Abstract
Introduction
One out of six adults has symptoms of overactive bladder (OAB). Antimuscarinic medication is the main pharmacological group used in the treatment of OAB. In preclinical studies, antimuscarinic compounds have been found to inhibit cell proliferation in lung cancer and colon cancer.
Objective
The aim of this study was to investigate the association between exposure to anti-muscarinic medication and the risk of lung cancer and colon cancer.
Methods
Individuals in Sweden who first filled a prescription for an antimuscarinic medication used to treat OAB (ie, oxybutynin, solifenacin, darifenacin, fesoterodine, or tolterodine) between July 1, 2006, and December 31, 2012, were identified and classified as exposed. Each exposed individual was individually matched with up to ten unexposed individuals from the Swedish general population, based on year of birth, sex, and county of residence. Cox proportional hazard models with follow-up time as the underlying time scale were used to estimate HRs with 95% CIs.
Results
In total, 164,000 exposed and 1,446,472 unexposed individuals were included in this study. The estimated HRs for lung cancer, in follow-up time intervals of <1 year, 1–4 years, and ≥4 years, were as follows: 0.86 (95% CI: 0.75–0.98), 0.63 (95% CI: 0.56–0.70), and 0.43 (0.34–0.55), respectively. The corresponding estimates for colon cancer were as follows: 0.91 (95% CI: 0.80–1.03), 0.81 (95% CI: 0.74–0.88), and 0.61 (95% CI: 0.51–0.73), respectively.
Conclusion
There was an inverse association between exposure to antimuscarinic medications, used in the treatment of OAB, and a diagnosis of colon cancer or lung cancer, which is in line with the findings in preclinical studies.
Introduction
Overactive bladder (OAB) is defined as urgency with or without incontinence, usually with frequency and nocturia.Citation1 One out of six adults has symptoms of OAB, and the prevalence increases with age and is more common among women than among men.Citation2,Citation3 Alzheimer’s disease, previous stroke, overweight, and obesity are important risk factors for OAB.Citation4,Citation5
Antimuscarinic medication is the main pharmacological group used in the treatment of OAB, and the selected study medications are solely approved for OAB in Sweden.Citation6 Antimuscarinic medications can also be used in other conditions, eg, asthma. They act by blocking acetylcholine from binding to the muscarinic receptors present on the detrusor muscle, resulting in a decreased contraction of the bladder.Citation6,Citation7 In preclinical studies, activation of the muscarinic receptor subtype 3 (M3) by acetylcholine, or other M3-receptor agonists, has been shown to affect cell proliferation and cancer cell growth in prostate, colon, pancreatic, lung, brain, breast, ovarian, skin (melanocytes), stomach, bone, and blood (lymphoma and leukemia).Citation8–Citation21 Preclinical studies have also shown that inhibition of the M3-receptor by antagonists inhibits cell proliferation in lung cancer (both small-cell lung cancer and non-small-cell lung cancer) and colon cancer.Citation22–Citation26 These findings suggest that muscarinic antagonists may have an inhibitory effect on cell proliferation and cancer cell growth in the lung and colon in humans.
Smoking, exposure to radon and asbestos, and genetic factors are well-established risk factors for lung cancer.Citation28 Inflammatory intestinal conditions, diet, a sedentary lifestyle, obesity smoking, and alcohol are risk factors for colon cancer.Citation29,Citation30
To the best of our knowledge, this is one of the first studies with data from population-based registers investigating the association between antimuscarinics for OAB and the risk of lung cancer and colon cancer.Citation27 A Danish study, including 72,917 patients, investigated the association between antimuscarinic medications for OAB and different cancers, including lung cancer and colon cancer, and estimated age- and sex-standardized incidence rates (SIRs) for the associations, and the findings indicated a protective effect.Citation27 However, as SIRs are less precise measures as compared to HRs, we will use HRs to assess associations between exposure to antimuscarinics and the risk of lung cancer and colon cancer.
The aim of the study was to investigate the association.
Patients and methods
Study population
The exposed individuals in the study population were patients in Sweden who first filled a prescription for an antimuscarinic medication used to treat OAB (ie, oxybutynin, solifenacin, darifenacin, fesoterodine, or tolterodine) between July 1, 2006, and December 31, 2012. A new user was defined as a patient who filled the first prescription for a study medication, without a filled prescription for a study medication during the previous 12 months. The first filled prescription had to be for a tablet formulation to enable calculation of accumulated use.
To ensure a homogeneous study population, only anti-muscarinic medications solely approved for the treatment of OAB were included.
Each exposed eligible individual was individually matched with up to ten unexposed individuals with the same characteristics such as year of birth, sex, county of residence, and vital status.
Individuals, both exposed and unexposed, younger than 18 years at the time of the first filled prescription, with a history of lung cancer or colon cancer at any time before the first filled prescription, were excluded from the study cohort. A look back period of 5 years was used to identify comorbid conditions.
Data sources
All Swedish residents are assigned a unique personal identification number (PIN) at birth or upon immigration, which is kept unchanged throughout life.Citation31,Citation32 The information from the different registers was linked using the PIN.
Data on filled prescriptions were obtained from the Swedish Prescribed Drug Register (PDR) with information available from July 2005, on filled prescriptions from community pharmacies. The PDR contains information on the substance name, product name, formulation, amount, date of prescribing and date of filling the prescription, and the prescriber’s profession.Citation33 The PDR does not include data on over-the-counter medications or medications on requisition used in hospitals or nursing homes.
The Swedish Cancer Register (SCR) was used to identify individuals with a diagnosis of lung cancer or colon cancer, both for exclusion and to identify the end points. The SCR was established in 1958 and records individual data on all newly diagnosed malignant tumors in Sweden.Citation34 The register uses the ICD 7th revision (ICD-7). Since 2005, the site and histological type of the cases have been coded in ICD Oncology third edition (ICD-O-3) codes.Citation35
The National Patient Register (NPR) and the PDR were used to obtain information on comorbid conditions (ie, cerebrovascular disease, COPD, diabetes mellitus [type 1 or 2], hypertension, inflammatory bowel disease, peptic ulcer disease, and obesity) for up to 5 years before the date of the first filled prescription. The data on filled prescriptions from the PDR were used as proxies for a diagnosis of hypertension, diabetes mellitus, or obesity. The specific medications used as proxies are listed in . The NPR started collecting data in 1964 and has national coverage regarding in-patient care since 1987.Citation36,Citation37 Since 2001, information on outpatient visits is also recorded and the coverage increased over subsequent years. Primary care is not covered in the register. For each admission, the NPR records information on health care establishment, date, duration of care, and personal data (sex, age, PIN, and place of residence) and contains a main and up to 30 contributory diagnoses using ICD codes. From 1997 the tenth revision of the ICD codes (ICD-10) is used in the NPR.
Information for censoring individuals who died was obtained from the Swedish Cause of Death Register (CDR). The CDR was established in 1961 and records causes of death of Swedish citizens independently of whether the death occurred in Sweden or abroad.Citation38 Data on income, education, and emigration were obtained from the population registers held by Statistics Sweden.Citation39,Citation40
End points and follow-up
Primarily, ICD-O-3 codes were used to identify the end points, and the ICD-7 codes were used in the case of missing ICD-O-3 codes. The two end points of interest were diagnoses of colon cancer (ICD-7 code 153 and ICD-O-3 code C18) and lung cancer (ICD-7 codes 162.1 and 163, and ICD-O-3 codes C34 and C39).
This was an intention-to-treat analysis and follow-up started at the date of the first filled prescription between July 1, 2006, and December 31, 2012 (index date). Follow-up ended on the date of one of the following events: a filled prescription for a non-tablet formulation of a study medication, death, a diagnosis of one of the cancer end points of interest, date of emigration, or on December 31, 2013, whichever occurred first. Follow-up of the unexposed individuals started on the same day as for the exposed individual they were matched to.
Statistical analysis
The standardized difference was calculated to quantify the difference in demographics and clinical characteristics between the compared groups, defined as the difference in the mean between the two groups divided by the SD.Citation41
Continuous variables are presented with median and IQR or with mean and SD, whereas categorical variables are presented as numbers and proportions.
Differences in the incidence of the two cancers of interest between exposed and unexposed were compared by estimating incidence rate difference (IRD) and 95% CIs.
Cox proportional hazard models, with follow-up time as the underlying time scale, were used to estimate HRs with 95% CIs for the association between exposure and the cancer end points of interest. HRs were estimated using unadjusted, base, and fully adjusted models. The base model was adjusted for the matching variables by stratification. The full model was additionally adjusted for “a priori” selected variables, such as income, the level of attained education the year before the cohort inclusion date, and smoking status using a COPD diagnosis or a filled prescription for smoking cessation medication during up to 5 years prior to the index date as a proxy.Citation42 In addition, all the reported comorbidities, Charlson comorbidity index,Citation43 history of any cancer, and the number of observations in the NPR were evaluated for inclusion in the full model and only included in the model if they changed the point estimate by at least 10%.Citation44 Analyses were done overall, and stratum-specific by sex, follow-up time, and index year. In addition, overall analyses were performed using a 6-month lag time and a 12-month lag time. The proportional hazard assumption was tested for all analyses, and if violated, an interaction term between the exposure status and the follow-up time was included in the model.
A separate analysis that included only the exposed individuals was performed to compare individuals by cumulative defined daily dose (DDD) intervals (≤90, 91–182, 183–364, and ≥365). In this analysis, the person time contributed by each exposed individual was separated by subsequent and cumulative DDD intervals.
A sensitivity analysis was performed and included patients who started treatment with tolterodine, which is the most common antimuscarinic medication for the treatment of OAB in Sweden. Apart from the criteria for end of follow-up used in the main analysis, a filled prescription for a study medication other than tolterodine was added in the sensitivity analysis.
All data were analyzed with SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC, USA) and STATA 14 (StataCorp LP, College Station, TX, USA).
Ethical approval
This study was approved by the regional ethical board in Stockholm, Sweden (record numbers 2014/1478-31 and 2015/1669-32).
Results
Descriptive data
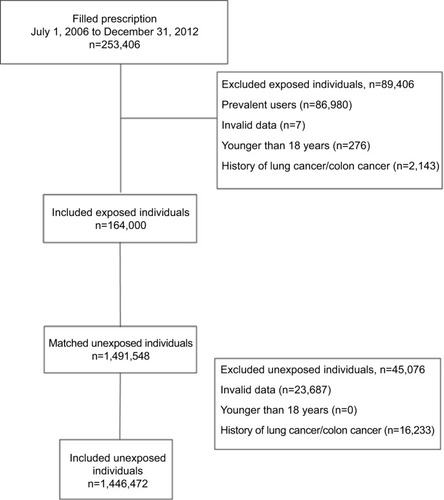
Overall, 253,406 exposed individuals were identified in the PDR (). Of those, 86,980 were considered to be prevalent users, 7 were excluded due to invalid data, 276 were younger than 18 years, and further 2,143 were excluded due to the diagnosis of lung cancer or colon cancer before the index date. In total, 164,000 exposed individuals were included. The exposed individuals were individually matched to a total of 1,491,548 unexposed individuals. There were 1,446,472 unexposed individuals included in the study after applying the exclusion criteria. On average, 8.8 unexposed individuals were matched to each exposed individual.
Among the exposed, 57.0% were women, with a corresponding proportion of 54.3% in the unexposed group (). The median age at index was 69 years in the exposed group and 68 years in the unexposed group. The median follow-up time, for both groups, was 47 months. A diagnosis of OAB was recorded in the NPR for 5.5% of the exposed individuals and for 1.2% of the unexposed individuals. The most common comorbid condition, in both groups, was hypertension, which was present in 45.6% of the exposed group and 39.0% in the unexposed group, with a standardized difference of 13%. All the investigated comorbid conditions were more common among the exposed individuals.
Table 1 Demographics and clinical characteristics of study subjects, both exposed and unexposed individuals
Outcomes
There were 659 (0.4%) and 911 (0.6%) exposed individuals who developed lung cancer and colon cancer, respectively (). The corresponding numbers in the unexposed group were 8,394 (0.6%) for lung cancer and 9,537 (0.7%) for colon cancer. The estimated IRD for lung cancer was −44.9 per 100,000 person-years (95% CI: −53.3, −36.5) and for colon cancer −26.1 per 100,000 person-years (95% CI: −35.8, −16.3) ().
Table 2 HRs and 95% CIs for the end points, overall, by sex and cumulative DDD
Lung cancer
The point estimates and 95% CIs of the HRs for the overall analysis of lung cancer in the three different intervals with time since index date (<1 year, 1–4 years, and ≥4 years) were 0.86 (95% CI: 0.75–0.98), 0.63 (95% CI: 0.56–0.70), and 0.43 (0.34–0.55), respectively (). When applying lag times, a tendency of decreasing point estimates was observed compared to the overall HRs observed in the main analysis, for which no lag time was used ().
In the stratum-specific analysis by sex, the observed point estimates of the HRs for men were all <1 in the follow-up time intervals (<1 year, 1–4 years, and ≥4 years): 0.90 (95% CI: 0.75–1.08), 0.62 (95% CI: 0.53–0.72), and 0.51 (95% CI: 0.37–0.70), respectively (). Similar observations were found for women. The observed HRs were as follows: 0.81 (95% CI: 0.66–0.99), 0.64 (95% CI: 0.54–0.74), and 0.37 (95% CI: 0.26–0.51), respectively.
When the HRs were estimated by cumulative DDD (≤90, 91–180, 181–364, and ≥365) using the lowest group as the reference group, the observed HRs were as follows: 0.96 (95% CI: 0.76–1.20), 1.01 (95% CI: 0.79–1.29), and 0.82 (95% CI: 0.67–1.00), respectively ().
In the stratum-specific analyses by groups of index years (2006–2008, 2009–2010, and 2011–2012), the observed HRs, as in the overall analysis, indicate an inverse association between fillings for antimuscarinic medications and lung cancer ().
Colon cancer
For colon cancer, the point estimates and 95% CIs for the overall HRs for the three different intervals for time since index date (<1 year, 1–4 years, and ≥4 years) were as follows: 0.91 (95% CI: 0.80–1.03), 0.81 (95% CI: 0.74–0.88), and 0.61 (95% CI: 0.51–0.73), respectively (). The observed estimated overall HRs were lower in the analyses where lag times were applied compared to the main analysis.
In the stratum-specific analyses by sex, all HRs for both men and women were <1. For men, the HRs in the different time intervals (<1 year, 1–4 years, and ≥4 years) were as follows: 0.94 (95% CI: 0.79–1.13), 0.81 (95% CI: 0.71–0.93), and 0.60 (95% CI: 0.46–0.78), respectively (). The observed estimates for women were 0.87 (95% CI: 0.72–1.05), 0.80 (95% CI: 0.70–0.91), and 0.62 (95% CI: 0.49–0.78), respectively.
The observed estimated HRs for the different cumulative DDD groups (≤90, 91–180, 181–364, and ≥365), using the lowest group as the reference group, were as follows: 0.99 (95% CI: 0.82–1.20), 1.02 (95% CI: 0.83–1.25), and 0.73 (95% CI: 0.61–0.87), respectively.
An inverse association was observed between filling a prescription for antimuscarinic medications and colon cancer for all the three index year groups ().
Sensitivity analyses
The point estimates of the HRs in the sensitivity analyses for lung cancer and for colon cancer were <1 in all the time intervals (<1 year, 1–4 years, and ≥4 years) (). For lung cancer, the HRs were as follows: 0.84 (95% CI: 0.69–1.03), 0.70 (95% CI: 0.61–0.82), and 0.40 (95% CI: 0.28–0.47), respectively. The corresponding estimates for colon cancer were as follows: 0.92 (95% CI: 0.77–1.11), 0.90 (95% CI: 0.80–1.02), and 0.75 (95% CI: 0.59–0.95), respectively.
Discussion
Key findings
This study observed an inverse association between filling prescriptions for antimuscarinic medications and a diagnosis of lung cancer or colon cancer. Inverse associations confirm the result from the previous Danish study, investigating the same associations and estimating SIRs.Citation27 The inverse association was stronger with the longest follow-up time of at least 4 years. During the first year, there were in general no substantial differences between the two exposure groups and the risk of the cancers of interest. Inverse associations between both follow-up time >1 year and filling 365 DDDs or more and the cancers of interest were observed. This indicates that it is of no or small importance if the exposure is reported as time since treatment start or as cumulative DDDs. However, this association was more pronounced for colon cancer than for lung cancer. A possible dose–response association is consistent with results from a study based on Danish data.Citation27 The observed estimates were on similar levels for both men and women, in both cancer types, indicating that there is no sex-based difference in the association between filling prescriptions for antimuscarinic medications and the cancer end points of interest. Similarities in observed associations between men and women are consistent with the findings from Denmark.Citation27 The observed inverse associations are in accordance with previous findings from preclinical studies showing an inhibitory effect of antimuscarinic substances, blocking the M3-receptor, on cell proliferation and cancer cell growth in lung cancer and colon cancer cell lines.Citation22,Citation24–Citation26
In the sensitivity analyses, inverse associations between exposure and lung or colon cancer were observed. The point estimates were on similar levels as observed in the main analyses, indicating no difference between the different antimuscarinic medications, demonstrating the robustness of our results.
Using no lag time for the main analysis was decided based on the preclinical studies reporting an inverse association. With an inverse association, there is no reason to suspect protopathic bias, the main reason for having a lag time.Citation45 However, when lag times were used, the point estimates decreased, indicating a stronger inverse association. The effect of lag times was most prominent during the first year of follow-up. Our observations of a decrease in the point estimates of the effect size with longer lag time confirm the results from a similar study investigating the association between proton pump inhibitors and gastric cancer.Citation45
Only about 6% of the exposed individuals had a recorded diagnosis of OAB, which is likely explained by the fact that the majority of the diagnoses of OAB are given in primary care, which is not covered by the NPR. The overall higher prevalence of comorbid conditions among the exposed individuals could, to some extent, be due to surveillance bias.
The results of this study indicate a potential protective effect of antimuscarinic medications for OAB on lung cancer and colon cancer. However, further studies are required to confirm the results found in this study. The included study medications should possibly, in future studies, be studied regarding a potential cancer-protective effect.
Strengths
A major strength of this study is the use of the Swedish population-based registers with high validity and close to complete coverage of the entire Swedish population, which contributed to the quality of the study, the large sample size, and the generalizability of the results.Citation37,Citation46,Citation47
The PDR records medications from filled prescriptions, which makes it more probable that the patient was actually exposed to the medication and eliminates the risk of recall bias regarding the exposure classification.Citation48 However, a filled prescription does not necessarily imply the use of a medication.
Limitations
One important limitation of this study is the lack of data on lifestyle factors such as smoking history and alcohol intake, well-known risk factors for cancer. However, the lack of information on smoking history was approached by using a diagnosis of COPD or a filled prescription for smoking cessation medication as a proxy for being a current or former smoker. However, this approach will most likely only have covered heavy smokers. The short follow-up time is also an important limitation of this study. As different cancer types have varying latency periods and the time from initiation to manifest malignancy is usually several years, ideally a long follow-up time is needed to make different assumptions about the risk of the outcome of interest and relevant exposure periods. Another possible limitation is the timing of the matching in relation to the application of exclusion criteria. The matching of unexposed individuals to the exposed individuals was done before exclusion. This lead to an uneven number of unexposed individuals matched to exposed individuals. However, this was done to avoid potential problems assigning index dates to the unexposed individuals since their index date was the same as the index date for the exposed individual they were matched to. Also, the inverse associations may to some extent be explained by the introduction of censoring due to filling a prescription of a non-tablet formulation of the study medication and as censoring only applied to the exposed individuals. It should, however, be noted that few individuals filled prescriptions for non-tablet formulations.
Conclusion
An inverse association between exposure to antimuscarinic medications, used in the treatment of OAB, and a diagnosis of colon cancer or lung cancer was observed. However, it is important to consider the relatively short follow-up time.
Author contributions
LL performed the statistical analysis and wrote the first draft of the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to express their gratitude to Sarah Burkill for her help with the language copy editing.
Supplementary materials
Table S1 ATC codes used to identify medications in the PDR used as disease proxies
Table S2 HR and 95% CIs for the end points, overall using 6-month and 12-month lag time, and by inclusion year using no lag time
Table S3 HR and 95% CIs for the end points for tolterodine
Disclosure
All the authors are employees of the Centre for Pharmacoepidemiology at Karolinska Institutet, which receives grants from several funding bodies for the performance of drug safety and drug utilization studies. These funding bodies had no role in the data collection and analysis and were not involved in the interpretation of results, writing, revision, or approval of the manuscript. The authors report no other conflicts of interest in this work.
References
- AbramsPCardozoLFallMThe standardisation of terminology of lower urinary tract function: report from the standardisation subcommittee of the international continence societyNeurourol Urodyn200221216717811857671
- MilsomIAbramsPCardozoLRobertsRGThüroffJWeinAJHow widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence studyBJU Int200187976076611412210
- IrwinDEMilsomIHunskaarSPopulation-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC studyEur Urol20065061306131517049716
- DallossoHMMcGrotherCWMatthewsRJDonaldsonMMLeicestershire MRC Incontinence Study GroupThe association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in womenBJU Int2003921697712823386
- Mayo ClinicOveractive bladder – risk factors2014 https://www.mayoclinic.org/diseases-conditions/overactive-bladder/symptoms-causes/syc-20355715Accessed May 22, 2016
- AnderssonKEAntimuscarinics for treatment of overactive bladderLancet Neurol200431465314693111
- WangPLuthinGRRuggieriMRMuscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteinsJ Pharmacol Exp Ther199527329599667752101
- RayfordWNobleMJAustenfeldMAWeigelJMebustWKShahGVMuscarinic cholinergic receptors promote growth of human prostate cancer cellsProstate19973031601669122040
- FruchtHJensenRTDexterDYangWLXiaoYHuman colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptorClin Cancer Res1999592532253910499630
- SpindelERMuscarinic receptor agonists and antagonists effects on cancerHandb Exp Pharmacol2012208451468
- SongPSekhonHSJiaYAcetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinomaCancer Res200363121422112517800
- AshkenaziARamachandranJCaponDJAcetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypesNature198934062291461502739737
- GuizzettiMCostaPPetersJCostaLGAcetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cellsEur J Pharmacol199629732652738666059
- EspañolAJde La TorreEFiszmanGLSalesMERole of non-neuronal cholinergic system in breast cancer progressionLife Sci20078024–252281228517276463
- FritzSWesslerIBreitlingRExpression of muscarinic receptor types in the primate ovary and evidence for nonneuronal acetylcholine synthesisJ Clin Endocrinol Metab200186134935411232023
- OppitzMMöbusVBrockSDrewsUMuscarinic receptors in cell lines from ovarian carcinoma: negative correlation with survival of patientsGynecol Oncol200285115916411925137
- BatraSPopperLDIosifCSCharacterisation of muscarinic cholinergic receptors in human ovaries, ovarian tumours and tumour cell linesEur J Cancer199329913021306
- BossAOppitzMLippertGDrewsUMuscarinic cholinergic receptors in the human melanoma cell line SK-Mel 28: modulation of chemotaxisClin Exp Dermatol200530555756416045692
- WangLZhiXZhangQMuscarinic receptor M3 mediates cell proliferation induced by acetylcholine and contributes to apoptosis in gastric cancerTumour Biol20163722105211726346168
- LiuPSChenYYFengCKLinYHYuTCMuscarinic acetylcholine receptors present in human osteoblast and bone tissueEur J Pharmacol20116501344020888332
- KawashimaKFujiiTExtraneuronal cholinergic system in lymphocytesPharmacol Ther2000861294810760545
- SongPSekhonHSLuAM3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretionCancer Res20076783936394417440109
- SongPMaierMSpindelJOlivasASpindelEInhibition of lung cancer cell growth by tiotropium: mechanism of actionB28 Lung Cancer Biomarkers of RiskAm Thoracic Soc2009A2675
- von RosenvingeECChengKDrachenbergCBBedside to bench: role of muscarinic receptor activation in ultrarapid growth of colorectal cancer in a patient with pheochromocytomaMayo Clin Proc201388111340134624100192
- XuRShangCZhaoJActivation of M3 muscarinic receptor by acetylcholine promotes non-small cell lung cancer cell proliferation and invasion via EGFR/PI3K/AKT pathwayTumour Biol20153664091410025964092
- HuaNWeiXLiuXA novel muscarinic antagonist R2HBJJ inhibits non-small cell lung cancer cell growth and arrests the cell cycle in G0/G1PLoS One2012712e5317023285263
- HallasJMargulisAVPottegårdAIncidence of common cancers in users of antimuscarinic medications for overactive bladder: a Danish nationwide cohort studyBasic Clin Pharmacol Toxicol2018122661261929345103
- Mayo Clinic [webpage on the Internet]Lung cancer2018 Available from: https://www.mayoclinic.org/diseases-conditions/lung-cancer/symptoms-causes/syc-20374620Accessed November 19, 2018
- Mayo Clinic [webpage on the Internet]Colon cancer2018 Available from: https://www.mayoclinic.org/diseases-conditions/colon-cancer/symptoms-causes/syc-20353669Accessed November 19, 2018
- National Cancer Institute [webpage on the Internet]Alcohol and cancer risk2018 Available from: https://www.cancer.gov/about-cancer/causes-prevention/risk/alcohol/alcohol-fact-sheetAccessed December 7, 2018
- LudvigssonJFOtterblad-OlaussonPPetterssonBUEkbomAThe Swedish personal identity number: possibilities and pitfalls in healthcare and medical researchEur J Epidemiol2009241165966719504049
- FuruKWettermarkBAndersenMMartikainenJEAlmarsdottirABSørensenHTThe Nordic countries as a cohort for pharmacoepidemiological researchBasic Clin Pharmacol Toxicol20101062869419961477
- WettermarkBHammarNForedCMThe new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six monthsPharmacoepidemiol Drug Saf200716772673516897791
- Socialstyrelsen – Swedish National Board of Health and Welfare [webpage on the Internet]The Swedish Cancer Register2017 Available from: https://www.socialstyrelsen.se/register/halsodataregister/cancerregistret/inenglishAccessed October 26, 2017
- Socialstyrelsen – Swedish National Board of Health and WelfareKodning i Cancerregistret – Handledning 2015Stockholm, SwedenSocialstyrelsen – Swedish National Board of Health and Welfare2015
- Socialstyrelsen – Swedish National Board of Health and Welfare [webpage on the Internet]The Swedish National Patient Register2017 Available from: https://www.socialstyrelsen.se/register/halsoda-taregister/patientregistret/inenglishAccessed October 26, 2017
- LudvigssonJFAnderssonEEkbomAExternal review and validation of the Swedish national inpatient registerBMC Public Health201111145021658213
- Socialstyrelsen – Swedish National Board of Health and Welfare [webpage on the Internet]The Swedish Cause of Death Register2017 Available from: http://www.socialstyrelsen.se/register/dodsorsaksreg-istretAccessed October 26, 2017
- Statistiska Centralbyrån (SCB) – Statistics Sweden [webpage on the Internet]The Total Population Register2017 Available from: http://www.scb.se/sv_/Vara-tjanster/Bestalla-mikrodata/Vilka-mikrodata-finns/Registret-over-totalbefolkningen-RTB/Accessed October 26, 2017
- Statistiska Centralbyrån (SCB) – Statistics Sweden [homepage on the Internet]Longitudinal integration database for health insurance and labour market studies2017 Available from: https://www.scb.se/contentassets/8f66bcf5abc34d0b98afa4fcbfc0e060/rtb-bar-2016-eng.pdfAccessed May 30, 2017
- AustinPCUsing the standardized difference to compare the prevalence of a binary variable between two groups in observational researchCommun Stat Simul Comput200938612281234
- TerzikhanNVerhammeKMHofmanAStrickerBHBrusselleGGLahousseLPrevalence and incidence of COPD in smokers and non-smokers: the Rotterdam StudyEur J Epidemiol201631878579226946425
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- GreenlandSModeling and variable selection in epidemiologic analysisAm J Public Health19897933403492916724
- TamimHMonfaredAALelorierJApplication of lag-time into exposure definitions to control for protopathic biasPharmacoepidemiol Drug Saf200716325025817245804
- LudvigssonJFAlmqvistCBonamyAKRegisters of the Swedish total population and their use in medical researchEur J Epidemiol201631212513626769609
- RosénMNational health data registers: a Nordic heritage to public healthScand J Public Health2002302818512028856
- StephanssonOGranathFSvenssonTHaglundBEkbomAKielerHDrug use during pregnancy in Sweden – assessed by the Prescribed Drug Register and the Medical Birth RegisterClin Epidemiol20113435021386973