Abstract
Background
Each year more than 4,000 cases of campylobacteriosis are reported in Denmark, making it the most common bacterial gastrointestinal infection. Here we describe a case-control study to identify sources of infection with a focus on environmental factors.
Methods
From January to December 2016, we conducted a prospective case-control study among Danish persons aged 1–30 years. Participants were invited by letter to complete an online questionnaire. Crude and adjusted ORs were calculated and final parsimonious multivariate models developed using logistic regression.
Results
The study recruited 1366 cases and 4,418 controls, of whom 65% and 66%, respectively, completed the questionnaire. A multivariate model for domestically acquired cases showed, among others, increased risk of infection with bathing in fresh water (OR=5.1), contact to beach sand (OR=1.8), owning a pet dog with diarrhea (OR=4.6), and eating minced beef (OR=2.6) or chicken (OR=2.5). The model for children highlighted similar risk factors but also included bathing in a paddling pool (OR=13.6) and eating fresh strawberries (OR=5.3). A separate analysis for persons reporting foreign travel showed increased infection risk when traveling to Asia, Africa, or Turkey and that eating from street kitchens and having contact to water during traveling were also risk factors.
Conclusion
Environmental factors and animal contact account for a sizeable proportion of domestic Campylobacter infections in the age group studied. The study also re-confirmed handling/consumption of chicken as an important risk factor while highlighting minced beef as a potential new risk factor. Overall, these results contribute to a better understanding of the transmission dynamics of Campylobacter and will be used to improve national guidelines for prevention of infection.
Introduction
Campylobacter spp. are a global cause of gastroenteritis in humans, particularly in industrialized countries. In Denmark, incidences have increased since 2012 and campylobacteriosis is now the most frequently reported gastrointestinal infectionCitation1 with 4,243 reported cases in 2017, corresponding to 73.7 cases per 100,000 population. This pattern is repeated throughout Europe, Australia, and the US where incidences remain high and have even increased during the past decade.Citation2–Citation4 Campylobacteriosis is a zoonotic disease with poultry, wild birds, and domestic pets as the main reservoirs.Citation5,Citation6 Symptoms in humans manifest as acute watery or bloody diarrhea and treatment is usually only required for severe cases or if infection triggers Guillain–Barré syndrome. Infection occurs in all age groups but incidences are higher in young persons, particularly children younger than 5 years.Citation1,Citation7 This age pattern of infection is most likely influenced by acquired immunity; children are repeatedly exposed to Campylobacter, through food and the immediate environment, developing partial immunity which allows them to remain asymptomatic (following most exposures) if infected as adults.Citation8
Campylobacteriosis is a mainly sporadic disease. When outbreaks occur, they have often been linked to contaminated water, raw (unpasteurized) milk, animal contact, and environmental exposures such as mud and sand.Citation9–Citation14 In Denmark, recent evidence from next-generation sequencing suggests that case clustering – and even outbreaks – may be more common than assumed.Citation15 To identify risk factors for sporadic cases, three DanishCitation16–Citation18 and numerous foreign case-control studies have been undertaken.Citation19–Citation26 These consistently identified traveling abroad, poor handling, and/or consumption of raw or undercooked chicken, consumption of raw milk, and animal contact as important determinants of infection, but a large proportion of cases remained unexplained. The current gray areas of Campylobacter epidemiology in particular cover the true spectrum of risk factors and the relative importance of poultry in relation to other exposures.Citation27–Citation29 Further, most case-control studies included persons of all ages which, considering the age-related partial immunity, may introduce bias as they are not all at equal risk of developing disease.Citation30 In other words, including controls from older age groups are likely to underestimate the importance of some risk factors and potentially miss others.
In this paper, we describe a national case-control study on Campylobacter risk factors in Denmark undertaken to identify the most important sources of infection, focusing on possible non-food determinants. To reduce the potential bias caused by partial immunity, we included only persons aged 1–30 years which also covers individuals believed to be at highest risk of disease.
Methods
Study design and population
The study was a national prospective case-control frequency-matched study with a population representative control group, conducted over a 12-month period beginning in January 2016. The total population of Denmark at that time was 5,700,000, of which 2,100,000 (37%) persons were aged between 1 and 30 years. Based on historical notification data, we expected approximately 1,300 cases to be reported in this age group during the study period.
Cases
A case was a person with a confirmed Campylobacter infection (all species), diagnosed using either culture, polymerase chain reaction, or serology, who was aged older than 1 and younger than 31 years and who lived in Denmark at the time of diagnosis. Cases were excluded in two steps: firstly from being invited to participate and secondly from being included in the analysis. The first exclusion step included cases who: 1) did not have a valid Danish address, 2) had their address and/or name protected by law, 3) were aged under 18 years and did not live with either parent, or 4) were not alive at the time of invitation. In the second step, we excluded cases who did not coherently answer the questionnaire. New cases were identified on a weekly basis through the national case notification register based on extraction of data from the Danish Microbiology Database (MiBa)Citation31 and assessed for inclusion using the criteria presented above. Each notification contains as a minimum information on name, age, gender, date of sample received in the laboratory, and the unique personal Civil Registry System (CPR) number. The CPR allows identification of the person’s address and familial status (eg, name and address of parents).
Controls
In total, 5,102 population controls aged 1–30 years living in Denmark were randomly extracted from the CPR System at the beginning of the study. From this group, a random sample of controls was selected for participation in the study each month. To account for the seasonal variation of campylobacteriosis, the number of controls asked for participation in any given month was correlated to the expected number of cases in that month (resulting in approximately four times more controls than cases each month).
Controls were also excluded in two steps. Firstly for the same reasons as for cases and if they resided in the same household as a previously included control. Secondly, controls who reported symptoms of gastrointestinal illness (diarrhea [bloody or non-bloody] and/or vomiting) during the past month and who did not coherently complete the questionnaire were excluded.
Recruitment and questionnaire
We recruited all cases and controls using a postal letter-based invitation, containing a rationale for the study, a short description of the questionnaire, and a personalized link (including username and password) to an online questionnaire. At the same webpage, invitees could decline participating in the study. For persons younger than 18 years, the invitation was sent to a parent living at the same address (the mother as a default). Parents completing questionnaires on behalf of children aged 12–17 were encouraged to answer the questions along with the child. Before recruitment, the vital status of each participant and – for persons under 18 years of age – their parents was assessed through the CPR System. Two postal reminders were sent 7 and 14 days, respectively, after the initial invitation to persons who had not completed the questionnaire and who had not actively declined to participate.
The questionnaire collected information on a range of exposures in the 5 days prior to symptom onset (cases) or the 5 days prior to completion of the questionnaire (controls) and as habits/baseline. Exposures included medical history, demographic information, overseas travel, recreational activities, dining locations, food and drink, kitchen hygiene, and animal contact. Questions on medical history and use of medication were asked based on a 4-week history and questions on travel on a 14-day history. Cases and controls reporting travel abroad provided further information about their journey, including exposures, after which they were excluded from the rest of the questionnaire. Participants could access their questionnaire at any time, save completed parts, and return at a later point. Passwords and usernames were valid for 8 weeks.
Seasonality
Because campylobacteriosis is highly seasonal in DenmarkCitation1 and exposures relating to the environment in particular are dependent on season, a season variable was included as a confounder. For cases, this variable was the month of self-reported symptom onset or, if they did not provide a symptom onset, the month when their sample was received in the laboratory. For controls, the season variable was defined as the month in which their questionnaire was completed (as they provided answers relating to their activities immediately prior to completing the questionnaire rather than at the time of inclusion in the study).
Data analysis
We performed univariate analyses on all explanatory variables to generate crude and adjusted odds ratios (ORs) with 95% confidence intervals (CIs). Multivariate analyses were performed by backward stepwise logistic regression modeling with elimination of nonsignificant variables based on the model deviance statistics and P-values. Two models were constructed: one for all participants in the study (ages 1–30 years) and one for small children (ages 1–5 years). Potential confounders were selected based on knowledge of determinants for Campylobacter infectionCitation1 and included age, sex, residential area (urban or rural), and season (as described above). Adjustments were made for these potential confounders and for two-factor interactions identified from investigative analysis of all explanatory variables.
Population attributable fractions (PAFs) were calculated using adjusted ORs from the final logistic regression models for each explanatory variable associated with an increased risk of infection.
All data were analyzed using STATA version 14 (Stata Corp, College Station, TX, USA).
Results
Study population
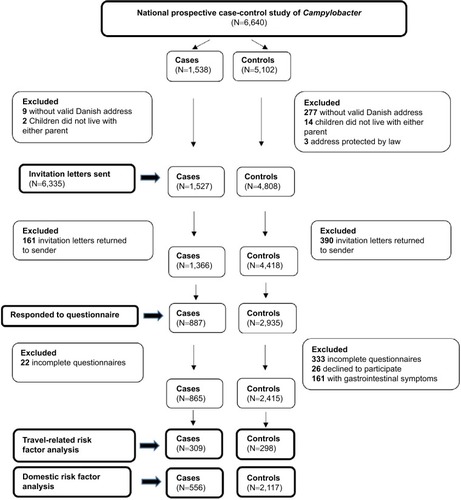
During 2016 in Denmark, 1,538 cases of Campylobacter were reported among persons aged 1–30 years. After exclusions (), we invited 1,527 of these (99.3%) to participate. A total of 161 cases (11%) returned the invitation letter to sender and were excluded from the study. Of the remaining 1,366 cases, 887 (65%) responded to the questionnaire, resulting in 556 cases available for the domestic risk factor analysis and 309 cases for the travel risk factor analysis (). Of the 5,102 randomly selected controls, 4,808 were eligible for invitation (). In total, 390 (8%) of these returned the invitation letter to sender and 4,418 controls therefore had a possibility to fill in the questionnaire. Of these, 2,935 (66%) persons responded, and this resulted in 2,117 controls for the domestic risk factor analysis and 298 for the travel risk factor analysis ().
There was no significant difference between cases and controls with respect to the distribution of gender, residential area, and – for persons older than 18 – occupation status (working/studying or unemployed). However, there were proportionally more cases than controls in the age group 1–4 years and more controls than cases in the age groups 5–9 and 10–14 years ().
Table 1 Frequency and percentage of study cases and controls by demographic characteristics (excluding persons reporting foreign travel), Denmark 2016
Univariate analysis of risk factors
Travel abroad
Traveling abroad was the single most important risk factor for infection for all cases (OR=4.6, 95% CI 3.7–5.7). Participants had traveled to 68 different countries with 29 countries (43%) being represented with a frequency of more than five visits. Cases exhibited a greater variation in the number of countries visited; 62 (91%) of the listed countries were visited by at least one case while for controls this was 37 (54%) countries. Risk of infection was higher for visitors to Asian countries and Turkey, whereas visiting Northern Europe and Scandinavia was associated with a reduced risk of infection (). For travelers, staying in a Bed & Breakfast (compared to all other types of accommodation, including outdoor camping), consuming food from street kitchens, having contact to sand, soil, and/or mud, and bathing in sea water carried an increased risk of infection, whereas cooking one’s own food was inversely associated with campylobacteriosis ().
Table 2 Determinants for Campylobacter infection associated with foreign travel, Denmark 2016
All subjects reporting travel abroad were excluded from the risk factor analysis for domestic factors presented below.
Environmental exposures
Contact to water from natural sources was associated with infection. Specifically, cases were more likely to have bathed in sea- or freshwater or in a paddling pool and have consumed water from a stream or spring in nature (). There was also a higher risk of infection associated with contact to beach sand and with fishing.
Table 3 Univariate determinants for Campylobacter infection, Denmark 2016
Animals
There was no association between illness and contact to animals or having a pet in general; however, cases were more likely to have had contact with animal feces (). Further, there was also an association between illness and having a pet dog and in particular a dog who had diarrhea in the 5-day exposure period. Lastly, cases were more likely to have had contact with cattle.
Eating habits and kitchen hygiene
Eating in a café/restaurant or fast food restaurant carried an increased risk of infection as did eating food served outdoors and own food consumed during a picnic in the countryside or forest (). Eating meat cooked on barbecue (at home) was also associated with infection.
Considering hygiene practices at home, handling of fresh chicken in the 5-day exposure period was associated with an increased risk of infection.
Food and drink
There was no association between illness and consumption of pork, duck, goose, game, or any deli meat as a habit or during the 5-day exposure period. Cases were more likely to consume chicken in general and to have consumed whole chicken, chicken fillet, or chicken thighs in the exposure period. The analyses further identified an increased risk of illness associated with consumption of beef mince and steaks ().
Consumption of specific vegetables was not associated with illness. However, fresh strawberries, raspberries, and blueberries increased the risk of infection as did consumption of smoothies prepared with frozen berries.
Lastly, cases were more likely than controls to have consumed unpasteurized milk and to live in a household with drinking water supplied from a private water supply.
Other exposures
Participants were asked about their own and family members’ medical history and use of medication. Among these, the use of antibiotics and proton pump inhibitors (PPIs) in the last month/month prior to developing symptoms and a household member suffering from diarrhea in the 5-day exposure period were associated with illness.
Specific exposures for adults and children
To account for age-specific exposures, adults (over 18 years of age) and children were asked different questions relating to occupation or schooling. Analyses showed that adults who worked – and in particular work involving patient contact in non-hospital settings (eg, home care) – were at higher risk of infection. Children who had an after school job with contact to animals (eg, dog walking) also had a higher risk of campylobacteriosis.
Protective factors
A number of factors were independently associated with a reduced risk of infection, including running (on both soil and asphalt) and having a pet cat (). With respect to food and drink, consumption of turkey and tap water (at home) also carried a lower risk of infection.
Multivariate analysis of risk factors
All study participants, ages 1–30 years
A range of both food- and non-food-related exposures were associated with illness after adjusting for the effect of other variables (). The explorative analyses identified an interaction between minced beef and barbecued meat which was included in this model. Consumption of chicken fillets, whole chicken, beef mince, and meat prepared on a barbecue were all associated with an increased risk of illness. Of environmental factors, bathing in fresh water, contact to beach sand, and having household drinking water from a private well carried increased risk of disease in the final model. In addition, the model showed that contact to animal feces, owning a pet dog with diarrhea, and the use of PPIs were associated with illness.
Table 4 Multivariable risk factors for Campylobacter infection, Denmark 2016
These independent determinants predicted almost half of the variation in illness (R2=0.48).
The calculated PAFs showed that avoiding consumption of chicken and minced beef, and contact to animals and pet dogs with diarrhea would result in the highest reduction in the number of infections (). The remaining variables accounted for a smaller proportion of the number of cam-pylobacteriosis cases.
Small children, ages 1–5 years
The separate model constructed for small children indicated that both food and non-food exposures were important and independently associated with campylobacteriosis (). Contact to animal feces and bathing in a paddling pool were the two sole environmental factors included. Having a pet dog was also associated with illness. With respect to food, the model included consumption of minced beef, whole chicken, and fresh strawberries.
These independent determinants predicted slightly more than half of the variation in illness (R2=0.51).
For children, not consuming chicken and minced beef and not having contact with animal feces and dogs would also result in a notable reduction in the number of infections (). Further, consumption of strawberries alone accounted for a relative 16% of cases.
Discussion
In this national case-control study of campylobacteriosis determinants, we found that exposures related to the environment, animal contact, and food were associated with an increased risk of illness. The study is the largest ever undertaken in Denmark. Because it focuses on the younger age groups, the results are less influenced by bias from persons with partial immunity. The investigation generated response rates at high levels (65% for cases and 66% for controls) for a non-telephone-based survey, highlighting the usefulness of online questionnaires but also the validity of the results.Citation32 Lastly, we asked persons who reported traveling to provide specific information regarding exposures during their trip, rather than immediately excluding them from the study.
One of the primary aims of this study was to identify environmental risk factors for campylobacteriosis. The results show that contact to water in the environment was particularly important. There is a well-established link between Campylobacter infection and recreational water contact,Citation22,Citation25,Citation33 especially in outbreak situations.Citation12,Citation34 Both fresh and sea water harbor Campylobacter spp.Citation35,Citation36 and our study suggests that 4% of sporadic Danish campylobacteriosis cases may be caused by recreational water contact – even double that for children using a paddling pool. Ingestion of water from a private (rather than public) household well also increased the risk of infection. Drinking water was implicated in several Campylobacter outbreaksCitation37,Citation38 and associated with disease in case-control studies from other countries.Citation21,Citation23,Citation39 However, as the public water supply in Denmark, unlike most other European countries, is drawn almost exclusively from ground water rather than surface water,Citation40 it is not unexpected that our study associates disease with drinking water from a private well. The final environmental determinant identified in this study was beach sand. Although not previously identified in any case-control study, it is not surprising as Campylobacter spp. are present in beach sandCitation41 and indeed several Campylobacter outbreaks have been linked to incidental ingestion of mud.Citation13,Citation14
Another known risk factor for Campylobacter infection is interaction with animals. We found that contact to animal feces was a particular determinant for infection, accounting for as much as 12% of cases. Further, we confirm that contact to dogs, especially if the dog has diarrhea, also increases the risk of infection. For small children, having a pet dog was the second most important determinant identified in the study. On the contrary, having a pet cat was associated with a reduced risk of infection, possibly indicating the distinction “cat people” vs “dog people” (although this was not confirmed by interactions between the variables). These results confirm previous findings that contact to dogs and their feces carries an increased risk of campylobacteriosis for humans.Citation20,Citation23 In general, the importance of proper hygiene measures during and after contact to dogs and their feces, especially for children, needs to be emphasized in public health settings.
For food-related exposures, consumption of chicken (whole chicken and chicken fillets) was associated with domestic Campylobacter infection. Our study showed that almost one third of Campylobacter cases in Denmark each year may be attributed to chicken. Although chicken liver, in the form of liver paté, has been identified as the source of several outbreaks as well as a risk factor for sporadic disease in other countries,Citation42 it was not a determinant for infection in our study. Rather than eliminating chicken livers as a potential risk factor, we attribute this result to the low risk of exposure among our study population – which most likely reflects the age group studied (the frequency of chicken liver consumption increases with age).Citation42 As an unexpected outcome, the results show that consumption of minced beef may be associated with campylobacteriosis. Campylobacter spp. have been isolated from cattle in both Denmark and other European countries,Citation43–Citation47 but the prevalence in beef is reported as minor.Citation48–Citation51 Minced beef/hamburger meat was identified as a risk factor in other case-control studiesCitation52,Citation53 and even as a source of outbreaks,Citation54–Citation56 but it is not considered an important transmission route for Campylobacter in Denmark. The risk associated with minced beef may be a recent occurrence due to the introduction of Modified Atmosphere Packaging (MAP). Minced meat in a MAP has lower concentrations of O2, improving the shelf life and reducing discoloration, but during preparation the meat rapidly turns brown, increasing the risk of consumption before properly cooked. The separate model for small children indicated fresh strawberries as a source of infection. This is also an unexpected result given previous findings that fresh berries reduce the risk of infection.Citation52,Citation57 The risk from strawberries may be linked to hygiene practices (not washing the berries) as Danish children frequently eat strawberries either from the field when picking them or directly from the box if bought in retail. Our results point to minced beef and fresh strawberries as two new possible sources of infection to be investigated, and both of these are presently being examined by the Danish Food and Veterinary Administration as part of the national action plan against Campylobacter.
The questionnaire also examined medical history and use of medication, and our results confirm previous findings that the use of PPIs increases the risk of Campylobacter infection.Citation58,Citation59 Use of over-the-counter PPIs has increased during recent years, and this may be one of the driving factors behind the observed increase in Campylobacter in many countries.Citation28,Citation60 Interestingly, our study found an age-independent risk for the use of PPIs with very young children also reporting use of this medication. PPIs are not contraindicated for the use in children, and are prescribed for treatment of complicated reflux.Citation61 For this age group in particular, our results highlight a potential public health concern correlated with the use of PPIs.
As a new addition to case-control studies of sporadic campylobacteriosis, we included an expanded analysis of travel-related cases. Our results confirm that infection abroad primarily occurs in Indonesia, Thailand, Africa, and Turkey. Cases in general could be classified as “adventurous” travelers, visiting more countries, and in particular countries outside Europe. Here, eating in street kitchens and staying in a Bed & Breakfast carried the highest risk of infection, but also contact to water, sand soil, or mud were determinants of infection. On the other hand, persons who traveled to Scandinavia or Northern Europe and who cooked their own food had lower risk of infection. These countries are not “protective” in themselves as campylobacteriosis rates are also high in Northern Europe, but the low infection risk is most likely an indicator for better hygiene and consumption of safer foods when traveling closer to home. Several studies report that travelers in more exotic locations, frequently Asia, often do not follow the rules of eating and drinking safely.Citation62–Citation64
Campylobacteriosis is a disease of many unanswered questions, in particular with respect to determination of risk factors and their relative importance. For instance, persistently high incidences of Campylobacter in many countries despite intense poultry control efforts has been an argument for chicken not being the primary source of human infections.Citation65,Citation66 Overall, our results confirm that chicken meat is an important risk factor for campylobacteriosis. However, they also cast new light on the ongoing question of whether infections arise from more complex transmission routesCitation66 – such as those from other food sources and the environment. The results presented here suggest that campylobacteriosis is not attributable to one primary food source but rather a combination of non-food and food factors. The relative importance of these factors is likely to vary between persons, locations, and even throughout the year.
The study size, age-specific inclusion criteria, and high response rates are all important strengths when interpreting our findings. Additionally, the independent determinants identified in the analyses all confirm previous knowledge about Campylobacter – albeit in more detail. Nevertheless, it is necessary to consider the potential biases commonly affecting case-control studies. Firstly, participation rates among cases and controls were high, resulting in an even distribution of exposures between the two groups. Secondly, using an online questionnaire eliminated interviewer bias. The third issue to consider is imperfect recall. Cases probably received their invitation 14–20 days after symptom onset and may have forgotten exposures in the period before symptom onset. However, all questions relating to the 5-day exposure period were also asked as “habit” questions (ie, “how often do you…”). Including these questions helps generate an overall image of each participant. For instance, a case who reports generally eating chicken up to five times per week is also likely to have eaten it in the 5 days before symptom onset. We therefore argue that the impact of recollection bias is negligible. In this study, we chose to exclude controls who reported suffering from symptoms of a gastrointestinal illness in the month prior to completing the questionnaire. Although often a standard practice in case-control studies, it has been suggested that such exclusions create bias as the control group has been amended to not exactly represent the population which gave rise to the cases.Citation67 This may have resulted in the identification of artificial associations – particularly if the unknown gastrointestinal illness was associated with some of the determinants identified in this study. However, considering that only 5% of controls were excluded for this reason, this bias is unlikely to have had an impact on the results. Another source of bias was only including persons aged 1–30 years and effectively omitting around 60% of all notified campylobacteriosis cases from the study. This improves the estimates for the identified determinants but may also have caused bias by missing specific determinants in the older population. The potential effect of bias is also visible in the wide confidence intervals calculated for some risk factors in the multivariate models, particularly for small children. This indicates a higher degree of uncertainty associated with the results and most likely reflects the smaller sample size for some exposures. Another limitation of the study is not distinguishing between different Campylobacter spp. which may have overlooked species-specific risk factors. However, as 95% of all reported infections in Denmark are Campylobacter jejuni,Citation68 variation between species is unlikely to have impacted the results. Indeed, omitting known Campylobacter coli infections from the analyses did not alter the outcomes (results not shown). Seasonality had the potential for causing bias in the results presented. Both Campylobacter infection ratesCitation1 and recreational/environmental exposures are highly seasonal. We aimed to minimize this bias by frequency-matching, ensuring that the number of controls included in any given month proportionally reflected the number of reported cases and that the relevant exposures were given appropriate weight in relation to the season. Finally, interpreting the results for risks of traveling need to be interpreted with the caution that all exposures whilst traveling were assumed to be equal, irrespective of destination – something which is most likely not the case.
Conclusion
Overall, the results from this study underpin that Campylobacter infection remains primarily a foodborne infection albeit with an important environmental component. The role of environmental factors in relation to Campylobacter infection is poorly understood and the Population Attributable Fractions calculated in this study indicate that environmental factors – primarily recreational water contact and contact to sand – could account for a large proportion of campylobacteriosis cases in this young population.
With respect to food, our findings confirm published evidence that chicken remains an important risk factor for campylobacteriosis. However, they also suggest minced beef as a potentially new source of infection. In order to confirm or reject this result, minced beef needs to be closely investigated for Campylobacter contamination at several levels of the food chain.
Combined, our results contribute significantly to a better understanding of the marked peak in cases during summer and of the “unexplained” cases of Campylobacter infection which are not related to chicken. Our results will be used to guide not only further research and control efforts but also to improve national guidelines for prevention of infection.
Ethical considerations
The Danish Data Protection Agency approved the study (journal 2012-54-0029). According to Danish regulations, ethical committee approval is not required for this study, as it did not involve analysis of biological material from human subjects.
Acknowledgments
We are very grateful to all subjects and parents who kindly participated in the study and to the Interview Center at Statens Serum Institut for initial screening of patients. We would also like to thank Gudrun Sandø, Mette Rørbæk Gantzhorn, Hanne Rosenquist, and Louise Boysen for providing constructive comments on the questionnaire and the study design. This project was supported by the Danish Food and Veterinary Administration, under the Ministry of Environment and Food of Denmark.
Disclosure
The authors report no conflicts of interest in this work.
References
- KuhnKGNielsenEMMølbakKEthelbergSEpidemiology of campylobacteriosis in Denmark 2000-2015Zoonoses Public Health2018651596628597535
- European Food Safety Authority, European Centre for Disease Prevention and ControlThe European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015Efsa J20161412
- MarderEPCieslakPRCronquistABIncidence and Trends of Infections with Pathogens Transmitted Commonly Through Food and the Effect of Increasing Use of Culture-Independent Diagnostic Tests on Surveillance - Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2013-2016MMWR Morb Mortal Wkly Rep2017661539740328426643
- MoffattCRMGlassKStaffordRD’EsteCKirkMDThe campylobacteriosis conundrum – examining the incidence of infection with Campylobacter sp. in Australia, 1998–2013Epidemiol Infect20171450483984727938447
- HaldBSkovMNNielsenEMCampylobacter jejuni and Campylobacter coli in wild birds on Danish livestock farmsActa Vet Scand2016581126842400
- CampagnoloERPhilippLMLongJMHanshawNLPet-associated Campylobacteriosis: A persisting public health concernZoonoses Public Health201865330431128834267
- KaakoushNOCastaño-RodríguezNMitchellHMManSMGlobal Epidemiology of Campylobacter InfectionClin Microbiol Rev201528368772026062576
- HavelaarAHvan PeltWAngCWImmunity to Campylobacter: its role in risk assessment and epidemiologyCrit Rev Microbiol200935112219514906
- Multistate Outbreak of Multidrug-Resistant Campylobacter Infections Linked to Contact with Pet Store Puppies | September 2017| Salmonella | CDC Available from: https://www.cdc.gov/campylobacter/outbreaks/puppies-9-17/index.html, Published December 14, 2017Accessed January 29, 2018
- KuhnKGFalkenhorstGEmborgHDEpidemiological and serological investigation of a waterborne Campylobacter jejuni outbreak in a Danish townEpidemiol Infect2017145470170927903324
- LahtiERehnMOckbornGOutbreak of Campylobacteriosis Following a Dairy Farm Visit: Confirmation by GenotypingFoodborne Pathog Dis201714632633228350214
- Harder-LauridsenNMKuhnKGErichsenACMølbakKEthelbergSGastrointestinal illness among triathletes swimming in non-polluted versus polluted seawater affected by heavy rainfall, Denmark, 2010-2011PLoS One2013811e7837124244306
- StuartTLSandhuJStirlingRCampylobacteriosis outbreak associated with ingestion of mud during a mountain bike raceEpidemiol Infect2010138121695170320334726
- ZeiglerMClaarCRiceDOutbreak of campylobacteriosis associated with a long-distance obstacle adventure race–Nevada, October 2012MMWR Morb Mortal Wkly Rep2014631737537824785983
- JoensenKGKuhnKGMüllerLWhole-genome sequencing of Campylobacter jejuni isolated from Danish routine human stool samples reveals surprising degree of clusteringClin Microbiol Infect2018242201.e5201.e8
- NeimannJEngbergJMølbakKWegenerHCA case-control study of risk factors for sporadic campylobacter infections in DenmarkEpidemiol Infect2003130335336612825719
- EthelbergSSimonsenJGerner-SmidtPOlsenKEMølbakKSpatial distribution and registry-based case-control analysis of Campylobacter infections in Denmark, 1991-2001Am J Epidemiol2005162101008101516207804
- WingstrandANeimannJEngbergJFresh chicken as main risk factor for campylobacteriosis, DenmarkEmerg Infect Dis200612228028416494755
- Carrique-MasJAnderssonYHjertqvistMSvenssonATornerAGie-seckeJRisk factors for domestic sporadic campylobacteriosis among young children in SwedenScand J Infect Dis200537210111015764201
- StaffordRJSchluterPKirkMA multi-centre prospective case-control study of campylobacter infection in persons aged 5 years and older in AustraliaEpidemiol Infect2007135697898817134530
- DominguesARPiresSMHalasaTHaldTSource attribution of human campylobacteriosis using a meta-analysis of case-control studies of sporadic infectionsEpidemiol Infect2012140697098122214729
- Mughini GrasLSmidJHWagenaarJARisk factors for campylobacteriosis of chicken, ruminant, and environmental origin: a combined case-control and source attribution analysisPLoS One201278e4259922880049
- MacdonaldEWhiteRMexiaRRisk Factors for Sporadic Domestically Acquired Campylobacter Infections in Norway 2010-2011: A National Prospective Case-Control StudyPLoS One20151010e013963626431341
- BassalROvadiaABrombergMRisk Factors for Sporadic Infection With Campylobacter Spp. Among Children in Israel: A Case-control StudyPediatr Infect Dis J201635324925226569191
- RavelAPintarKNesbittAPollariFNon food-related risk factors of campylobacteriosis in Canada: a matched case-control studyBMC Public Health2016161101627677338
- RosnerBMSchielkeADidelotXA combined case-control and molecular source attribution study of human Campylobacter infections in Germany, 2011-2014Sci Rep201771513928698561
- CaseyEFitzgeraldELuceyBTowards understanding clinical campylobacter infection and its transmission: time for a different approach?Br J Biomed Sci2017742536428367739
- StrachanNJRotariuOMacraeMOperationalising factors that explain the emergence of infectious diseases: a case study of the human campylobacteriosis epidemicPLoS One2013811e7933124278127
- AilesEScallanEBerkelmanRLKleinbaumDGTauxeRVMoeCLDo differences in risk factors, medical care seeking, or medical practices explain the geographic variation in campylobacteriosis in Foodborne Diseases Active Surveillance Network (FoodNet) sites?Clin Infect Dis201254Suppl 5S464S47122572671
- HavelaarAHSwartAImpact of waning acquired immunity and asymptomatic infections on case-control studies for enteric pathogensEpidemics201617566327915211
- VoldstedlundMHaarhMMølbakKMiBa Board of Representatives. The Danish Microbiology Database (MiBa) 2010 to 2013Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull2014191
- MortonLMCahillJHartgePReporting participation in epidemiologic studies: a survey of practiceAm J Epidemiol2006163319720316339049
- Schönberg-NorioDTakkinenJHänninenMLSwimming and Campylobacter infectionsEmerg Infect Dis20041081474147715496253
- DaleKKirkMSinclairMHallRLederKReported waterborne outbreaks of gastrointestinal disease in Australia are predominantly associated with recreational exposureAust N Z J Public Health201034552753021040184
- KovanenSKivistöRLlarenaAKTracing isolates from domestic human Campylobacter jejuni infections to chicken slaughter batches and swimming water using whole-genome multilocus sequence typingInt J Food Microbiol2016226536027041390
- MooreJECaldwellPSMillarBCMurphyPGOccurrence of Campylobacter spp. in water in Northern Ireland: implications for public healthUlster Med J200170210210711795758
- Guzman-HerradorBCarlanderAEthelbergSWaterborne outbreaks in the Nordic countries, 1998 to 2012Euro Surveill201520242116026111239
- NicholsGLaneCAsgariNVerlanderNQCharlettARainfall and outbreaks of drinking water related disease and in England and WalesJ Water Health2009711818957770
- FriedmanCRHoekstraRMSamuelMRisk factors for sporadic Campylobacter infection in the United States: A case-control study in FoodNet sitesClin Infect Dis200438Suppl 3S285S29615095201
- VoutchkovaDDHansenBErnstsenVKristiansenSMNationwide Drinking Water Sampling Campaign for Exposure Assessments in DenmarkInt J Environ Res Public Health2018153467
- BoltonFJSurmanSBMartinKWareingDRHumphreyTJPresence of Campylobacter and Salmonella in sand from bathing beachesEpidemiol Infect1999122171310098779
- JonesAKRigbyDBurtonMRestaurant Cooking Trends and Increased Risk for Campylobacter InfectionEmerg Infect Dis20162271208121527314748
- NielsenEMFussingVEngbergJNielsenNLNeimannJMost Campylobacter subtypes from sporadic infections can be found in retail poultry products and food animalsEpidemiol Infect2006134475876716316490
- ThépaultAPoezevaraTQuesneSRoseVChemalyMRivoalKPrevalence of Thermophilic Campylobacter in Cattle Production at Slaughterhouse Level in France and Link Between C. jejuni Bovine Strains and CampylobacteriosisFront Microbiol2018947129615999
- KovacJStesslBČadežNPopulation structure and attribution of human clinical Campylobacter jejuni isolates from central Europe to livestock and environmental sourcesZoonoses Public Health2018651515828755449
- EscherRBrunnerCvon SteigerNClinical and epidemiological analysis of Campylobacter fetus subsp. fetus infections in humans and comparative genetic analysis with strains isolated from cattleBMC Infect Dis20161619827177684
- Grove-WhiteDHLeatherbarrowAJCrippsPJDigglePJFrenchNPMolecular epidemiology and genetic diversity of Campylobacter jejuni in ruminantsEpidemiol Infect2011139111661167121134320
- NielsenEMEngbergJMadsenMDistribution of serotypes of Campylobacter jejuni and C. coli from Danish patients, poultry, cattle and swineFEMS Immunol Med Microbiol199719147569322068
- HakkinenMHeiskaHHänninenMLPrevalence of Campylobacter spp. in cattle in Finland and antimicrobial susceptibilities of bovine Campylobacter jejuni strainsAppl Environ Microbiol200773103232323817369335
- LammerdingAMGarciaMMMannEDPrevalence of Salmonella and Thermophilic Campylobacter in Fresh Pork, Beef, Veal and Poultry in CanadaJ Food Prot19885114752
- ZhaoCGeBde VillenaJPrevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the Greater Washington, D.C., areaAppl Environ Microbiol200167125431543611722889
- KapperudGSkjerveEBeanNHOstroffSMLassenJRisk factors for sporadic Campylobacter infections: results of a case-control study in southeastern NorwayJ Clin Microbiol19923012311731211452694
- GallayABousquetVSiretVRisk factors for acquiring sporadic Campylobacter infection in France: results from a national case-control studyJ Infect Dis2008197101477148418444804
- GreigJDRavelAAnalysis of foodborne outbreak data reported internationally for source attributionInt J Food Microbiol20091302778719178974
- Marler Clark NetworkFoodborne Illness Outbreak Database Available from: http://www.outbreakdatabase.com/Accessed October 16, 2018
- Public Health EnglandCampylobacter data 2006 to 2015 National laboratory data for residents of England and Wales Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/597752/Campylobacter_2016_Data.pdfAccessed October 16, 2018
- WhileyHvan den AkkerBGiglioSBenthamRThe role of environmental reservoirs in human campylobacteriosisInt J Environ Res Public Health201310115886590724217177
- DoorduynYvan den BrandhofWEvan DuynhovenYTBreukinkBJWagenaarJAvan PeltWRisk factors for indigenous Campylobacter jejuni and Campylobacter coli infections in The Netherlands: a case-control studyEpidemiol Infect2010138101391140420223048
- HafizRAWongCPaynterSDavidMPeetersGThe Risk of Community-Acquired Enteric Infection in Proton Pump Inhibitor Therapy: Systematic Review and Meta-analysisAnn Pharmacother201852761362229457492
- BouwknegtMvan PeltWKubbingaMEWedaMHavelaarAHPotential association between the recent increase in campylobacteriosis incidence in the Netherlands and proton-pump inhibitor use - an ecological studyEuro Surveill201419322087325139075
- BellJCSchneuerFJHarrisonCAcid suppressants for managing gastro-oesophageal reflux and gastro-oesophageal reflux disease in infants: a national surveyArch Dis Child2018103766066429472195
- KassBTraveller’s diarrhoeaAust Fam Physician200534424324715861744
- BryantHECsokonayWMLoveMLoveEJSelf-reported illness and risk behaviours amongst Canadian travellers while abroadCan J Public Health19918253163191768989
- MigaultCKanagaratnamLNguyenYPoor knowledge among French travellers of the risk of acquiring multidrug-resistant bacteria during travelJ Travel Med2017241
- NelsonWHarrisBFlies, fingers, fomites, and food. Campylobacteriosis in New Zealand–food-associated rather than food-borneN Z Med J20061191240U212816924279
- NelsonWHarrisBCan we change the hymn sheet? Campylobacteriosis not just from chickenN Z Med J20061191244U229917072370
- PooleCControls who experienced hypothetical causal intermediates should not be excluded from case-control studiesAm J Epidemiol1999150654755110489992
- LitrupETorpdahlMNielsenEMMultilocus sequence typing performed on Campylobacter coli isolates from humans, broilers, pigs and cattle originating in DenmarkJ Appl Microbiol2007103121021817584467