Abstract
Importance
Prevention of primary breast cancer (BCa) in women is of great public health importance. The existing results from observational epidemiologic studies focused on the association between bisphosphonates and primary BCa risk have been inconsistent.
Objective
To update this systematic review and meta-analysis to assess the effect of bisphosphonates on primary BCa risk.
Data sources
We comprehensively searched MEDLINE, EMBASE, Cochrane libraries, ProQuest, and Web of Science through June 25, 2018 for relevant studies.
Study selection
Epidemiological studies that assessed the effect of bisphosphonates on the risk of primary BCa in women.
Data extraction and synthesis
We reported this meta-analysis according to the PRISMA guidelines. Available multivariable-adjusted effect estimates and corresponding 95% CIs were pooled with a random-effects model.
Main outcomes and measures
The prespecified main outcome was the risk of primary BCa.
Results
In total, five cohort studies involving 657,558 women and 12,991 primary BCa patients, three population-based case-control studies involving 54,701 primary BCa cases and 237,962 healthy controls and two randomized controlled trials (RCTs) involving 13,774 women and 165 primary BCa patients were included in this meta-analysis. Bisphosphonates were associated with a 12% decreased risk of primary BCa (RR, 0.88; 95% CI, 0.83–0.94). However, when we analyzed study designs separately, the pooled results from observational studies were inconsistent with that from RCTs. The observed association of primary BCa risk with long-term use (≥1 year) of bisphosphonates seemed to be more robust and stronger than that of short-term use (<1 year) (RR, 0.75; 95% CI, 0.66–0.84; and 0.90; 95% CI, 0.84–0.97; respectively).
Conclusion
This meta-analysis adds to the body of evidence for an association between bisphosphonates and a significantly decreased risk of primary BCa. However, future large-scale RCTs are required to validate this concern.
Introduction
Breast cancer (BCa) is the most frequently diagnosed cancer and the leading cause of cancer deaths among females worldwide,Citation1 with an estimated 2,088,849 newly diagnosed cases in 2018. Incidence rates are highest in economically developed countries but are now increasing rapidly in developing countries as well.Citation2 Prevention of primary BCa in women is of great public health importance. Bisphosphonates are widely used for the prevention and treatment of osteoporosis.Citation3,Citation4 Recently, an increasing body of evidence has shown that bisphosphonates have potential anticancer properties against the risk of developing primary BCa. Although the precise biologic mechanisms that link bisphosphonates and BCa risk remain unclear, an increasing body of evidence supports the notion that bisphosphonates may modify the tumour cell microenvironment and directly affect tumour cells through a variety of pathways, including inhibition of osteolysis and release of bone-derived growth factors,Citation5 inhibition of protein prenylation,Citation6,Citation7 and angiogenesis inhibition,Citation8,Citation9 enhanced immune surveillance by activation of gamma delta T cells,Citation10,Citation11 direct inhibition of tumour cell growth, proliferation, adhesion and invasion, and induction of apoptosis.Citation12,Citation13
To date, several epidemiologic studies have assessed the relationship between bisphosphonates and BCa risk. However, the findings have been inconsistent. Recently, a report from two randomized controlled trials (RCTs, Fracture Intervention Trial (FIT) and Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly–Pivotal Fracture Trial (HORIZON-PFT)) has showed that bisphosphonates could not decrease the incidence risk of primary BCa.Citation14 In our previous meta-analysis, which included four observational studies, we reported a significant protective effect of bisphosphonates on the risk of BCa.Citation15 Since then, however, three large cohort studiesCitation16–Citation18 involving 95,701 participants and 1,079 BCa patients and one large nested case-control studyCitation19 involving 49,933 BCa patients and 232,780 controls have been published. We therefore performed an updated comprehensive systematic review and meta-analysis to determine and quantify the potential antitumor effect of bisphosphonates on the risk of primary BCa.
Methods
Search strategy and selection criteria
This meta-analysis was registered on PROSPERO (CRD42014014901) and was reported according to the PRISMA guidelines (the checklist was provided as Appendix A).Citation20 We systematically searched the electronic databases of MEDLINE, EMBASE, ProQuest, and Web of Science through August 15, 2016 to identify relevant publications. We used a combination of Medical Subject Headings (MeSH) terms and corresponding free-text terms as follows: (diphosphonates[MeSH Terms] or diphosphonate or bisphosphonate or alendron* or etidron* or clodron* or zoledron* or risedron* or ibandron* or pamidron* or tiludron* or neridron* or olpadron*) and (breast neoplasms[MeSH Terms] or breast neoplasm or breast cancer or breast tumor or mammary carcinoma or mammary neoplasm). The reference sections and citation lists of the retrieved literature, including original research articles, reviews, editorials, and letters, were manually reviewed for potentially relevant articles. An updated search was performed (from August 16, 2016, through June 25, 2018) to identify new literatures.
We included studies that met the following inclusion criteria: 1) epidemiological studies (including case-control or cohort studies, or RCTs) that addressed the potential effect of bisphosphonates on primary BCa risk; 2) studies that reported effect estimates, which included the RRs, ORs and HR with 95% CIs, or reported sufficient information to calculate these values; 3) when multiple articles were published from the same population, we included the most recent or complete publication or the one with the highest quality score; and 4) studies that addressed the association between bisphosphonates and contralateral BCa risk in women with primary BCa were excluded from the main analysis but included in a sensitivity analysis. There were no restrictions on language, sample size, or participant characteristics.
Data extraction and quality assessment
The eligibility determination, data extraction, and quality assessment for each study were performed independently by two authors (YP.L. and XS.Z.). Disagreements were resolved by discussion and consensus with a third author (HR.S.). Standard electronic forms specifically created for the present study were used to record the following information: authors, publication date, characteristics of participants (country, age and menopausal status), bisphosphonate exposure (type, dose, frequency, and duration), and studies (study design, sample size, and the controlled or adjusted potential confounding factors). Both the maximally and minimally adjusted effect sizes with 95% CIs were recorded, if available. The extracted data from each study was carefully checked and verified before performing meta-analysis. When necessary, we contacted authors of studies for missing information.
A quality-scoring system, which was partially derived from that developed by Voskuil and colleagues and included 15 items (5 on selection bias, 8 on misclassification bias, and 2 on confounding bias) with a maximum score of 95, was adopted to assess the methodological quality of observational epidemiological studies.Citation21 The quality score of each study was presented as a percentage of the maximum score, and studies with a score of >70% were categorized as high-quality studies (Appendix B). The Jadad scoreCitation22 and Cochrane risk of bias toolCitation23 were used to assess the quality of RCTs.
Statistical analysis
In this meta-analysis, the maximally adjusted effect sizes and 95% CIs were pooled using random-effects models, rather than fixed-effects models, to analyze the results in a conservative manner. When an individual study only reported effect estimates for each type of bisphosphonate separatelyCitation24 or separately reported risk estimates in ductal carcinoma in situ and invasive BCa,Citation25 likely to account for a higher relative weight in the pooled analysis, we initially combined the subgroup datasets into a study-specific effect estimate with a fixed-effects model.Citation26 To evaluate whether a duration-dependent threshold effect of bisphosphonates on primary BCa risk exists, we conducted a cumulative meta-analysis based on the medication duration across the included studies. We estimated the between-study heterogeneity using the Q test and the I2 Statistic.Citation27,Citation28 To investigate potential sources of the between-study heterogeneity, we performed subgroup and meta-regression analyses using random-effects models.
For sensitivity analyses, we removed the most relatively weighted study from each subgroup analysis to assess its influence on the summarized estimates and to explore potential sources of heterogeneity. We then conducted another sensitivity analysis using the fixed-effects model. In addition, to test whether the underlying confounders that were adjusted or controlled in the original observational studies could have influenced the effect of bisphosphonates on primary BCa risk, we conducted repetition analyses using the minimally adjusted data and subsequently analyzed the confounding RR, which was defined as the ratio of the pooled results of the maximally and minimally adjusted data.Citation29 Potential impact of an unmeasured confounder on the observed results was calculated presenting E-values for point estimates and the upper limit of the CIs as recently suggested by VanderWeele and colleagues.Citation30 We performed a sensitivity analysis to assess the association between bisphosphonates and the risk of developing contralateral BCa among primary BCa patients. We also performed a sensitivity analysis to include only the prospective studies (including cohort studies, RCTs and nested case-control studies), because the prospective nature of cohort studies and RCTs is invaluable for confirming the temporal sequence of bisphosphonate use and primary BCa onset and therefore helps to examine causal associations.
To determine the evidence strength of the association between bisphosphonates and the incidence risk of primary BCa, an evidence synthesis modified from previous studies was developed.Citation21 To assess potential publication bias, we used funnel plots for asymmetry and, formally, used the Begg’s rank correlation and Egger’s linear regression tests.Citation31 Furthermore, we robustly adjusted for the summarized results by applying the Duval and Tweedie’s trim and fill method.Citation32 We did all analyses with Comprehensive Meta Analysis version 2.2.046 (Biostat, Englewood, NJ, USA). All tests were two-sided. Statistical significance was defined as P-values <0.05.
Results
Characteristics and reporting quality of included studies
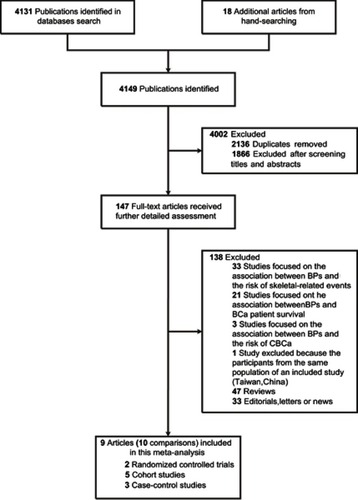
The search strategy identified 10 eligible studies (). The main characteristics of 10 studies included are provided in Tables S1–S3. Briefly, there were five cohort studies,Citation16–Citation18,Citation24,Citation25 three population-based case-control studiesCitation19,Citation33,Citation34 and two RCTs.Citation14 The two RCTs enrolled 13,774 participants, with a total of 165 primary BCa cases. The five cohort studies enrolled 657,558 participants, with mean follow-up periods ranging from 2.9 to 8.5 years and 12,991 cases, while the three case-control studies included 54,701 cases and 237,962 controls. Overall, these 10 studies enrolled 67,857 primary BCa cases and 188,685 bisphosphonate users. Of these, seven studiesCitation14,Citation18,Citation24,Citation25,Citation33,Citation34 focused on BCa risk, and the other three studiesCitation16,Citation17,Citation19 assessed the association between bisphosphonates and the risk of developing various cancers, including BCa. Among these studies, the majority of the participants were postmenopausal women, and the mean age ranged from 54.2 to 73.5 years. All but one studyCitation33 reported the type of bisphosphonate used, of which alendronate was the most common (range, 51.8–100%).
Bisphosphonates and primary BCa risk
Overall, we noted a 12% risk reduction of primary BCa in women who used bisphosphonates compared with non-users (RR, 0.88; 95% CI, 0.83–0.94; and ). Considering the study design separately, we found similar associations in cohort studies and case-control studies (OR, 0.83; 95% CI, 0.71–0.98; RR, 0.87; 95% CI, 0.80–0.95; respectively); however, the association was not found in RCTs (RR, 1.13; 95% CI, 0.81–1.57). When restricted to invasive BCa or postmenopausal women, the pooled RRs were 0.83 (95% CI, 0.69–0.99; Figure S1) and 0.89 (95% CI, 0.80–0.99; Figure S2), respectively.
Figure 2 Forest plot for the association between bisphosphonates and primary breast cancer risk. Square markers indicate effect sizes of each study; horizontal lines, the 95% CI. The diamond data marker indicates the summarized effect size. The vertical solid line indicates the overall pooled effect. Note that the studies are ranked in order of their relative weights from random effects analysis.
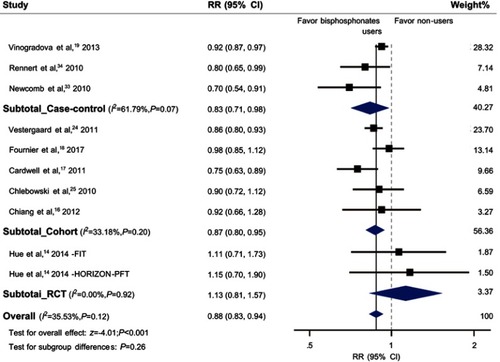
Table 1 The pooled association between bisphosphonates and breast cancer risk
Duration-response analysis of bisphosphonates and primary BCa risk
Eight studiesCitation14,Citation17–Citation19,Citation25,Citation33,Citation34 were included in this duration-response analysis. Subgroup analyses ( and Figure S3) showed that women who used bisphosphonates for ≥1 year before diagnosis had a 25% reduced risk of BCa (RR, 0.75; 95% CI, 0.66–0.84), whereas those who received bisphosphonates for <1 year had an attenuated risk reduction (RR, 0.90; 95% CI, 0.84–0.97). Similarly, cumulative meta-analysis based on duration of bisphosphonate use demonstrated that a 10% risk reduction of primary BCa was observed when the duration of medication usage reached 1 year (RR, 0.90; 95% CI, 0.84–0.97; Figure S4). Thereafter, the observed protective effect did not change substantially with increasing years of medication usage, although subsequent datasets have increased the precision of the magnitude of the effect.
Sensitivity analyses
We performed a repetition analysis with fixed-effects models and found no significant change in the pooled results (). Furthermore, a repetition analysis using the minimally adjusted data from original studies was also carried out, and confounding RRs demonstrated that the summarized results did not significantly differ from those with the maximally adjusted data (Table S4). Moreover, confounding RR values indicated that the summarized results with the maximally adjusted data were more conservative. According to the results from E-value sensitivity analyses, E-values for both the observed point estimates and the upper limit of the CIs were all small (), indicating that little unmeasured confounding would be needed to explain away the observed association estimates. Another sensitivity analysis excluding the study with the most relative weight in each subgroup did not result in a substantial change of the summarized results (Table S5), except for subgroup analyses of cohort studies, bisphosphonate use duration of <1 year, 2–3 years or >4 years. We also performed a sensitivity analysis by excluding retrospective case-control studies and found a RR of 0.90 (95% CI, 0.85–0.95; Figure S5). Another sensitivity analysis that excluded the RCTs showed a RR of 0.87 (95% CI, 0.82–0.93; Figure S6).
Another three observational studies assessed the impact of bisphosphonates on risk of second primary contralateral BCa among BCa survivors.Citation35–Citation37 The characteristics of these studies were shown in Tables S6 and S7. When these results were pooled together, the RR was 0.84 (95% CI, 0.66–1.07; Figure S7). By including these studies, we performed another sensitivity analysis and found a RR of 0.88 (95% CI, 0.84–0.93; Figure S8).
Heterogeneity
In general, there was no obvious between-study heterogeneity (P=0.12; I2=35.53%). When considering study design, we observed moderate heterogeneity across the case-control studies (P=0.07; I2=61.79%), but not across the cohort studies (P=0.20; I2=33.18%) or the RCTs (P=0.92; I2=0.00%). Based on results from meta-regression analyses, we found that the between-study heterogeneity was not relevant to the mean age of participants (P=0.65, Figure S9A), quality score (P=0.76, Figure S9B), the number of BCa patients (P=0.11, Figure S9C), or bisphosphonate users (P=0.56, Figure S9D).
Publication bias
We found limited evidence for publication bias using funnel plots (Figure S10), Begg’s rank correlation or Egger’s regression test (Table S8). In addition, the adjusted summary effect size analyzed using the trim and fill method did not demonstrate a substantial change, which also implies no evidence of publication bias.
Discussion
This meta-analysis has strengthened the evidence for anticancer effects of bisphosphonates in BCa primary prevention. We found a 12% risk reduction of BCa associated with bisphosphonates. However, according to the evidence synthesis, there is a general “indecisive” evidence for this association (Table S9).
Bisphosphonates have predominantly been used in women with osteoporosis who typically have a low bone mineral density (BMD) level, which is caused by a low level of circulating estrogen that is associated with BCa riskCitation38 and may result in confounding by indication in observational studies.Citation14 Consequently, it is vital to determine whether the association of risk reduction of BCa with bisphosphonates is merely responsible for the inverse relationship between osteoporosis, the indication of bisphosphonate therapy, and BCa risk. Eight of the 10 analyzed studies have adjusted for BMD statusCitation14 or other factors that reflect the level of BMD between bisphosphonate users and non-users, including the 5-year hip fracture scoreCitation25 and history of osteoporosis or fracture.Citation16–Citation19,Citation33 When we pooled the results of these eight studies together, we found that a decreased risk of BCa remained associated with the use of bisphosphonate, which supports the evidence for an authentic inverse association between bisphosphonates and BCa risk, independent of indications of bisphosphonates. Menopausal status can influence the levels of estrogens in women, as circulating concentrations of estrogens decrease markedly after menopause.Citation39 Among the studies included, enrolled participants were predominantly postmenopausal women, and none of these studies reported an effect in premenopausal women separately. When we limited participants to postmenopausal women, we found a similar inverse association between bisphosphonates and BCa risk.
Two previous meta-analyses have focused on the same issues. In our previous meta-analysis that included only 4 studies, we reported a 15% risk reduction of primary BCa related with bisphosphonates.Citation15 Thereafter, another meta-analysis by Ou et al reported a very similar effect estimate of 16% risk reduction associated with bisphosphonates.Citation40 The pooled effect size of our current study is smaller than those in these two previous meta-analyses. The possible reason may be that neither of the previous meta-analyses had taken into account the RCT results.
As commonly known, results from observational epidemiological studies cannot confirm causality.Citation41 However, it is possibly difficult to conduct an RCT to address the effect of bisphosphonates in the primary prevention of BCa due to ethical challenges and the requirement for a considerably large sample size.Citation41 Recently, the results from two RCTs (FIT and HORIZON-PFT) were published.Citation14 Hue and colleagues showed that 3–4 years of bisphosphonate treatment did not decrease the risk of invasive BCa in postmenopausal women.Citation14 Of note, the two RCTs were at high risk of selective reporting bias according to the Cochrane Collaboration recommendation (Figures S11A and B), since they were not initially designed to study BCa outcomes as the authors stated.Citation14 The data about incident BCa were collected from adverse event databases, thus, the results were prone to detection bias. Because women assigned to bisphosphonate therapy group were more likely to visit clinics or be hospitalized and subsequently had higher incidence rate of newly diagnosed BCa, since the number of patients with adverse events were significantly higher in the bisphosphonate treatment group compared with the placebo control group (95.5% vs 93.9%, P=0.002).Citation42 Additionally, given the limited number of BCa patients during the follow-up period and the limited statistical power (in the Supplementary), the findings from the two RCTs (even those that were very well done) are not sufficient to overturn the findings from observational studies.
Three observational studies assessed the impact of bisphosphonates on contralateral BCa risk among BCa survivors.Citation35–Citation37 When pooling these results together, we did not find an obvious association, which is consistent with the results from a meta-analysis of individual patient data from RCTs that showed no significant effect on contralateral BCa.Citation43 These findings provide no support for bisphosphonate treatment as BCa chemoprevention strategy. It is supposed that bisphosphonates may have different effects on healthy women (as average-risk populations of developing BCa) or on women with primary BCa.
EightCitation14,Citation17–Citation19,Citation25,Citation33,Citation34 of the studies included assessed the relationship between bisphosphonate use duration (from <1 to >5 years) and primary BCa risk, although the findings were inconsistent. The controversy has focused on the optimum duration of bisphosphonate use, the magnitude of the beneficial effect and possible harm from long-term use of bisphosphonates. Subgroup analyses based on the medication periods suggested that, compared to non-users, women who used bisphosphonates for <1 year had a 10% decreased risk of primary BCa, whereas women with ≥1 year of bisphosphonate use had a 25% lower risk of primary BCa. However, in the subgroup of <1 year of bisphosphonate use, a sensitivity analysis excluding Vinogradova’ study, which had 84.37% relative weight, showed no significant effect of bisphosphonates on primary BCa risk. Given the limited number of studies included in each subgroup, the results from subgroup analyses should not be overemphasized. Consistent with the findings from subgroup analyses, cumulative meta-analyses showed that this observed beneficial effect became robust only when the treatment duration reached 1 year, and thereafter the effect tended to stabilize over time. Interestingly, this result is in accordance with both the anti-resorptive properties of bisphosphonates, which were only observed after at least 6 months of use,Citation44 and the pharmacokinetics of bisphosphonates, by which, once entering bone, the drug sustains a stable anti-resorptive potency for several years.Citation45
Given the global use of bisphosphonates among millions of women over the past 20 years, it can be concluded that adverse effects related to bisphosphonates are relatively low.Citation46 Commonly reported side-effects are mild and include dyspepsia, nausea, and oesophagitis, whereas serious side-effects including osteonecrosis of the jaw, renal toxicity, and oesophageal cancer are rare. However, whether these serious side-effects are attributable to bisphosphonates alone remain inconclusive.Citation26,Citation47–Citation49 Another concern is the long-term (>5 years) side-effects of bisphosphonates, and few studies have addressed this issue.Citation50 Additional well-designed prospective studies are still needed to evaluate potential long-term adverse effects and determine whether the benefit of bisphosphonate therapy could outweigh possible disadvantages. Based on the current evidence, the benefits from bisphosphonate treatment for 1–5 years significantly outweigh the potential harm from these drugs.
Unlike tamoxifen, which has previously been shown to be efficient in the primary and relapse prevention of BCaCitation51–Citation53 but has “exceptionally low” acceptance and prevalence of use for chemoprevention,Citation51,Citation54 bisphosphonates should have a substantial and extensive public health benefit for the prevention of primary BCa because bisphosphonates are already widely prescribed for the prevention and treatment of osteoporosis or cancer-induced bone lossCitation10–Citation13 and an increasing number of bisphosphonate users worldwide are expectable. For instance, approximately 40 million prescriptions for bisphosphonates are written every year in the United States alone.Citation55
The current meta-analysis had some strengths. First, a large number of participants were enrolled in the studies included, which added validity to this analysis. Second, we performed extensive sensitivity analyses, including confounding RR, E-value and subgroup analyses, to test the robustness of our results. Finally, we performed subgroup analysis and meta-regression analysis to explore the potential causes of heterogeneity. However, several limitations of this study should be considered. First, various types of bisphosphonates were given to women in the individual studies, which could have contributed to heterogeneity; of these drugs, alendronate, clodronate, and zoledronic acid were the major bisphosphonates prescribed. Although the type of bisphosphonate used may have differing antitumor activities between nitrogen-containing bisphosphonates and non-nitrogen-containing bisphosphonates, as demonstrated by mechanistic studies in vitro and in vivo models,Citation56,Citation57 we could not explore this differential effect due to the lack of available data. Although currently unclear, researches on which bisphosphonates are better at reducing the risk of primary BCa than others are needed. Another potential limitation was the residual confounding factors that had not been taken into account in the original studies. This concern was assessed with E-values. Importantly, the E-values of the effect estimates are small, indicating that our findings are not very robust and incontrovertible. In this respect, RCTs are invaluable for controlling various biases including residual confounding biases. Additionally, given that results from some sensitivity analyses did not support this evidence of the association, our findings should be interpreted with caution.
Conclusion
In conclusion, this meta-analysis adds to the body of evidence for an association between bisphosphonates and a decreased risk of primary BCa. However, due to the lack of consistency between the results from observational studies and that from RCTs, future large-scale RCTs that focus on BCa primary prevention are needed.
Key points
Question: Does the use of bisphosphonates reduce the risk of primary breast cancer in women?
Findings: In this updated systematic review and meta-analysis that included five large cohorts, three large population-based case-control studies and two large-randomized controlled trials, in which 67,857 primary breast cancer patients and 188,685 bisphosphonate users involved, a 12% lower risk of primary breast cancer was associated with the use of bisphosphonates.
Meaning: These findings provide support for the use of bisphosphonates as BCa chemoprevention strategy, however, future randomized controlled trials with large sample sizes are required to validate this concern.
Author contributions
All authors had full access to the data in the study and take responsibility for the integrity of the data and accuracy of data analysis. YL, XZ and HS contributed equally to this work and should be considered co-first authors. Study concept and design: YL, SZ, YXZ, XZ, QZ and YSZ. Acquisition, analysis, or interpretation of data: YL, XZ, HS, SZ and YXZ. Drafting of the manuscript: YL, XZ, QZ and YSZ. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: YL, SZ, HS, YXZ and YSZ. Administrative, technical, or material support: YL, QZ and YSZ. Study supervision: YL, QZ and YSZ. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors acknowledge Justina Ucheojor Onwuka for her help in English language copy editing. This work was supported by The Seed Project Funds of Public Health School of Harbin Medical University (grant number 2012-05 to YL) and by a grant from The Harbin Medical University Innovation Research Foundation (2016JCZX14 to YL; 2016JCZX20 to YXZ). The funders and sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. doi:10.3322/caac.21492
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 V1.1, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2014 Available from: http://globocan.iarc.fr, Accessed January 16, 2018
- Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2005;353(6):595–603. doi:10.1056/NEJMcp04380116093468
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom. 2008;11(4):473–477. doi:10.1016/j.jocd.2008.04.00318562228
- Bedard PL, Body JJ, Piccart-Gebhart MJ. Sowing the soil for cure? Results of the ABCSG-12 trial open a new chapter in the evolving adjuvant bisphosphonate story in early breast cancer. J Clin Oncol. 2009;27(25):4043–4046. doi:10.1200/JCO.2008.21.490819652062
- Green JR. Antitumor effects of bisphosphonates. Cancer. 2003;97(3 Suppl):840–847. doi:10.1002/cncr.1112812548584
- Wiemer AJ, Hohl RJ, Wiemer DF. The intermediate enzymes of isoprenoid metabolism as anticancer targets. Anticancer Agents Med Chem. 2009;9(5):526–542.19519294
- Santini D, Vincenzi B, Galluzzo S, et al. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res. 2007;13(15 Pt 1):4482–4486. doi:10.1158/1078-0432.CCR-07-055117671133
- Stresing V, Fournier PG, Bellahcene A, et al. Nitrogen-containing bisphosphonates can inhibit angiogenesis in vivo without the involvement of farnesyl pyrophosphate synthase. Bone. 2011;48(2):259–266. doi:10.1016/j.bone.2010.09.03520920623
- Dieli F, Gebbia N, Poccia F, et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102(6):2310–2311. doi:10.1182/blood-2003-05-165512959943
- Benzaid I, Monkkonen H, Stresing V, et al. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011;71(13):4562–4572. doi:10.1158/0008-5472.CAN-10-386221646473
- Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34(5):453–475. doi:10.1016/j.ctrv.2008.02.00418423992
- Clezardin P. Bisphosphonates’ antitumor activity: an unravelled side of a multifaceted drug class. Bone. 2011;48(1):71–79. doi:10.1016/j.bone.2010.07.01620655399
- Hue TF, Cummings SR, Cauley JA, et al. Effect of bisphosphonate use on risk of postmenopausal breast cancer: results from the randomized clinical trials of alendronate and zoledronic Acid. JAMA Intern Med. 2014;174(10):1550–1557. doi:10.1001/jamainternmed.2014.363425111880
- Liu Y, Zhao S, Chen W, et al. Bisphosphonate use and the risk of breast cancer: a meta-analysis of published literature. Clin Breast Cancer. 2012;12(4):276–281. doi:10.1016/j.clbc.2012.04.00322622199
- Chiang CH, Huang CC, Chan WL, et al. Oral alendronate use and risk of cancer in postmenopausal women with osteoporosis: anationwide study. J Bone Miner Res. 2012;27(9):1951–1958. doi:10.1002/jbmr.164522532232
- Cardwell CR, Abnet CC, Veal P, Hughes CM, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of cancer. Int J Cancer. 2012;131(5):E717–E725. doi:10.1002/ijc.2738922161552
- Fournier A, Mesrine S, Gelot A, et al. Use of bisphosphonates and risk of breast cancer in a French cohort of postmenopausal women. J Clin Oncol. 2017;35(28):3230–3239. doi:10.1200/JCO.2016.71.433728708471
- Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to bisphosphonates and risk of common non-gastrointestinal cancers: series of nested case-control studies using two primary-care databases. Br J Cancer. 2013;109(3):795–806. doi:10.1038/bjc.2013.38323868009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269.19622511
- Voskuil DW, Monninkhof EM, Elias SG, Vlems FA, van Leeuwen FE. Physical activity and endometrial cancer risk, a systematic review of current evidence. Cancer Epidemiol Biomarkers Prev. 2007;16(4):639–648. doi:10.1158/1055-9965.EPI-06-074217416752
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.8721797
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi:10.1136/bmj.d592822008217
- Vestergaard P, Fischer L, Mele M, Mosekilde L, Christiansen P. Use of bisphosphonates and risk of breast cancer. Calcif Tissue Int. 2011;88(4):255–262. doi:10.1007/s00223-011-9463-721253712
- Chlebowski RT, Chen Z, Cauley JA, et al. Oral bisphosphonate use and breast cancer incidence in postmenopausal women. J Clin Oncol. 2010;28(22):3582–3590. doi:10.1200/JCO.2010.28.209520567009
- Sun K, Liu JM, Sun HX, Lu N, Ning G. Bisphosphonate treatment and risk of esophageal cancer: a meta-analysis of observational studies. Osteoporos Int. 2013;24(1):279–286. doi:10.1007/s00198-012-2158-823052941
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi:10.1002/sim.118612111919
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.55712958120
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi:10.1093/oxfordjournals.epirev.a0362983678409
- VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi:10.7326/M16-260728693043
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi:10.1136/bmj.315.7109.6299310563
- Duval S, Tweedie R. Trim and fill: asimple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463.10877304
- Newcomb PA, Trentham-Dietz A, Hampton JM. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br J Cancer. 2010;102(5):799–802. doi:10.1038/sj.bjc.660555520160722
- Rennert G, Pinchev M, Rennert HS. Use of bisphosphonates and risk of postmenopausal breast cancer. J Clin Oncol. 2010;28(22):3577–3581. doi:10.1200/JCO.2010.28.111320567021
- Monsees GM, Malone KE, Tang MT, Newcomb PA, Li CI. Bisphosphonate use after estrogen receptor-positive breast cancer and risk of contralateral breast cancer. J Natl Cancer Inst. 2011;103(23):1752–1760. doi:10.1093/jnci/djr39922021667
- Kwan ML, Shi JM, Habel LA, et al. Effectiveness of bisphosphonate use and risk of contralateral breast cancer and recurrence in women with early-stage breast cancer treated with tamoxifen. Breast Cancer Res Treat. 2016;156(2):379–389. doi:10.1007/s10549-016-3763-627002508
- Korde LA, Doody DR, Hsu L, Porter PL, Malone KE. Bisphosphonate use and risk of recurrence, second primary breast cancer, and breast cancer mortality in a population-based cohort of breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2018;27(2):165–173. doi:10.1158/1055-9965.EPI-17-055629254937
- Wiseman M. The second world cancer research fund/american institute for cancer research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253–256. doi:10.1017/S002966510800712X18452640
- Coleman RE, Rathbone E, Brown JE. Management of cancer treatment-induced bone loss. Nat Rev Rheumatol. 2013;9(6):365–374. doi:10.1038/nrrheum.2013.3623507900
- Ou YJ, Chiu HF, Wong YH, Yang CC, Yang YH. Bisphosphonate use and the risk of breast cancer: a meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2017;26(10):1286–1295. doi:10.1002/pds.430228857419
- Dreyfuss JH. Oral bisphosphonate use associated with a decreased risk of breast cancer. CA Cancer J Clin. 2010;60(6):343–344. doi:10.3322/caac.2009120959402
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. doi:10.1056/NEJMoa06731217476007
- Early Breast Cancer Trialists’ Collaborative G. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361. doi:10.1016/S0140-6736(15)60908-426211824
- Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19(6):733–759. doi:10.1007/s00198-007-0540-818214569
- Licata AA. Discovery, clinical development, and therapeutic uses of bisphosphonates. Ann Pharmacother. 2005;39(4):668–677. doi:10.1345/aph.1E35715755793
- Hollick RJ, Reid DM. Role of bisphosphonates in the management of postmenopausal osteoporosis: an update on recent safety anxieties. Menopause Int. 2011;17(2):66–72. doi:10.1258/mi.2011.01101421693503
- Reid IR, Cornish J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat Rev Rheumatol. 2012;8(2):90–96. doi:10.1038/nrrheum.2011.181
- Andrici J, Tio M, Eslick GD. Meta-analysis: oral bisphosphonates and the risk of oesophageal cancer. Aliment Pharmacol Ther. 2012;36(8):708–716. doi:10.1111/apt.1204122966908
- Oh YH, Yoon C, Park SM. Bisphosphonate use and gastrointestinal tract cancer risk: meta-analysis of observational studies. World J Gastroenterol. 2012;18(40):5779–5788. doi:10.3748/wjg.v18.i40.577923155320
- Hermann AP, Abrahamsen B. The bisphosphonates: risks and benefits of long term use. Curr Opin Pharmacol. 2013;13(3):435–439. doi:10.1016/j.coph.2013.02.00223434194
- Dreyfuss JH. Tamoxifen infrequently used by women at risk for breast cancer. CA Cancer J Clin. 2010;60(4):204–206. doi:10.3322/caac.2008020530798
- Powles TJ. Extended adjuvant tamoxifen for breast cancer – anew era? Lancet. 2013;381(9869):782–783. doi:10.1016/S0140-6736(12)62038-823219287
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi:10.1016/S0140-6736(12)61963-123219286
- Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 2010;19(2):443–446. doi:10.1158/1055-9965.EPI-09-093020142242
- Kirk R. Oral bisphosphonate use may protect women from breast cancer. Nat Rev Clin Oncol. 2010;7(9):482. doi:10.1038/nrclinonc.2010.126
- Coleman R, Gnant M, Morgan G, Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104(14):1059–1067. doi:10.1093/jnci/djs26322752060
- Gnant M, Clezardin P. Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat Rev. 2012;38(5):407–415. doi:10.1016/j.ctrv.2011.09.00321983264