circumference at 34 weeks’ gestation were associated with decreased risks of atopic eczema (eczema OR/SD increase 0.81 [95% CI 0.69–0.96], P=0.017 and 0.78 [95% CI 0.65–0.93], P=0.006, respectively), while every SD increase in head to abdominal circumference ratio (indicating disproportionate growth) was associated with an increase in risk of atopic eczema (1.37 [1.15–1.63], P=0.001). Lower velocities of linear growth from 11 weeks’ gestation to birth and birth to age 6 months were associated with atopic eczema (atopic eczema OR/SD increase 0.80 [0.65–0.98], P=0.034 and 0.8 [1 0.66–1.00], P=0.051, respectively). Infants with atopic eczema at age 12 months had a larger head circumference in early gestation and faltering of abdominal growth velocity from 19 to 34 weeks’ gestation (atopic eczema OR/SD increase 0.67 [0.51–0.88], P=0.003).
Conclusion
Infants with atopic eczema demonstrate altered patterns of fetal growth, including faltering of linear growth in utero, prior to the clinical onset of atopic eczema. These findings suggest growth falters prior to the start of clinical atopic eczema and its treatment.
Keywords:
Introduction
Linear growth impairment in children with atopic eczema is a clinical concern.Citation1–Citation3 National recommendations in USA,Citation4 UK,Citation5,Citation6 and other settings are that growth is monitored as part of clinical care for children with atopic eczema. Possible reasons for growth faltering have been proposed and include effects of the inflammatory disease,Citation7 corticosteroid treatment,Citation8 poor nutrition as a result of an inappropriately restrictive diet,Citation9 and eczema associated sleep disturbance.Citation10 Hitherto, little attention has been paid to the possibility of premorbid changes in growth trajectory in infants with atopic eczema. Any such changes in premorbid growth may help explain the growth impairment in children with atopic eczema while also providing insights into etiology of the skin disorder. The development of inflammatory diseases such as atopic eczema is influenced by both genetic determinants and environmental exposures in early life, including poor nutrition,Citation11,Citation12 maternal stress,Citation13,Citation14 smoking,Citation15 and microbiome-related exposures.Citation16 With increasing evidence that atopic disease is partly determined by the fetal environment, better understanding of early life environment becomes crucial for identifying potential preventative strategies.
Studies examining infant birthweight in relation to atopic eczema have been inconsistent,Citation17,Citation18 and it is now recognized that infant birthweight is only a crude proxy for patterns of fetal growth, which may show stronger associations with later outcomes than cross-sectional assessments of size at single time points.Citation19 A previous study reported that fetuses with below average crown-rump length at 11 weeks gestation and an above average biparietal diameter at 19 weeks’ gestation were more likely to have eczema ascertained by postal questionnaire at age 10 years,Citation20 but no previous study has examined longitudinal measures of fetal and infant size in relation to infantile atopic eczema. More information is available for other atopic outcomes, including evidence that a large neonatal head circumference and higher abdominal circumference growth velocity between 11 and 19 weeks’ gestation have been linked to an increased risk of atopy.Citation19
In this study, we examined fetal and infant anthropometric measurements and growth velocities in relation to the risk of atopic eczema at ages 6 and 12 months, to look for evidence of altered growth prior to the clinical onset of atopic eczema which might support a prenatal developmental influence on the disorder.
Methods
Southampton Women’s Survey (SWS)
In the UK SWS, between 1998 and 2002, 12,583 women aged 20–34 years who were not pregnant were recruited from the general population through general practitioners in Southampton, UK. Information on maternal diet, lifestyle, socioeconomic status, and body composition was collected.Citation21 Women who became pregnant were followed up through their pregnancies; ultrasound measurements of fetal size were performed at 11, 19, and 34 weeks. A total of 3,158 live-born singleton infants were delivered. Further anthropometry was performed at birth and at ages 6 and 12 months. The findings reported here are based on 1,759 term, live singleton births with no congenital abnormalities, who were assessed for atopic eczema at 6 and/or 12 months and had fetal and infant anthropometric measurements and known maternal menstrual data. Neonatal deaths and infants with major congenital anomalies, gestational age <37 weeks, and missing atopic eczema data at 6 and/or 12 months were excluded from the analyses (). All phases of the SWS were approved by the Southampton and South West Hampshire Local Research Ethics Committee, and parents gave written informed consent.
Outcome assessment
Case definition of atopic eczema was based on the UK Working Party diagnostic criteria for the definition of atopic eczema,Citation22 using information collected by trained research nurses who administered a standard questionnaire and ascertained other information required for the diagnostic criteria (a combination of history of itchy skin condition and two or more of the following: history of involvement of the skin creases such as folds of elbows, behind the knees, fronts of ankles, cheeks, or around the neck; a history of a general dry skin in the last year; and visible flexural eczema or eczema involving the cheeks/forehead and outer limbs). All infants were assessed for atopic eczema before the age of 2 years, thus this UK Working Party criterion was met by all infants in the study cohort. However, as the infants were not old enough to have developed clearly defined atopic disorders, a personal history of atopy was omitted as a criterion.
Fetal and infant anthropometric measurements
At 11, 19, and 34 weeks’ gestation, women underwent high-resolution ultrasound scanning by experienced research staff using Kretz Voluson® 730 (GE Kretz Ultrasound, Tiefenbach, Austria) or Acuson Sequoia® 512 (Siemens, Erlangen, Germany) systems, which was cross-calibrated. Measurements of fetal linear size (crown-rump length at 11 weeks, femur length at 19 and 34 weeks), head circumference (at 11, 19, and 34 weeks), and abdominal circumference (at 11, 19, and 34 weeks) were made according to an internationally accepted and validated methodology.Citation23 Postnatal anthropometry was performed by trained research nurses according to standardized procedures, with each measurement repeated three times and the mean value used for analysis. Crown-heel length at birth was measured using a neonatometer (Harpenden, Wrexham, UK) and at ages 6 and 12 months using an infantometer (Seca Ltd, Birmingham, UK); head and abdominal circumferences were measured using unmarked tapes read off against a metal ruler at birth, 6, and 12 months.
Statistical analyses
Summary statistics are presented as mean (SD) or median (IQR) for continuous variables, and percentages for categorical variables.
As measurements were taken close to but not at the exact ages specified, the associations between anthropometric measures and age were modeled using Cole’s LMSCitation24 in LMSchartmaker,Citation25 to create sex-specific size-for-age z-scores. This method provides smooth centile curves to potentially skewed data, enabling calculation of z-scores at exact ages. The method summarizes the changing distribution of the anthropometric parameters by age using three curves representing the skewness (L), the median (M), and the coefficient variation (S). These parameters are all used together to create the preferred percentiles. LMS has been adopted in many studies and is considered a reliable method of smoothing growth curves.Citation26–Citation29
Conditional models of change were built using linear regression analysis: thus size z-score at 11 weeks was the starting point. Conditional change in z-score from 11 to 19 weeks was defined as the standardized residuals resulting from the linear regression model of z-score at 19 weeks on z-score at 11 weeks. The conditional change in z-score from 19 to 34 weeks was obtained from the standardized residuals resulting from regressing z-score at 34 weeks on both z-score at 19 weeks and z-score at 11 weeks simultaneously. This process was continued for each subsequent time point, resulting in independent measures of conditional growth. Using the measures of linear size at 11 weeks, birth, and 6 months, additional conditional growth measures using this subset of time points were created: linear size at 11 weeks, linear growth from 11 weeks to birth, from birth to 6 months, and from 6 to 12 months. Measures of conditional growth were mutually uncorrelated and yielded SD scores, enabling comparison of relationships between growth in different time intervals and risk of atopic eczema at ages 6 and 12 months.
Potential confounding variables were determined prior to the analysis using a directed acyclic graph (DAG) (). DAGs provide a robust and objective means of selecting confounders. DAGs are a graphical representation of causal effects between variables. Based on prior knowledge and usual convention for the causal effects studied, these graphs identify potential confounding variables which are then adjusted for multivariate analysis to minimize confounding bias.Citation30 Additionally, the graphs identify competing exposures that can be adjusted for to improve the precision of the model. The resulting factors that were included as confounding variables in our analyses were maternal body mass index (BMI) at initial assessment, educational attainment (maternal age and parity were also considered, but the DAG indicated their inclusion was not appropriate), infant gestational age, and breastfeeding duration. Competing exposures identified and adjusted for were maternal eczema in the 12 months prior to the initial assessment, maternal smoking, and infant sex. P<0.05 was considered statistically significant. Logistic regression analyses were performed (Stata version 14.1; StataCorp LP, College Station, TX, USA) to relate fetal and infant anthropometric measures (in SD) and growth velocities (in SD) to infant atopic eczema at ages 6 and 12 months, with results presented as atopic eczema OR per SD increase (OR/SD).
Results
Cohort characteristics
summarizes maternal, fetal, and infant characteristics. Among the study group, the mothers’ mean (SD) age at their child’s birth was 31.0 (3.7) years, 52.2% were primiparous, 11.7% smoked during pregnancy, 6.7% of mothers had eczema in the past 12 months, 51.1% of infants were male, mean (SD) infant birthweight was 3.52 (0.47) kg, and median gestational age at birth was 40.1 weeks (IQR 39.3–41.0). A total of 1,698 infants were assessed for atopic eczema at age 6 months, 9.5% of them had atopic eczema. At age 12 months, 1,684 infants were assessed and 10.0% had atopic eczema. shows the characteristics of the 1,759 participants in the study group in comparison with the overall SWS pregnancy cohort; the study group mothers were slightly older at child’s birth, higher proportions were primiparous or had attained A level or higher education, and smoking was less prevalent.
Table 1 Characteristics of the study population
Univariate (unadjusted) and multivariate (adjusted) analyses of atopic eczema at ages 6 and 12 months in relation to fetal and infant size measurements are shown in . and show the ORs of atopic eczema in relation to fetal and infant size measurements at ages 6 and 12 months, respectively. Postnatal anthropometry showed that infants with atopic eczema at 6 months were shorter at age 6 months (eczema OR/SD increase 0.78, 95% CI 0.65–0.93, P=0.006) and that those with atopic eczema at 12 months were shorter at ages 6 and 12 months (eczema OR/SD increase 0.81, 95% CI 0.67–0.97, P=0.021 and OR 0.82, 95% CI 0.69–0.98, P=0.028, respectively).
Figure 1 Size measurements in relation to atopic eczema at age 6 months.
Notes: (A) Linear size, (B) head circumference, (C) abdominal circumference, and (D) head:abdominal circumference ratio. *Controlling for gestation, sex, breastfeeding, maternal BMI, qualification, maternal eczema, and smoking in pregnancy.
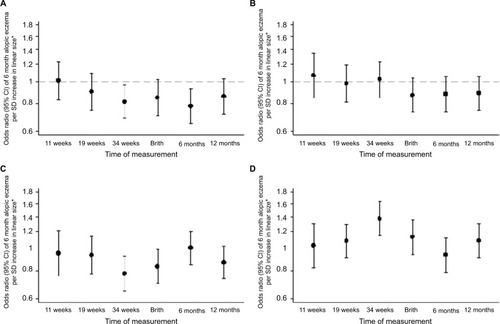
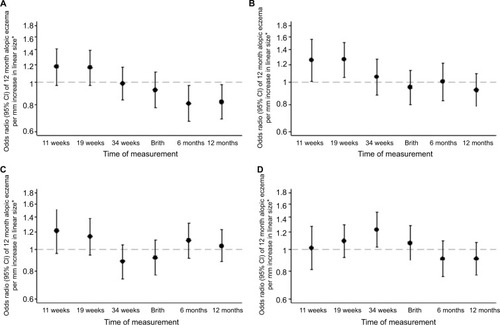
Figure 2 Size measurements in relation to atopic eczema at age 12 months.
Note: (A) Linear size, (B) head circumference, (C) abdominal circumference, and (D) head:abdominal circumference ratio. *Controlling for gestation, sex, breastfeeding, maternal BMI, qualification, maternal eczema, and smoking in pregnancy.
Associations of fetal size and growth velocities with infant atopic eczema at age 6 months
At 34 weeks’ gestation, a shorter femur length, smaller abdominal circumference, and higher head to abdominal circumference ratio were associated with increased risks of atopic eczema at age 6 months. Expressed per SD increase, higher femur length and abdominal circumference were associated with decreased risks of atopic eczema (eczema OR/SD increase 0.81, 95% CI 0.69–0.96, P=0.017 and 0.78, 95% CI 0.65–0.93, P=0.006, respectively), while every SD increase in head to abdominal circumference ratio (indicating disproportionate growth) was associated with an increase in risk of atopic eczema (1.37, 95% CI 1.15–1.63, P=0.001) (). Fetal head circumference was not related to infant atopic eczema at age 6 months ().
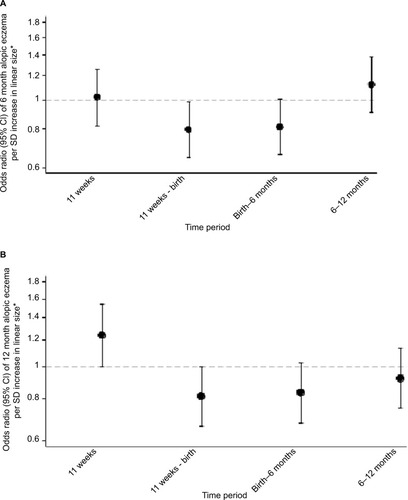
A lower velocity of linear growth from 11 weeks’ gestation to birth was associated with atopic eczema at age 6 months (atopic eczema OR/SD increase 0.80, 95% CI 0.65–0.98, P=0.034) (); this particularly reflected lower linear growth velocity from 11 to 19 weeks’ gestation (). Lower velocity of linear growth from birth to 6 months also showed a trend toward significance (atopic eczema OR/SD increase 0.81, 95% CI 0.66–1.00, P=0.051). A lower abdominal circumference growth velocity from 19 to 34 weeks’ gestation was associated with an increased risk of atopic eczema at age 6 months (eczema OR/SD increase 0.71, 95% CI 0.55–0.92, P=0.009), but there were no associations with head circumference growth velocities ().
Figure 3 Linear growth velocities in relation to atopic eczema at ages 6 and 12 months.
Abbreviations: CRL, crown-rump length; FL, femur length; CHL, crown-heel length.
Associations of fetal size and growth velocities with infant atopic eczema at age 12 months
Infants with atopic eczema at age 12 months had a larger head circumference in early pregnancy (at 11 and 19 weeks’ gestation eczema OR/SD increase 1.25, 95% CI 1.00–1.56, P=0.045 and 1.26, 95% CI 1.05–1.51, P=0.012, respectively), faltering of abdominal circumference growth velocity from 19 to 34 weeks’ gestation (eczema OR/SD increase 0.67 [0.51–0.88], P=0.003) and a higher head to abdominal circumference ratio at 34 weeks (eczema OR/SD increase 1.22, 95% CI 1.03–1.46, P=0.025), with trends toward faltering of linear growth velocity from 11 weeks to birth and birth to age 6 months (eczema OR/SD increase 0.81 [0.66–1.00], P=0.051 and 0.83 [0.68–1.03], P=0.087, respectively) ( and ).
Discussion
We found that infants with atopic eczema at age 6 months have faltering of linear growth beginning after 11 weeks’ gestation, with a higher head to abdominal circumference ratio at 34 weeks’ gestation. Infants with atopic eczema at age 12 months had a larger head circumference in early pregnancy and faltering of abdominal growth in the second half of pregnancy. Postnatally, the infants with atopic eczema at ages 6 and 12 months were shorter than infants without atopic eczema, but the longitudinal measurements of fetal size suggest that this growth faltering commenced prior to birth. These associations were robust to adjustment for potentially confounding variables, notably maternal age, BMI, education, smoking in pregnancy, and eczema in the 12 months prior to the initial assessment at preconception and infant sex, and duration of breastfeeding.
Our study presents the first longitudinal data examining fetal and infant growth velocities to infant atopic eczema at ages 6 and 12 months. Previous studies have generally focused on anthropometric measurements at birth or during infancy, and have found that they were not related to the prevalences of reported eczema or hay fever by the age of 13 years,Citation31 or to eczema at age 7 years.Citation32 The same infant size at birth can be achieved through different patterns of fetal growth, and few previous studies have examined patterns of fetal growth in relation to atopic outcomes. An increase in size between first trimester crown-rump length and second trimester biparietal diameter has been associated with higher risks of eczema and asthma at age 10 years.Citation20 Pike et alCitation19 reported that rapid early gestation fetal abdominal growth followed by late gestation faltering of abdominal circumference growth was associated with later atopy at age 3 years, and late gestation abdominal growth faltering with atopic wheeze.
Mechanistically, it is known that intrauterine growth restriction leads to disproportionate fetal growth and a high head to abdominal circumference ratio as a result of “brain sparing” responses, which direct nutrient-rich blood to maintain brain growth away from truncal organs including the thymus, with potential impact on immune development. Animal and human studies have linked fetal and birth anthropometric parameters indicative of undernutrition during pregnancy with smaller thymic size and impaired thymic development.Citation12,Citation33,Citation34 We found that a larger fetal head circumference at 11 and 19 weeks’ gestation was linked with a higher risk of atopic eczema, with evidence of disproportionate head to abdominal circumference at 34 weeks. Therefore, we suspect that these growth alterations might influence thymus development, resulting in a diminished population of T helper (Th) 1 lymphocytes, favoring Th2 populations and consequently raised serum IgE,Citation35 an immune reaction that is seen in atopic eczema and other atopic conditions.Citation36 However, the exact mechanisms responsible for the association of altered fetal growth with development of atopic eczema are unknown, the observation points to involvement of periconception or early pregnancy factors in the etiology of infantile atopic eczema. The patterns of association between anthropometric measurements and infant atopic eczema at ages 6 and 12 months differed; this could reflect a chance finding or heterogeneity in the etiology and pathogenesis of atopic eczema in early childhood.Citation37
Hitherto, it has been thought most likely that the chronic inflammatory process associated with atopic eczema results in growth impairment as proinflammatory cytokines such as IL-6, which promotes Th2 differentiation and simultaneously inhibits Th1, can act at the level of the growth plate, or may alter the growth hormone insulin-like growth factor 1 (IGF-1) axis.Citation7 Animal studies show growth impairment in juvenile chronic arthritis and chronic inflammatory bowel disease independent of nutrition as a result of an IL-6-mediated decrease in IGF-1.Citation38,Citation39 Alternatively, it has been proposed that infants with atopic eczema may be susceptible to postnatal growth impairment due to treatment of the condition with topical or systemic corticosteroids,Citation8 poor nutrition as a result of an inappropriately restrictive diet,Citation9 and associated disturbance in sleep.Citation10 However, our findings suggest that intrinsic and intrauterine factors that influence growth may modify the risk of developing atopic eczema as opposed to growth faltering developing postnatally as a result of the inflammatory skin condition or its treatment. These findings have important clinical implications and suggest that improved control of the inflammatory process in infantile atopic eczema or avoidance of topical corticosteroids may not necessarily resolve the growth impairment seen in many infants with atopic eczema.
Strengths of this study are its large sample size, its prospective nature, and the standardized assessment of fetal/infant size and atopic eczema by trained staff. Only a sub-sample of the SWS cohort was studied as calculation of fetal growth velocities in early gestation requires secure menstrual information; otherwise the estimated date of conception has to be “set” from a measurement of fetal size in the first half of pregnancy, thereby negating the scope for examination of early gestation effects. The mother–offspring pairs included in the study were therefore those with known menstrual data information and consistent ultrasound data, allowing for accurate size-for-age fetal and infant measurements and calculation of growth velocities. Mothers in the study group were slightly older at child’s birth, higher proportions were primiparous or had attained A-level or higher education, and smoking was less prevalent when compared with the overall SWS pregnancy cohort. These factors were considered in the DAG, which indicated that maternal education and smoking during pregnancy were potential confounding variables and were therefore corrected for the statistical analysis. Numerous potentially confounding factors were considered, and the DAG identified those that should be included in the statistical analyses. This objective method provides a robust means of assessing the causal relationship between exposure and outcome. Residual confounding cannot, however, be completely excluded. Limitations of the study include the use of questionnaire-based assessments for part of the assessment for the diagnosis of atopic eczema, which may introduce bias; however, the assessment also involved a clinical examination undertaken by trained staff. The UK Working Party Diagnostic Criteria for Diagnosis of Atopic Dermatitis are highly sensitive and specific for identifying cases of atopic eczema, particularly if applied to developed countries;Citation40–Citation44 although they do not assess the severity of the disease, they represent the most comprehensively validated criteria for the diagnosis of atopic eczema, in both community and hospital settings.Citation45,Citation46 The criteria were modified to omit atopic disease in a first-degree relative from our case definition to avoid too narrow focus on familial cases of atopic eczema and to prevent excluding an important group of infants from such studies as we were seeking to disentangle the apparent heterogeneous phenotypes that “atopic eczema” is now thought to represent.Citation34 Maternal history of atopic eczema in the 12 months prior to the initial assessment at preconception, however, was considered as a confounding variable as determined by the DAG (). Severity of atopic eczema was not assessed, and it may be possible that those with clinically mild atopic eczema may not exhibit the same growth impairment as those with a more severe condition.Citation10 Although exploratory and hypothesis-generating methods were used to determine the described growth patterns and multiple statistical testing is a potential limitation of this study, only three parameters (linear size, abdominal circumference, and head circumference) were examined to lessen the inherent risks.
Conclusion
Our study demonstrates links between fetal and infant anthropometric measurements and growth patterns with risk of atopic eczema at ages 6 and 12 months. The findings suggest that growth falters prior to the onset of the inflammatory process associated with atopic eczema or its treatment and provide additional support for important prenatal influences on this skin condition.
Acknowledgments
This work was supported by grants from the Medical Research Council, British Heart Foundation, Food Standards Agency, Arthritis Research UK, National Osteoporosis Society, International Osteoporosis Foundation, Cohen Trust, European Union’s Seventh Framework (FP7/2007-2013), projects EarlyNutrition and ODIN under grant agreement numbers 289346 and 613977, NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, NIHR Musculoskeletal Biomedical Research Unit, University of Oxford, and British Lung Foundation. Abstracts containing some material presented in this manuscript were presented as poster presentations at the 10th World Congress of Developmental Origins of Health and Disease (published in the Journal of Developmental Origins of Health and Disease, Volume 8, Issue s1 [10th World Congress October 15–18, 2017, Rotterdam, the Netherlands], https://doi.org/10.1017/S2040174417000848) and at the International Investigative Dermatology 2018 Meeting (published in the Journal of Investigative Dermatology, Volume 138, Issue 5, Supplement [Abstract Supplement May 16, 2018–May 19, 2018]).
Supplementary materials
Figure S1 Selection of study group sample from the Southampton Women’s Survey (SWS) cohort.
Note: *Non assisted conception, regular cycle, sure/certain of last menstrual period (LMP), not on oral contraceptive pill prior to LMP, dating range scan data available, LMP consistent with date of conception, first positive pregnancy test, scan data, and gestation at birth.

Figure S2 Fetal and infant growth and atopic eczema DAG.
Notes: Confounding variables: maternal BMI, maternal education, gestational age, and breastfeeding duration. Competing exposures (variables adjusted for to improve precision of model): maternal eczema, smoking during pregnancy, and infant sex.
Abbreviations: BMI, body mass index; DAG, directed acyclic graph.
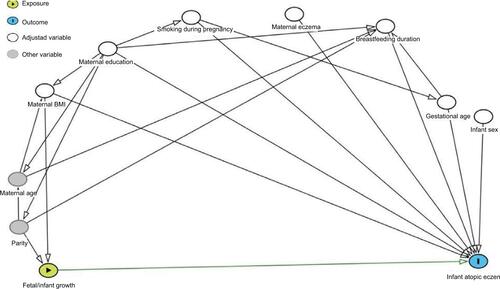
Table S1 Comparison of the study population with the remainder of the SWS participants
Table S2 Static size measurements in relation to eczema at ages 6 and 12 months
Table S3 Growth velocities in relation to eczema at age 6 and 12 months
Disclosure
KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. The other authors report no conflicts of interest in this work.
References
- KristmundsdottirFDavidTJGrowth impairment in children with atopic eczemaJ R Soc Med1987801912
- MassaranoAAHollisSDevlinJDavidTJGrowth in atopic eczemaArch Dis Child19936856776798323339
- ParkMKParkKYLiKSeoSJHongCKThe short stature in atopic dermatitis patients: are atopic children really small for their age?Ann Dermatol2013251232723467580
- EichenfieldLFTomWLChamlinSLGuidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitisJ Am Acad Dermatol201470233835124290431
- Royal College of Paediatrics and Child HeathRCPCH Care Pathway Eczema2011 Available from: http://www.rcpch.ac.uk/system/files/protected/page/2011_RCPCH-CarePathway-Eczema_v3_(19.23).pdfAccessed March 31, 2017
- The National Institute for Health and Care Excellence, National Collaborating Centre for Women’s and Children’s Health (UK) [homepage on the Internet]Atopic Eczema in Children: Management of Atopic Eczema in Children from Birth up to the Age of 12 YearsLondonRCOG Press200712 (NICE Clinical Guidelines, No. 57.) 2007. Available from: https://www.ncbi.nlm.nih.gov/books/NBK49365/Accessed March 8, 2017
- WongSCDobieRAltowatiMAWertherGAFarquharsonCAhmedSFGrowth and the growth hormone-insulin like growth factor 1 axis in children with chronic inflammation: current evidence, gaps in knowledge, and future directionsEndocr Rev20163716211026720129
- AylettSEAthertonDJPreeceMAThe treatment of difficult atopic dermatitis in childhood with oral beclomethasone dipropionateActa Derm Venereol Suppl (Stockh)19921761231251476023
- KellerMDShukerMHeimallJCianferoniASevere malnutrition resulting from use of rice milk in food elimination diets for atopic dermatitisIsr Med Assoc J2012141404222624441
- SilverbergJIPallerASAssociation between eczema and stature in 9 US population-based studiesJAMA Dermatol2015151440140925493447
- BeachRSGershwinMEHurleyLSGestational zinc deprivation in mice: persistence of immunodeficiency for three generationsScience198221845714694717123244
- VargINescakovaETothFUhrinovaAAdamkovMNutrition and immune system: the size of the thymus as an indicator of the newborn’s nutrition statusAnthropol Anz201168326527421905416
- AnderssonNWHansenMVLarsenADHougaardKSKolstadHASchlünssenVPrenatal maternal stress and atopic diseases in the child: a systematic review of observational human studiesAllergy2016711152626395995
- El-HeisSCrozierSRHealyEMaternal stress and psychological distress preconception: association with offspring atopic eczema at age 12 monthsClin Exp Allergy201747676076928218994
- WangIJChenSLLuTPChuangEYChenPCPrenatal smoke exposure, DNA methylation, and childhood atopic dermatitisClin Exp Allergy201343553554323600544
- WestCERydénPLundinDEngstrandLTulicMKPrescottSLGut microbiome and innate immune response patterns in IgE-associated eczemaClin Exp Allergy20154591419142925944283
- PanduruMSalavastruCMPanduruNMTiplicaGSBirth weight and atopic dermatitis: systematic review and meta-analysisActa Dermatovenerol Croat2014222919625102793
- OlesenABEllingsenAROlesenHJuulSThestrup-PedersenKAtopic dermatitis and birth factors: historical follow up by record linkageBMJ19973147086100310089112844
- PikeKCCrozierSRLucasJSPatterns of fetal and infant growth are related to atopy and wheezing disorders at age 3 yearsThorax201065121099110620956394
- TurnerSPrabhuNDanielanPFirst- and second-trimester fetal size and asthma outcomes at age 10 yearsAm J Respir Crit Care Med2011184440741321642247
- InskipHMGodfreyKMRobinsonSMCohort profile: the Southampton Women’s SurveyInt J Epidemiol2006351424816195252
- WilliamsHCBurneyPGPembrokeACHayRJThe U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validationBr J Dermatol199413134064167918017
- ChittyLSAltmanDGHendersonACampbellSCharts of fetal size: 4. Femur lengthBr J Obstet Gynaecol199410121321358305387
- ColeTJGreenPJSmoothing reference centile curves: the LMS method and penalized likelihoodStat Med19921110130513191518992
- PanHColeTJhomepage on the InternetLMSchartmaker, a program to construct growth references using the LMS method. Version 2.542011 Available from: http://www.healthforallchildren.co.uk/Accessed October 25, 2018
- InokuchiMHasegawaTAnzoMMatsuoNStandardized centile curves of body mass index for Japanese children and adolescents based on the 1978–1981 national survey dataAnn Hum Biol200633444445317060068
- RoelantsMHauspieRHoppenbrouwersKReferences for growth and pubertal development from birth to 21 years in Flanders, BelgiumAnn Hum Biol200936668069419919503
- SilvaSMaiaJClaessensALBeunenGPanHGrowth references for Brazilian children and adolescents: healthy growth in Cariri studyAnn Hum Biol2012391111822092114
- StanojevicSWadeAColeTJAsthma UK Spirometry Collaborative GroupSpirometry centile charts for young Caucasian children: the Asthma UK Collaborative InitiativeAm J Respir Crit Care Med2009180654755219574442
- GreenlandSPearlJRobinsJMCausal diagrams for epidemiologic researchEpidemiology199910137489888278
- LeadbitterPPearceNChengSRelationship between fetal growth and the development of asthma and atopy in childhoodThorax1999541090591010491453
- CarringtonLJLangley-EvansSCWheezing and eczema in relation to infant anthropometry: evidence of developmental programming of disease in childhoodMatern Child Nutr200621516116881914
- LangUBakerRSKhouryJClarkKEEffects of chronic reduction in uterine blood flow on fetal and placental growth in the sheepAm J Physiol Regul Integr Comp Physiol20002791R53R5910896864
- FulfordAJMooreSEArifeenSEDisproportionate early fetal growth predicts postnatal thymic size in humansJ Dev Orig Health Dis20134322323125054841
- GodfreyKMBarkerDJPOsmondCDisproportionate fetal growth and raised IgE concentration in adult lifeClin Exp Allergy19942476416487953946
- PrescottSLMacaubasCSmallacombeTHoltBJSlyPDHoltPGDevelopment of allergen-specific T-cell memory in atopic and normal childrenLancet199935391481962009923875
- LooEXShekLPGohAAtopic dermatitis in early life: evidence for at least three phenotypes? Results from the GUSTO StudyInt Arch Allergy Immunol2015166427327925925088
- BallingerABAzoozOEl-HajTPooleSFarthingMJGrowth failure occurs through a decrease in insulin-like growth factor 1 which is independent of undernutrition in a rat model of colitisGut2000465695700
- de BenedettiFAlonziTMorettaAInterleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammationJ Clin Invest19979946436509045866
- WilliamsHCBurneyPGHayRJThe U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitisBr J Dermatol199413133833967918015
- FlemingSBodnerCDevereuxGAn application of the United Kingdom Working Party diagnostic criteria for atopic dermatitis in Scottish infantsJ Invest Dermatol200111761526153011886518
- SaekiHIizukaHMoriYCommunity validation of the U.K. diagnostic criteria for atopic dermatitis in Japanese elementary schoolchildrenJ Dermatol Sci200747322723117544635
- PopescuCMPopescuRWilliamsHForseaDCommunity validation of the United Kingdom diagnostic criteria for atopic dermatitis in Romanian schoolchildrenBr J Dermatol199813834364429580796
- GirolomoniGAbeniDMasiniCThe epidemiology of atopic dermatitis in Italian schoolchildrenAllergy200358542042512752329
- BrenninkmeijerEESchramMELeeflangMMBosJDSpulsPIDiagnostic criteria for atopic dermatitis: a systematic reviewBr J Dermatol2008158475476518241277
- GuHChenXSChenKEvaluation of diagnostic criteria for atopic dermatitis: validity of the criteria of Williams et al. in a hospital-based settingBr J Dermatol2001145342843311531832
