Abstract
Background
The combination of organized cervical cancer screening and childhood HPV vaccination programs has the potential to eliminate cervical cancer in the future. However, only women participating in both programs gain the full protection, and combined non-attenders remain at high risk of developing cervical cancer. Our aim was to analyze the association between non-adherence to HPV vaccination and non-participation in cervical cancer screening for the total population and stratified by native background and parental education.
Participants
Women born in 1993 eligible for both childhood HPV vaccination and first cervical cancer screening.
Analysis
Logistic regression models were used to estimate the odds ratio (OR) of non-participation in cervical cancer screening with 95% confidence intervals (CI). Stratified and adjusted logistic regression models were used along with the Wald test in order to test for interaction.
Results
24,828 women were included in the study. Among vaccinated women, 61.4% participated in cervical cancer screening; only 39.0% of unvaccinated women participated in cervical cancer screening. Unvaccinated and unscreened women were often non-native and had the lowest socio-economic status, whereas vaccinated and screened women were often native and had the highest socio-economic status. The adjusted OR for non-participation in cervical cancer screening was 2.07 [95% CI: 1.88–2.28] for unvaccinated compared to vaccinated women. After stratifying by country of origin, unvaccinated natives had the highest adjusted OR of not participating in cervical cancer screening compared to non-native women from both western and non-western countries (adjusted ORs of 2.2 [95% CI: 2.0–2.4], 1.3 [95% CI: 0.6–2.8], and 1.5 [95% CI: 1.1–2.0], respectively) (Wald test p=0.019).
Conclusion
Among natives, non-adherence to HPV vaccination and non-participation in screening seem to be signs of generally poor health-preventive behavior, whereas among non-natives from non-western countries, non-attendance in HPV vaccination and cervical cancer screening seem to be influenced by unrelated factors. Therefore, a differentiated and culturally sensitive approach is needed to enhance overall cervical cancer preventive behavior across different nativities.
Plain Language Summary
Screening for cervical cancer combined with vaccination against HPV infection, which is the main cause of cervical cancer, has the potential to eliminate cervical cancer in the future. However, only women accepting both vaccination and screening will gain full protection, while those declining both will gain little to no protection, leaving these women at higher risk of developing cervical cancer.
The purpose of this study was to examine the association between childhood HPV vaccination and cervical cancer screening, in a free-of-charge Danish setting.
Our results illustrate that women who were neither HPV vaccinated nor screened, were often non-native and had the lowest socio-economic status. Furthermore, women who were not HPV vaccinated were also more likely not to be screened; this pattern remained even when taking the influence of socio-economic status into account. However, this pattern was not similar for non-native women.
In conclusion, among native Danish women, non-attendance to HPV vaccination and non-participation in screening seem to be signs of generally poor health-preventive behavior. Where as among non-native non-western women, non-attendance in HPV vaccination and cervical cancer screening seem to be influenced by separate factors. Socio-cultural factors therefore likely influence HPV vaccination and cervical cancer screening behavior in native and non-native women in different ways.
Introduction
Organized cervical cancer screening has considerably reduced cervical cancer incidence and mortality in many western countries.Citation1,Citation2 Effective vaccines targeting the most oncogene human papilloma virus (HPV) types 16/18,Citation3–Citation5 which cause >70% of all cervical cancers,Citation6 have been introduced in immunization programs worldwide since 2006.Citation7 In Denmark, publicly funded, clinically based, three-dose HPV vaccination was introduced in the late 2008 and early 2009, and was at this time recommended to all 12–15-year-old girls born in 1993–1996.Citation8
The combination of organized cervical cancer screening and childhood HPV vaccination programs has the potential to eliminate cervical cancer. However, only women participating in both programs full protection, leaving a group of unvaccinated and unscreened women with little to no protection and thus at a higher risk of developing cervical cancer.Citation9
A large number of national and international studies have described that low socio-economic status and non-native background are associated with non-adherence to HPV vaccinationCitation10–Citation18 and non-participation in cervical cancer screening.Citation9,Citation19–Citation23 However, the relationship between socio-economic status and nativity has not been explored in the context of combined non-attendance in both HPV vaccination and cervical cancer screening.
Previous studies,Citation24–Citation33 apart from one,Citation34 have shown a positive association between HPV vaccination and cervical cancer screening participation. However, most of these studies have been conducted on women who were HPV vaccinated as young adults,Citation29,Citation31,Citation34 though self-payment,Citation24,Citation29,Citation32 or who were from highly selectiveCitation25,Citation27,Citation30 or regionalCitation28,Citation33 populations. It is thus unknown whether these previous results may be generalizable to the growing number of countries implementing national childhood HPV vaccination.
The aim of this study was to analyze if non-adherence to a childhood HPV vaccination program was associated with non-participation in cervical cancer screening program in the total population and stratified by country of origin and parental education status. Furthermore, the aim was to identify potential underlying socio-economic factors related to combined non-attendance.
Materials And Methods
Study Design
A retrospective register-based nationwide-closed cohort study was conducted between 1 October 2008 and 31 December 2017.
Setting
The study was carried out in Denmark whose population then counted approximately 5.7 million citizens.Citation35 Denmark has two publicly financed national programs aiming to prevent cervical cancer: a childhood HPV vaccination program and a cervical cancer screening program.
Cervical cancer screening was introduced in Denmark in 1962 and reached national coverage in the late 1990s.Citation36 In the Danish National Cervical Cancer Screening Program, women receive their first invitation when they are 23 years old and are subsequently invited every third year until the age of 49, while women aged 50–64 are invited to participate every fifth year. General practitioners (GPs) obtain a liquid-based cytology sample from the cervix during a gynecological examination. Women who do not respond to the screening invitation letter receive up to two personal reminders after 3 and 6 months, respectively. In case of non-participation, the woman receives a new invitation after 3 or 5 years, depending on her age.
HPV vaccination was implemented in the Danish childhood vaccination program in January 2009, targeting girls born in 1996. A few months earlier a start-up program targeting girls born 1993–1995 was also introduced. The HPV vaccination program is now recommended to all girls aged 12–18 years. The types of vaccine and vaccination dozes have varied over time. Parents have to consult their GP for vaccination of their daughter and if this is not done before the age of 14 years, they receive one reminder.Citation8,Citation37
In the period from the time the HPV vaccine was released in 2006 until it was introduced in the childhood vaccination program almost 3 years later, all women had the opportunity to arrange HPV vaccination through self-payment (at this time approximately 500 USD).
Participants
The study population comprises all women born in 1993 who were resident in Denmark during the entire study period. The 1993 birth cohort was chosen as it represents the ever first population offered both national childhood HPV vaccination and invited for the first cervical cancer screening through 2016–2017.
Women with a cervical cytology obtained before the age of 22.5 were excluded as these cytologies were most likely obtained due to symptoms rather than for screening purposes, potentially influencing future screening participation. We allowed a 6-month window prior to the screening invitation (age 23) for appropriate “early screening” as we assumed that these cytologies had been performed in connection with a benign gynecological examination (i.e., contraception consultation) close to the screening invitation and thus would not influence future screening participation.
Furthermore, to ensure that the exposure was restricted to free-of-charge childhood HPV vaccination, women who were HPV vaccinated outside the childhood vaccination program (self-paid vaccination) were likewise excluded as they could have a different health-promoting behavior than those vaccinated in the program.
Data Collection And Definitions
The study population was defined in the Danish Civil Registration System,Citation38 which contains information on all citizens born in or having immigrated to Denmark. In the Danish Civil Registration System, all citizens are registered with a unique ten-digit identification number, allowing direct and complete linkage to other national registries.
Data on HPV vaccination status were collected from the Danish National Health Service Register,Citation39 which holds information on all tax-financed services. GPs obtain payment through a reimbursement system that includes information on the service provided and citizens receiving the service provided. Women with at least one HPV vaccination doze assigned a childhood HPV vaccination code (8328, 8329, 8330, 334, 8335, and 8336) between 1 October 2008 and the women’s 22.5-year birthday were considered “vaccinated” (exposed). All other women were considered “unvaccinated” (unexposed).
Data on self-paid redeemed HPV vaccination were collected through the Danish National Prescription Registry.Citation40 To identify these women, we used data on redeemed vaccine prescriptions holding the Anatomical Therapeutic Chemical Classification System codes of the two HPV vaccines available at the time (07BM01 and J07BM02). Women who were registered with at least one redeemed HPV vaccine prescription before they turned 22.5 years were defined as “self-paid vaccinated” and thus excluded from the main analyses.
Data on participation in the Danish National Cervical Cancer Screening Programme were collected from the Danish Pathology Register.Citation41 The Danish Pathology Register holds individual pathology data from all pathology departments classified according to the Systematized Nomenclature of Medicine (SNOMED),Citation42 which is a standardized glossary of clinical terminology used by healthcare providers for exchange of clinical health information. Cervical cytology samples were identified by the SNOMED codes T8X3* and material type 23. Women with at least one registered cytology sample between the age of 22.5 and 24 were considered “screened.” All women received a total of 18 months of follow-up. Those with no cervical cytology within this range were defined as “unscreened.”
Based on the above definitions, four groups of “combined attendance” were defined by linking HPV vaccination status and screening participation: 1) vaccinated and screened (combined attenders), 2) vaccinated but not screened, 3) screened but not vaccinated, and 4) neither vaccinated nor screened (combined non-attenders). For the purpose of this study, the latter group was considered a high-risk group.
Six variables were used as proxy measures for socio-economic status (parental civil status, highest parental education, and occupation, family disposable income and area of residence, and country of origin). These data were obtained from Statistics Denmark, which offers a research service linking data from different health registers to socio-economic status data.Citation43
Socio-economic status data were obtained for the year 2009, which was the calendar year where the study population was eligible for HPV vaccination (time of exposure). Since socio-economic status data commonly used in scientific work (completed education level, occupation, income, and civil status) are not fully established at age 15,Citation44 these variables were collected for the parents instead and supplemented with the participants’ data on area of residence and country of origin.
Parental civil status was categorized as 1) married/cohabiting or 2) single parents. Parental educational level was defined as the highest completed education level by either parent and classified as 1) low (<10 years), 2) middle (10–15 years), or 3) higher education (>15 years).Citation45 Family disposable household income based on the OECD-modified equivalence scaleCitation46 was used as an income measure. Based on tertiles, income was categorized as 1) low (lowest 33%), 2) middle (33–66%), or 3) high (highest 33%). Parental occupation was defined as the highest level of occupation by either parent and categorized as 1) working, 2) temporarily not working (including those receiving any kind of leave compensation, state education grant, or unemployment benefits), and 3) permanently not working (including early and ordinary retirement).
Area of residence was categorized according to the degree of urbanization as living in a densely, intermediate, or thinly populated area.
Native women were defined as those having Denmark as their country of origin and non-native women as those for whom Denmark was not their country of origin. Non-natives were furthermore sub-categorized according to their country of origin, into 1) western countries (EU, Andorra, Australia, Canada, Iceland, Liechtenstein, Monaco, New Zealand, Norway, San Marino, Switzerland, and the USA), and 2) non-western countries (all other countries).
Statistical Analyses
Differences in sample socio-economic characteristics between the four “combined attendance groups” were tabulated and provided with 95% confidence intervals (CI) to allow comparison.
In the subsequent analyses, exposure was defined as non-adherence to HPV vaccination and outcome as non-participation in cervical cancer screening.
Logistic regression models were used to estimate the odds ratio (OR) of non-participation in cervical cancer screening with 95% CI comparing vaccinated to unvaccinated women. Unadjusted logistic regression model was performed along with a model adjusting for all six confounding socio-economic variables.
For these logistic regression models, three sensitivity analyses were performed to test the robustness of our models. Firstly, we included women screened before the age of 22.5 in the study population. Secondly, we included “self-paid” HPV-vaccinated women in the study population. Both analyses were done in order to test the hypothesis that these groups of women would have a different health-promoting behavior than the background population. Thirdly, we expanded the follow-up period by 6 months (increasing follow-up to 2 years) in order to examine whether the association found in the main analyses was present only during the relatively short follow-up period.
Finally, to determine if the association between HPV vaccination status and screening participation was modified by country of origin or parental education level, stratified and adjusted logistic regression models were used along with the Wald test for interaction.
All statistical analyses were conducted using STATA version 15 (STATA Corp., College Station, TX, USA).
Results
A total of 31,228 women were eligible for study inclusion; a total of 6,400 (20.5%) were excluded, 1,828 (5.9%) due to self-paid HPV vaccination and 4,572 (14.6%) due to registered cervical cytology sample before the age of 22.5 years. This left 24,828 women in the main study population of whom 22,634 (91% [95% CI: 90.8-91.5%]) were vaccinated and 14,749 (59% [95% CI: 58.8–60.0%]) were screened. The mean age at HPV vaccination was 15.5 years [95% CI: 15.4–15.5], and the mean age at first cervical cancer screening was 23.4 years [95% CI: 23.4–23.5] (data not shown).
The majority of HPV-vaccinated women (13,893, 61.4% [95% CI: 60.7–62.0%]) participated in cervical cancer screening, whereas only 856 (39.0% [95% CI: 36.9–41.1%]) unvaccinated women participated in cervical cancer screening ().
Figure 1 Inclusion and exclusion flow-chart for the study population, including screening participation according to HPV vaccination status.
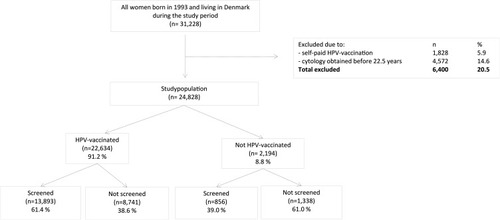
shows that 55.9% [55.3–56.6] of the total study population was both HPV-vaccinated and screened, while 5.4% [5.1–5.6] was neither HPV-vaccinated nor screened. Furthermore, HPV-vaccinated and screened women (combined attenders) had more often married (58.1% [57.3–58.8]), working (58.1% [57.4–58.7]) parents with higher education (61.4% [60.3–62.5]) and income (65.9% [64.7–67.1]), and lived in thinly populated area (59.4% [58.4–60.4]) and had native Danish background (59.6% [59.0–60.3]) than the three other combined attendance groups.
Table 1 Socio-Economic Characteristics Of The Study Population And Their Parents, Stratified By Combined Attendance In The Two Preventive Programs Against Cervical Cancer
On the other hand, un-vaccinated and un-screened women (combined non-attenders) had more often single (8.2% [7.5–8.9]), temporarily not working parents (15.0% [13.0–17.0]) with low education (9.9% [8.9–10.9]) and income (9.0% [8.4–9.6]), and lived in densely populated areas (7.0% [6.4–7.7]) and had non-native background (non-western 13.3% [11.9–14.7] and western 11.6% [7.7–16.5]).
After adjusting for socio-economic status, unvaccinated women had 2.1-fold higher odds of not participating in cervical cancer screening than vaccinated women (adjusted OR = 2.1[95% CI: 1.9–2.3]). The same adjusted model revealed that non-native women from non-western countries had 3.6-fold higher odds of not participating in cervical cancer screening than native women (adjusted OR=3.6 [95% CI: 3.2–4.0]) ().
Table 2 Unadjusted And Adjusted Odds Ratios With 95% Confidence Intervals (CIs) For Not Participation In Cervical Cancer Screening
In the three sensitivity analyses, we found that our conclusions did not change if we included women with cervical cytology before 22.5 years, “self-paid” vaccinated women, and expanding the follow-up period.
After stratifying by country of origin, unvaccinated native women had 2.2-fold higher odds of not participating in cervical cancer screening than vaccinated native women. Western-unvaccinated women had 1.3-fold higher odds and non-western unvaccinated 1.5-fold higher odds of not participating in cervical cancer screening than vaccinated women (adjusted ORs of 2.2 [95% CI: 2.00–2.4], 1.3 [95% CI: 0.6–2.8] and 1.5 [95% CI: 1.1–2.0], respectively). The test for homogeneity revealed a p-value of 0.019.
After stratifying by parental education level, unvaccinated women with highly educated parents had 1.9-fold higher odds of not participating in cervical cancer screening than vaccinated, those with middle-educated parent had 2.3-fold higher odds, and those with low-educated parents had 1.8-fold higher odds (adjusted ORs of 1.9 [95% CI: 1.6–2.3], 2.3 [95% CI; 2.0–2.6], and 1.8 [95% CI: 1.5–2.2], respectively). The test for homogeneity, however, revealed a p-value of 0.141 ().
Table 3 Models Testing The Interaction By Country Of Origin (Model 1) And Highest Parental Education (Model 2) On The Association Between Non-vaccination And Cervical Cancer Screening Non-participation
Discussion
Main Findings
This nationwide cohort study found that the best-protected women (combined attenders) belonged to the highest socio-economic status groups and were mostly native women, while the least protected women (combined non-attenders) belonged to the lowest socio-economic status groups and were mostly non-native women from non-western countries. Furthermore, it revealed that non-adherence to childhood HPV vaccination was associated with non-participation in cervical cancer screening. Thus, even after adjusting for socio-economic factors, unvaccinated women had higher odds of not participating in cervical cancer screening than vaccinated women.
The association between non-adherence to vaccination and non-participation in screening was stronger for natives than for non-natives from both western and non-western countries, indicating that natives and non-natives may encounter different barriers for HPV vaccination and cervical cancer screening.
Strengths And Limitations
A major strength of this study is the linkage of high-quality individual data on HPV vaccination, cervical cancer screening participation, and socio-economic status. This linkage eliminates the risk of differential misclassification, e.g., caused by recall bias or social desirability bias in self-reported data. The choice of a closed cohort study design gave us complete follow-up data; however, our study accounted only for citizens living permanently in Denmark over a longer period.
Our study is the first published study to use a strong register-based design with nationwide data to link associations between HPV vaccination status in a free-of-charge childhood HPV vaccination program and later organized cervical cancer screening participation adjusted for individual and parental socio-economic status.
Our results are thus relevant to the current and future situation in Denmark as well as in other countries with organized cervical cancer screening programs implementing childhood HPV vaccination programs. However, the present cohort represents women with a high HPV vaccination adherence of 91%, which is higher than what has later been reported in Denmark for younger cohorts and what has been seen in other countries.Citation47,Citation48 Thus, it may be relevant to further investigate “combined attendance” in cohorts with lower HPV vaccination adherence.
Overall, when interpreting our results, it is important not to perceive the odds ratios (OR) presented in the result section as an expression of relative risk of non-participation in screening, as this interpretation would lead to an overestimation of the results. Caution is also advised when interpreting the stratified estimates among different non-native groups, as both western and non-western women were small in sample size, leading to OR estimates with broad and overlapping 95% confidence intervals. It is possible that the estimates for western and non-western women shown in would have been different if the sample size had been larger.
The lack of data on indication for collecting cervical cytology was a limitation, as cytology registrations did not distinguish between cytologies taken for screening purpose and cytologies taken on medical indication. However, by excluding women with cytologies prior to screening invitation (<22.5 yrs.), we believe that we excluded the group most likely to consist of symptomatic women from the main analysis. This minimizes the risk of information bias in our definition of outcome.
Another limitation was the lack of information on reasons for not participating in cervical cancer screening. In light of the age at first screening invitation (23 years), some women in our cohort could likely have postponed participation in screening due to pregnancy, for example, as screening during pregnancy is not recommended in Denmark. Thus, some women would falsely be defined as unscreened, when, in fact, they participated in screening at a later time according to clinical recommendations. However, relatively few women resident in Denmark are pregnant at 23 yearsCitation49 as the mean age at first pregnancy is 29.2 years.Citation50 However, non-native women from non-western countries have a slightly higher fertility (0.2 live births pr. woman higher than native women) than native women.Citation43 This could mean that potentially more non-western women than native women have been misclassified as non-participants in screening in our results. Expanding the follow-up window with another 6 months did, however, not alter our main results.
Lastly, it was a limitation that our dataset had no information on non-native women, especially those from western countries, attending HPV vaccination or screening in their country of origin. Many western countries currently provide HPV vaccination and screening programs.Citation51 Therefore, for western women, it is possible that the combined non-attendance is actually lower than seen in our study. In contrast, it is not likely that non-western women attend vaccination or/and screening in their home countries, and the results regarding these women would therefore be accurate despite the lack of this information.Citation52
Furthermore, in order to prevent any misclassification on HPV vaccination status that could have occurred due to errors in registration, as demonstrated in a Danish study concerning measles, mumps, and rubella vaccination coverage,Citation53 we included all vaccination codes available for free HPV vaccination, and thus marked the first given vaccination code as the first given HPV vaccination.
Other Studies
Our main finding regarding the association between HPV vaccination and cervical cancer screening participation was in line with findings in previous studies.Citation24–Citation33 Only one Australian studyCitation34 from 2014 found that vaccinated women had a lower screening participation than unvaccinated women. This particular study did not link HPV vaccination to screening participation at the level of the individual, which may explain the conflicting result compared to other studies. It is, however, also possible that the organization of HPV vaccination in Australia (school-based opt-out program) could weaken the association to opt-in screening. However, it seems that in this particular study, participants were above the age for participation in a school-based vaccination program and thus were vaccinated mostly through a community-based opt-in catch-up program, like in many of the other studies showing a positive association between vaccination status and screening participation.
Our study demonstrated that age at HPV vaccination did not alter subsequent screening participation patterns, as our results on childhood HPV-vaccinated women mirror results reported by previous studies conducted on varied and often older populations.Citation24,Citation25,Citation27–Citation33 Screening behavior patterns were consistent whether the decision to be HPV-vaccinated was made by the women themselves or by their parents. It is therefore likely that a person’s health-promoting behavior may be shaped during childhood and persists throughout adulthood.
In a Swedish nationwide cohort study conducted in 2018, Kreusch et alCitation24 found that compared to unvaccinated women, opportunistically HPV-vaccinated women often had higher educational level, higher income, and were natives. In our study, this social inequality in health-promoting behavior was further demonstrated across both preventive programs available against cervical cancer. Thus, in our study, well-protected women (combined attenders) had the highest socio-economic status and native background, while unprotected women (combined non-attenders) had the lowest socio-economic status and non-native background.
Kreusch et al also found that educational level and native status interacted with HPV vaccination and cervical cancer screening participation.Citation24 Contrary to our results, Kreusch et al demonstrated proportionally increased attendance in HPV vaccination and cervical cancer screening with increasing level of education and a stronger association between HPV vaccination and cervical cancer screening among non-native women than among native women.
In our interaction analyses, the level of education was not an effect modifier. Moreover, the association between HPV vaccination status and screening participation was stronger for native women than for non-native women. Methodological differences in the two studies (i.e., categorization of non-natives or algorithms for HPV vaccination or cervical cancer screening) could potentially explain why education and native background played a different role in the two studies. However, the most likely reason for this difference is the highly selected population in the Swedish study, in which only 13.6% were HPV vaccinated and, moreover, had paid for their vaccination themselves. It is likely that vaccinated women in this study, especially non-natives, had a particularly active health-promoting behaviour and this may explain the opposing results in the two studies.
Furthermore, in line with our interaction results regarding the influence of native background on HPV vaccination and subsequent screening, two recent Danish studies showed both lower attendance and lower effect size of offering HPV self-sampling to non-native women than to native women,Citation54,Citation55 supporting that health-promoting behavior may be influenced by nativity/region of origin.
Among some non-natives, factors other than socio-economic status seem to influence attendance pattern in HPV vaccination and cervical cancer screening. Besides barriers in language and lack of knowledge, lower screening participation among non-native women from the Middle-East and South Asia has been linked to socio-cultural barriers such as fatalism, fear of cancer, embarrassment, modesty, and perceiving HPV infection as related to promiscuous behavior.Citation56,Citation57–Citation60
Some of these barriers are difficult to overcome, but lack of knowledge may be overcome through educational interventions and by improving women’s perception on their own disease susceptibility, their perception of disease severity, and finally their perception of benefits weighed against barriers toward attending, besides improving their understanding of the healthcare system.Citation61
It therefore could be beneficial to consider adding differentiated interventions for natives and non-natives in order to equalize participation across all populations.
Conclusion
It seems that among native Danish women, especially those with lower socio-economic status, non-adherence to HPV vaccination and non-participation in screening are both signs of a generally poor health-preventive behavior pattern. However, among non-western women, it seems that non-adherence in HPV vaccination and non-participation in cervical cancer screening are influenced by unrelated factors. It is likely that socio-cultural factors influence non-western women’s HPV vaccination and cervical cancer screening behavior in different ways than native women. Therefore, a differentiated approach to native and non-native women is needed to enhance overall cervical cancer preventive behavior across different nativities.
Ethics Approval And Informed Consent
According to Danish legislation and the Central Denmark Region Committees on Biomedical Research Ethics, the study did not require ethical approval because it was based on register data. The same institutions waived patient consent for use of register data. In accordance with Danish law and the EU’s General Data Protection Regulation, the project was listed at the Central Denmark Region internal list of research projects (J. No.: 1-16-02-400-16).
Author Contributions
All authors made substantial contributions to both design, achievement of data, analysis, and interpretation of data; took part in drafting the article or revising it critically; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
Data cleaning and initial analyses were performed with assistance from data manager Bo Søborg at the Department of Public Health Programmes, Randers Regional Hospital. This study was funded by the Family Hede Nielsen’s Foundation and Helsefonden. The sponsors had no influence on the scientific process.
Disclosure
Andersen B has received HPV test kits and HPV self-sampling devices from Roche and Axlab for other studies. Andersen B reports grants from Helsefonden, grants from Familien Hede Nielsens fond, during the conduct of the study; non-financial support from Roche, non-financial support from Axlab, outside the submitted work. Pedersen LK has received speaker’s fee from Merck and Sanofi Pasteur in relation to lectures on HPV vaccines. The authors report no other conflicts of interest in this work.
References
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. PMC3681632; Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev. 2013;2:35. doi:10.1186/2046-4053-2-3523706117
- Arbyn M, Raifu AO, Weiderpass E, Bray F, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. Eur J Cancer. 2009;45(15):2640–2648. doi:10.1016/j.ejca.2009.07.01819695864
- Arbyn M, Xu L, Simoens C, Martin-Hirsch P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi:10.1002/14651858.CD009069.pub329740819
- European Medicines Agency. Summary of the European Public Assessment Report (EPAR) for Cervarix. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000721/human_med_000694.jsp&mid=WC0b01ac058001d124 Accessed 628, 2019.
- European Medicines Agency. Summary of the European Public Assessment Report (EPAR) for Gardasil. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000703/human_med_000805.jsp Accessed 628, 2019.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:9590; ProQuest pg. 890. doi:10.1016/S0140-6736(07)61416-0
- World Health Organization. Countries with HPV Vaccine in the National Immunization Programme. Available from: https://www.who.int/immunization/diseases/hpv/decision_implementation/en/ Accessed 628, 2019.
- Statens Serum Institut. Available from: http://www.ssi.dk/~/media/Indhold/DK%20-%20dansk/Aktuelt/Nyhedsbreve/EPI-. NYT/2008/2008%20PDF/EPI-NYT%20-%202008%20-%20uge%2035.ashx Accessed 628, 2019
- Azerkan F, Sparen P, Sandin S, Tillgren P, Faxelid E, Zendehdel K. Cervical screening participation and risk among Swedish-born and immigrant women in Sweden. Int J Cancer. 2012;130(4):937–947. doi:10.1002/ijc.2608421437898
- Slattelid Schreiber SM, Juul KE, Dehlendorff C, Kjaer SK. Socioeconomic predictors of human papillomavirus vaccination among girls in the Danish childhood immunization program. J Adolesc Health. 2015;56(4):402–407. doi:10.1016/j.jadohealth.2014.12.00825659994
- Fisher H, Trotter CL, Audrey S, MacDonald-Wallis K, Hickman M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int J Epidemiol. 2013;42(3):896–908. doi:10.1093/ije/dyt04923620381
- Agenor M, Abboud S, Delgadillo JG, Perez AE, Peitzmeier SM, Borrero S. Intersectional nativity and racial/ethnic disparities in human papillomavirus vaccination initiation among U.S. women: a national population-based study. Cancer Causes Control. 2018;29(10):927–936. doi:10.1007/s10552-018-1069-130120642
- Perez AE, Agenor M, Gamarel KE, Operario D. Nativity disparities in human papillomavirus vaccination among U.S. adults. Am J Prev Med. 2018;54(2):248–258. S0749-3797(17)30642-6 [pii]. doi:10.1016/j.amepre.2017.10.019.29241719
- Cofie LE, Hirth JM, Guo F, Berenson AB, Markides K, Wong R. HPV vaccination among foreign-born women: examining the national health interview survey 2013-2015. Am J Prev Med. 2018;54(1):20–27. S0749-3797(17)30458-0 [pii]. doi:10.1016/j.amepre.2017.08.017.29074320
- Widgren K, Simonsen J, Valentiner-Branth P, Molbak K. Uptake of the human papillomavirus-vaccination within the free-of-charge childhood vaccination programme in Denmark. Vaccine. 2011;29(52):9663. doi:10.1016/j.vaccine.2011.10.02122015392
- Fernandez de Casadevante V, Gil Cuesta J, Cantarero-Arevalo L. Determinants in the uptake of the human papillomavirus vaccine: a systematic review based on european studies. Front Oncol. 2015;5:141. doi:10.3389/fonc.2015.0014126157706
- Fernandez de Casadevante V, Cantarero-Arevalo L, Cuesta JG, Valentiner-Branth P .Ethnic background and human papillomavirus vaccine uptake in Denmark: a countrywide retrospective cohort study including 274,154 women aged 19-28 years. Papillomavirus Res.2016;2:78–84. S2405-8521(15)30010-0 [pii]. doi:10.1016/j.pvr.2016.03.003.29074189
- Roberts SA, Brabin L, Stretch R, et al. Human papillomavirus vaccination and social inequality: results from a prospective cohort study. Epidemiol Infect. 2011;139(3):400–405. doi:10.1017/S095026881000066X20334731
- Kristensson JH, Sander BB, von Euler-Chelpin M, Lynge E. Predictors of non-participation in cervical screening in Denmark. Cancer Epidemiol. 2014;38(2):174. doi:10.1016/j.canep.2013.12.00724447699
- Harder E, Juul KE, Jensen SM, Thomsen LT, Frederiksen K, Kjaer SK. Factors associated with non-participation in cervical cancer screening – a nationwide study of nearly half a million women in Denmark. Prev Med. 2018;111:94–100. doi:10.1016/j.ypmed.2018.02.03529501474
- Broberg G, Wang J, Ostberg AL, et al. Socio-economic and demographic determinants affecting participation in the Swedish cervical screening program: a population-based case-control study. PLoS One. 2018;13(1):e0190171. doi:10.1371/journal.pone.019017129320536
- Moen KA, Kumar B, Qureshi S, Diaz E. Differences in cervical cancer screening between immigrants and nonimmigrants in Norway: a primary healthcare register-based study. Eur J Cancer Prev. 2017;26(6):521–527. doi:10.1097/CEJ.000000000000031127749381
- Leinonen MK, Campbell S, Ursin G, Trope A, Nygard M. Barriers to cervical cancer screening faced by immigrants: a registry-based study of 1.4 million women in Norway. Eur J Public Health. 2017;27(5):873–879. doi:10.1093/eurpub/ckx09328957477
- Kreusch T, Wang J, Sparen P, Sundstrom K. Opportunistic HPV vaccination at age 16–23 and cervical screening attendance in Sweden: a national register-based cohort study. BMJ Open. 2018;8(10):e024477-2018-024477. doi:10.1136/bmjopen-2018-024477
- Boone SD, Pinkston CM, Baumgartner KB, et al. Associations between prior HPV4 vaccine doses and cervical cancer screening participation. Cancer Epidemiol. 2016;42:108–114. doi:10.1016/j.canep.2016.04.00327100836
- Palmer TJ, McFadden M, Pollock KG, et al. HPV immunisation and increased uptake of cervical screening in Scottish women; observational study of routinely collected national data. Br J Cancer. 2016;114(5):576–581. doi:10.1038/bjc.2015.47326794278
- Chao C, Silverberg MJ, Becerra TA, et al. Human papillomavirus vaccination and subsequent cervical cancer screening in a large integrated healthcare system. Am J Obstet Gynecol. 2017;216(2):151.e1-151.e9 S0002-9378(16)30869-9 [pii]. doi:10.1016/j.ajog.2016.10.006.
- Kim J, Bell C, Sun M, et al. Effect of human papillomavirus vaccination on cervical cancer screening in Alberta. CMAJ. 2016;188(12):E281–E288. doi:10.1503/cmaj.15152827378467
- Herweijer E, Feldman AL, Ploner A, et al. The participation of HPV-vaccinated women in a national cervical screening program: population-based cohort study. PLoS One. 2015;10(7):e0134185. doi:10.1371/journal.pone.013418526218492
- Paynter CA, Van Treeck BJ, Verdenius I, et al. Adherence to cervical cancer screening varies by human papillomavirus vaccination status in a high-risk population. Prev Med Rep. 2015;2:711–716. doi:10.1016/j.pmedr.2015.07.01126844141
- Sauer AG, Jemal A, Simard EP, Fedewa SA. Differential uptake of recent papanicolaou testing by HPV vaccination status among young women in the United States, 2008–2013. Cancer Epidemiol. 2015;39(4):650–655. doi:10.1016/j.canep.2015.05.00226055147
- Kliewer EV, Mahmud SM, Demers AA, Lambert P. Human papillomavirus vaccination and pap testing profile in Manitoba, Canada. Vaccine. 2013;32(1):33–38. doi:10.1016/j.vaccine.2013.10.08224211170
- Beer H, Hibbitts S, Brophy S, Rahman MA, Waller J, Paranjothy S. Does the HPV vaccination programme have implications for cervical screening programmes in the UK? Vaccine. 2014;32(16):1828–1833. doi:10.1016/j.vaccine.2014.01.08724530938
- Budd AC, Brotherton JM, Gertig DM, Chau T, Drennan KT, Saville M. Cervical screening rates for women vaccinated against human papillomavirus. Med J Aust. 2014;201(5):279–282. 10.5694/mja14.00021[pii]. doi:10.5694/mja14.00021.25163380
- Statistics Denmark. Population. Available from: https://www.dst.dk/da/Statistik/emner/befolkning-og-valg/befolkning-og-befolkningsfremskrivning/folketal Accessed 628, 2019.
- Bigaard J, Hariri J, Lynge E. Cervical cancer screening in Denmark. Eur J Cancer. 2000;36(17):2198 S0959-8049(00)00309-9 [pii]. doi:10.1016/s0959-8049(00)00174-x.11072204
- The Danish Health and Medicines Authority. Reduction in the Risk of Cervical Cancer by Vaccination against Human Papillomavirus (HPV) – a Health Technology Assessment Copenhagen. 2007.
- Pedersen CB. the danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–25. doi:10.1177/140349481038796521775345
- Andersen JS, Olivarius Nde F, Krasnik A. The Danish national health service register. Scand J Public Health. 2011;39(7):34–37. doi:10.1177/140349481039471821775348
- Kildemoes HW, Sorensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi:10.1177/140349481039471721775349
- Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Public Health. 2011;39(7 Suppl):72–74. doi:10.1177/140349481039356321775357
- Côté RA. Progress in Medical Information Management Systematized Nomenclature of Medicine (SNOMED). Jama. 1980;243(8):756–762. doi:10.1001/jama.1980.033003400320156986000
- Statistics Denmark. English Front Page. Available from: https://www.dst.dk/en Accessed 909, 2019
- Galobardes B, Shaw M, Lawlor DA, Lynch JW, George DS. Indicators of socioeconomic position (part 1). 2006 0.1136/jech.2004.023531.
- UNESCO. International Standard Classification of Education, ISCED. Quebec, Canada: UNESCO Institute for Statistics; 2011 Avaiable from: http://www.uis.unesco.org/Education/Documents/isced-2011-en.pdf Accessed 628, 2019.
- OECD. Project on Income Distribution and Poverty. What are equivalence scales? Available from: http://www.oecd.org/els/soc/OECD-Note-EquivalenceScales.pdf Accessed 628, 2019
- Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Global Health. 2016;4(7):e453–e463. doi:10.1016/s2214-109x(16)30099-727340003
- Statens Serum Institut. HPV-Vaccination Coverage for Birth Cohorts 1993–2006. Available fom: https://statistik.ssi.dk//sygdomsdata#!/?vaccination=5&sex=0&landsdel=100&xaxis=Cohort&show=Graph&datatype=Vaccination Accessed 628, 2019
- Statistics Denmark. Mothers Age at First Child Delivery. Available from: https://www.statistikbanken.dk/statbank5a/default.asp?w=1920 Accessed 909, 2019
- Statistics Denmark. Mean Age at First Child Delivery. Avaialble from: https://www.statistikbanken.dk/statbank5a/default.asp?w=1920 Accessed 909, 2019.
- World Health Organization. Overview of Global National HPV Vaccination and Cervical Cancer Screenings Programs. Available from: https://www.who.int/ Accessed 730, 2019.
- Sancho-Garnier H, Khazraji YC, Cherif MH, et al. Overview of cervical cancer screening practices in the extended Middle East and North Africa countries. Vaccine. 2013;31(Suppl 6):G51. doi:10.1016/j.vaccine.2012.06.04624331820
- Holt N, Mygind A, Bro F. Danish MMR Vaccination Coverage Is Considerably Higher than Reported. 2017.
- Tranberg M, Larsen MB, Mikkelsen EM, Svanholm H, Andersen B. Impact of opportunistic testing in a systematic cervical cancer screening program: a nationwide registry study. BMC Public Health. 2015;15:681. doi:10.1186/s12889-015-2039-026194007
- Harder E, Thomsen LT, Hertzum-Larsen R, et al. Determinants for participation in human papillomavirus self-sampling among nonattenders to cervical cancer screening in denmark. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1342–1351. doi:10.1158/1055-9965.EPI-18-048030108095
- Marlow LAV, Waller J, Wardle J. Barriers to cervical cancer screening among ethnic minority women: a qualitative study. J Fam Plann Reprod Health Care. 2015;41(4):248–254. doi:10.1136/jfprhc-2014-10108225583124
- Islam N. Understanding Barriers and Facilitators to Breast and Cervical Cancer Screening among Muslim Women in New York City – Perspectives from Key Informants. 2018.
- Gele AA, Qureshi SA, Kour P, Kumar B, Diaz E. Barriers and facilitators to cervical cancer screening among Pakistani and Somali immigrant women in Oslo: a qualitative study. Int J Womens Health. 2017;9:487–496. doi:10.2147/IJWH.S13916028740435
- Ghebre RG, Sewali B, Osman S, et al. Cervical cancer: barriers to screening in the Somali community in Minnesota. J Immigr Minor Health. 2015;17(3):722–728. doi:10.1007/s10903-014-0080-125073605
- Ferdous M, Lee S, Goopy S, et al. Barriers to cervical cancer screening faced by immigrant women in Canada: a systematic scoping review. BMC Womens Health. 2018;18(1): 165-018-0654-5. doi:10.1186/s12905-018-0654-5
- Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Educ Q. 1988;15(2):175–183.3378902
