Abstract
Background
Prenatal exposures may contribute to male infertility in adult life, but large-scale epidemiological evidence is still lacking. The Fetal Programming of Semen quality (FEPOS) cohort was founded to provide means to examine if fetal exposures can interfere with fetal reproductive development and ultimately lead to reduced semen quality and reproductive hormone imbalances in young adult men.
Methods
Young adult men at least 18 years and 9 months of age born to women in the Danish National Birth Cohort living in relative proximity to Copenhagen or Aarhus and for whom a maternal blood sample and two maternal interviews during pregnancy were available were invited to FEPOS. Recruitment began in March 2017 and ended in December 2019. The participants answered a comprehensive questionnaire and underwent a physical examination where they delivered a semen, urine, and hair sample, measured their own testicular volume, and had blood drawn.
Results
In total 21,623 sons fulfilled eligibility criteria of whom 5697 were invited and 1058 participated making the response rate 19%. Semen characteristics did not differ between sons from the Copenhagen and Aarhus clinics. When comparing the FEPOS semen parameters to similar cohorts, the median across all semen characteristics was slightly lower for FEPOS participants, although with smaller variation.
Conclusion
With its 1058 young adult men, the FEPOS cohort is the largest population-based male-offspring cohort worldwide specifically designed to investigate prenatal determinants of semen quality. Wide-ranging information on maternal health, lifestyle, socioeconomic status, occupation, and serum concentrations of potential reproductive toxicants during pregnancy combined with biological markers of fertility in their sons collected after puberty allow for in-depth investigations of the ‘fetal origins of adult disease hypothesis’.
Introduction
Infertility is the most common chronic disease among people of reproductive age, and affects up to 15–25% of all couples trying to achieve a pregnancy.Citation1–Citation3 Male factor infertility is a contributing factor in up to half of these cases.Citation4 Around 40% of Danish men have a semen concentration below 40 million/mL, from where the probability for conception gradually decreases.Citation5 Several factors in adult life have been linked to reduced semen quality, including lifestyle such as smoking, chronic alcohol use, obesity, sleep, and nutritionCitation6–Citation12 and occupational and environmental exposures such as sedentary work, radiation, air pollution, bisphenol A, parabens, organophosphate pesticides, pyrethroids, and phthalates.Citation13–Citation17 Still, subfertility remains unexplained for many, and the underlying causes are far from understood. According to the ‘fetal origins of adult disease hypothesis’ suggested by Barker and Osmond in 1986, the environment encountered during fetal life is strongly related to the risk of developing non-communicable diseases later in life.Citation18 This was supported by Sharpe and Skakkebæk,Citation19 who subsequently proposed that hypospadias, cryptorchidism, poor semen quality, and testicular cancer are symptoms of one underlying entity with a common fetal origin.Citation20,Citation21 Although an increasing number of studies of maternal lifestyle and health during pregnancy, such as maternal diet, alcohol consumption, smoking, medication use, and obesity support this fetal programming hypothesis,Citation22–Citation27 large-scale epidemiological evidence is still lacking – especially regarding maternal exposure to endocrine disrupting chemicals at home and at work.Citation28 The limited body of evidence is likely explained by the complex logistics and high costs of establishing long-term longitudinal population-based studies of male reproductive function, with information on exposures during early life, such as the need for detailed maternal information and bio-specimens stored for decades before follow-up.
The Fetal Programming of Semen quality (FEPOS) cohort is nested within the Danish National Birth Cohort (DNBC)Citation29,Citation30 and was founded to provide means to examine if fetal exposures can interfere with fetal reproductive development and ultimately lead to reduced semen quality and reproductive hormone imbalances in young adult men.
Methods
Study Population
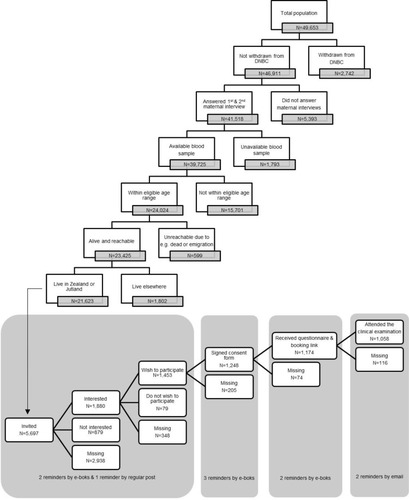
In March 2017, FEPOS was established as a male-offspring sub-cohort within the DNBC. In total, 49,653 sons were born into the DNBC cohort. A full flow chart of the sampling strategy is depicted in . Sons eligible to participate in FEPOS should still be enrolled in the DNBC (N=46,911) with mothers having participated in the two maternal computer-assisted telephone interviews conducted around gestational week 16 and 31 (N=41,518) and with a stored gestational blood sample in the DNBC biobank (N=39,725).Citation30 Further, the FEPOS participants had to be at least 18 years and 9 months of age within the study period (N=24,024) (this arbitrary age cut off was due to an 18-year follow-up in the original DNBC cohort), not dead or emigrated (N=23,425) and live in Zealand (N=8817) or Jutland (N=12,806) in relative proximity to the FEPOS clinics in Copenhagen or Aarhus. Thus, the total number of young men eligible for invitation was 21,623.
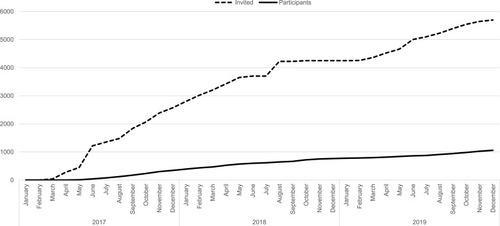
The Aarhus clinic included participants until June 2018 where it was closed due to a lack of funding. The Copenhagen clinic included participants in the entire study period that ended in December 2019. In total, 5697 men were invited and 1058 participated making the response rate 19%.
Recruitment Logistics
Using a digitalized and comprehensive recruitment system, eligible sons were randomly selected for invitation to participate on an ongoing basis during the study period (). The monthly number of sons invited varied in the two clinics according to capacity. An invitation letter was sent to the young men’s personal and secure digital mailbox “e-Boks” linked to the unique personal identification number. E-Boks is automatically created at the age of 15 years and used for bi-directional communication with public and private authorities, eg to receive pay checks or bank statements.Citation31 The invitation letter included information about the study and an electronic option to be contacted for additional information or decline participation. The invitation letter specified that participants who had undergone sterilization, cancer treatment, orchidectomy, or had one or no testicles were ineligible. Of the 5697 invited sons 1880 requested more information and were contacted by telephone by a member of the project group. Those still wishing to participate after the verbal information was given (N=1453), received an electronic informed consent form by e-Boks. Upon digitally consenting using their common secure login “NemID” (N=1248), participants received links to an online questionnaire and booking system to schedule an appointment for a clinical examination at the clinic closest to their home (). After answering the online questionnaire (N=1174) participants underwent a clinical examination (N=1058). At each step of contact, non-responders were sent two reminders by e-Boks, with 14 days interval, and at the initial invitation a final reminder was sent by regular mail. Participants were each remunerated 500 DKK (≈ 67 Euro).
Data and Measurements
An overview of data collected from the questionnaire and clinical examination data is presented in .
Table 1 Overview of Data Available on the FEPOS Cohort
Questionnaire Information
Participants filled out a comprehensive web-based questionnaire administered electronically using SurveyXact. The questionnaire included questions regarding education, work, health, and health behavior (). Nationally validated questionnaire scales were used where possible.Citation32–Citation35 To ensure that questions were explicit and easily understood by this age group, the questionnaire was tested and revised in a group of nine young men before being sent to the FEPOS participants.
Clinical Examination
The clinical examination was performed by a trained biomedical laboratory technician at either the Department of Occupational and Environmental Medicine at Bispebjerg and Frederiksberg Hospital (Copenhagen) or the Department of Occupational Medicine at Aarhus University Hospital (Aarhus).
Height and waist circumference in centimeters were measured using a seca® 213 Height Measure and seca® 201 measuring tape, respectively (seca®, Hamburg, Germany). Body weight in kilos, fat percentage (including visceral fat), fat mass, fat-free mass, water percentage, muscle mass, basal metabolic rate, metabolic age, and bone weight were measured using a MC-780MA Body Composition Analyzer (Tanita®, Tokyo, Japan). Blood pressure was measured three times with 2–3 min interval on the right arm, with a BP A3 Plus monitor (Microlife®, Taipei, Taiwan) at the Aarhus clinic and with an Omron M6 Comfort IT (OMRON Corporation, Kyoto, Japan) at the Copenhagen clinic. Measurements of pulmonary function forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) was measured using an EasyOne® spirometer with EasyOne® disposable Spirette® tubes (ndd Medical Technologies, Inc., Andover, Massachusetts, USA). Testicular size was measured in privacy at the clinic by the participants themselves using a Prader Orchidometer (Bayer AG, Leverkusen, Germany). This method has previously been found valid when compared to measurements by an experienced examiner.Citation36
Assessment of Reproductive Hormones
Nonfasting venous blood samples were collected using VACUETTE® SAFETY Blood Collection Set + Holder (Greiner Bio-One GmbH, Kremsmünster, Austria). Plasma, serum, and whole blood were stored in CryoPure Tubes (Sarstedt, Nümbrecht, Germany) at −80°C until analysis of reproductive hormones including hemoglobin A1c (HbA1c), follitropin (FSH), Insulin-like Growth Factor I (IGF-1), testosterone, Sex hormone-binding globulin (SHBG), insulin, lutropin (LH), thyrotropine (TSH), thyroxine (free T4 and T4), oestradial, and inhibin B. Analyses currently await funding.
Exposure Biomarkers of Xenobiotic Chemicals
Besides blood samples urine samples were collected at the clinics in 150 mL Frascos clear polypropylene containers with polyethylene leak proof screw caps (DELTALAB S.L., Barcelona, Spain), both free of phthalates. Samples were stored at 3–8°C for a maximum of 12 hours before transfer to polypropylene Microtubes (Sarstedt, Nümbrecht, Germany) and storage at −80°C.
Maternal plasma (100 µL) was retrieved from the Danish National Biobank (DNB). First trimester plasma samples were preferred, if these were not available, we used second or third trimester plasma samples, in that prioritized order.
Vitamin D, cotinine, and a range of exposure biomarkers of several xenobiotic chemicals () were analyzed in urine and blood, from both mother and son, using liquid chromatography-triple quadrupole mass spectrometry (QTRAP 5500, AB Sciex, Foster City, CA, USA; LC-MS/MS).Citation37–Citation40 The analysis was performed at the Division of Occupational and Environmental Medicine at Lund University, Sweden. Analyses are ongoing – thus far 559 urine samples, 555 blood samples and 533 maternal plasma samples have been analyzed. The laboratory in Lund is a reference laboratory for bisphenol A (BPA) analysis in urine and is part of Erlangen Round Robin inter-laboratory control program for analysis for triclosan, cotinine, trichloropyridinol (TCP), 3-phenoxybencoic acid (3-PBA) and perfluoroalkyl acid (PFAA). The laboratory has qualified as HBM4EU laboratory for the analysis of: BPA, Bisphenol F (BPF), Bisphenol S (BPS), 1-Hydroxypyren (1-HP) and Cyclohexane-1,2-dicarboxylate-mono(oxo-isononyl) ester (oxo-MINCH) and the phthalates; Monobenzyl (MBzP), Mono-(2-ethylhexyl) (MEHP), Mono-(4-methyl-7-carboxyheptyl) (cx-MiNP), Mono-(4-methyl-7-hydroxyloctyl) (OH-MiNP), Mono-(4-methyl-7oxo octyl) (oxo-MiNP), Mono (2-ethyl-5-oxohexyl) (5-oxo-MEHP), Mono(2-ethyl-5-hydroxyhexyl) (5-OH-MEHP), Mono-(2-ethyl-5-carboxypentyl) (5-cx-MEPP), Perfluoroheptanoic acid (PFHpA), Perfluorooctanoic acid (PFOA), Perfluorononanoic acid (PFNA), Perfluorodecanoic acid (PFDA), Perfluoroundecanoic acid (PFUnDA), Perfluorododecanoic acid (PFDoDA), Perfluorohexane sulfonic acid (PFHxS), and Perfluorooctane sulfonic acid (PFOS).
Hair Samples
Approximately 150 hair strands (~10–20 mg) were cut from the vertex posterior as close to the scalp as possible. The hair strands were stuck on paper with adhesive tape (Lyreco, Marly, France) with clear indication of the hair root, and stored in an envelope at room temperature. The thought was to conduct hair analysis as an indicator of eg stress,Citation41,Citation42 but no specific studies are planned yet.
Semen Samples
Participants had the option to collect the semen sample at the clinic or at home. When choosing the latter, detailed instructions on collection and transportation was sent to the participant together with a polypropylene sample container with a diameter of 79 mm and a height of 22mm, with polyethylene snap-lid (Nerbe Plus GmbH, Winsen/Luhe, Germany) for sample collection. This container was also used to collect the semen sample in the clinic. Participants were informed that they should be sexually abstinent 48–72 hours prior to semen collection. All semen analyses followed the recommendations by the World Health Organization (WHO) 2010.Citation43 Immediately upon receipt in the laboratory, the semen volume was measured by weighing (Scout® SKX222 Portable Precision Balance, OHAU®, Parsippany, New Jersey, USA) of the sample in the pre-weighed container. The sample was then placed in a 37°C HERATherm™ Compact Microbiological Incubator (Thermo Fisher Scientific, Waltham, Massachusetts, USA) for liquefaction. After liquefaction using a TrioMix (Triolab, Brøndby, Denmark), samples were analyzed manually for sperm concentration, total sperm count, and motility using a Compudiff 2000–16 Semen Analyzer (Molek AB, Arsta, Sweden). One trained biomedical laboratory technician affiliated to the clinic in Copenhagen and another to the clinic in Aarhus performed all in-house semen analyses. Sperm concentration was determined on two aliquots of semen samples using a BLAUBRAND® Improved Neubauer Hemocytometer (BRAND®, Wertheim, Germany) diluted with NaHCO3 resolution according to concentration. The diluted semen was transferred to each chamber of the hemocytometer and put in a humid chamber for 10 minutes. Each chamber was examined until at least 200 cells had been counted. Sperm cell motility was determined by counting the proportion of a) progressive sperm; b) non-progressive sperm; c) immotile sperm, on 200 spermatozoa within each of two fresh drops of semen, placed on a preheated (37°C), clean glass slide covered with a cover slip using a Compudiff 2000–16 Semen Analyzer (Molek AB, Arsta, Sweden). Morphology was analyzed at the Reproductive Medicine Centre, Skaane University Hospital, in Malmö, Sweden. DNA fragmentation Index (DFI), measured by flow cytometry semen chromatin structure assayCitation44 was analyzed at the Reproductive Medicine Centre, Skaane University Hospital, in Malmö, Sweden (no results yet).
The FEPOS biomedical laboratory technicians participated in a follow-up quality control with the Reproductive Medicine Centre in Malmö where they were originally trained to perform the semen analyses. In January 2018, based on five semen samples, the average coefficient of variation and range for the FEPOS biomedical laboratory technicians versus the reference laboratory were 18.4% (10.1–31.4%) versus 17.6% (1.3–28.3%) for sperm concentration and 12.7% (2.0–26.0%) versus 38.6% (9.1–81.6%) for sperm motility. As the Aarhus clinic would only include participants until June 2018, only the Copenhagen-based clinic followed the ESHRE External Quality Assessment scheme (Centre for Andrology, Karolinska University Hospital, Stockholm, Sweden). The average Z-scores based on four semen samples at each test period; winter 2017, spring 2018, and winter 2018, were −0.04 for semen concentration, −0.60 for motility, and 0.27 for all progressive compared to expert reference examiners. This was comparable to previous studies and well below ± 3, considered acceptable for semen quality measures.Citation45
Biobank
Additional quantities of biological material were collected for long-term storage at −80°C in the DNB at Statens Serum Institut, Copenhagen, Denmark, where all other samples collected within DNBC are also stored. The amounts sent to the DNB were three 2 mL MaxxLine tubes each containing 250 µL semen, three 2 mL MaxxLine tubes with 1.5 mL urine, four 2 mL MaxxLine tubes with 600 µL plasma, four 2 mL MaxxLine tubes containing 600 µL serum, and two 4.5 mL CryoPure tubes with 3.5 mL EDTA whole blood.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki. The establishment of the FEPOS cohort was approved by the Scientific Research Ethics Committee for Copenhagen and Frederiksberg (No. H-16,015,857) and the Danish Data Protection Agency (P-2019-503). Recruitment and data collection were also permitted by the Steering Committee of the DNBC (Ref. no. 2016–08).
Results
Cohort Characteristics
Selected maternal characteristics are presented in . Mean age of the mothers was 30.5 years. Smoking during first trimester was relatively common (23%) and most maternal plasma samples were from the first trimester (92%). Median concentration of mono-(4-methyl-7-carboxyheptyl) phthalate measured in blood was 0.3 ng/mL (10th-90th percentile (p10-p90): 0.2 ng/mL, 0.8 ng/mL) and median PFOS was 26.3 ng/mL (p10-p90: 17.0 ng/mL, 40.1 ng/mL). Selected characteristics of the sons are presented in . Besides more sons from the Aarhus clinic donating hair samples (90% versus 58% in the Copenhagen clinic) no big differences between sons from the Aarhus clinic and the Copenhagen clinic were observed. Of the 1054 sons who delivered a semen sample 87% chose to do so at the clinics. Azoospermia was detected in 17 (2%) young men. Median mono-(4-methyl-7-carboxyheptyl) phthalate measured in urine was 3.6 ng/mL (p10-p90: 1.0 ng/mL, 12.6 ng/mL) and median PFOS was 4.3 ng/mL (p10-p90: 2.6 ng/mL, 7.2 ng/mL).
Table 2 Selected Maternal Characteristics of the 1057 Mothers to the 1058 Included Sons in the FEPOS Cohort*
Table 3 Selected Characteristics of 1058 Sons Included in the FEPOS Cohort*
Semen parameters are presented in . The median total sperm count was 104.9 million for sons from the Copenhagen clinic compared to 95.9 million among sons from the Aarhus clinic. The median time from ejaculation until motility analysis was longer in the Copenhagen clinic (45 versus 35 minutes). The median values across all other semen parameters were similar among sons from the Copenhagen and Aarhus clinics.
Table 4 Semen Characteristics of 1054 Sons Who Delivered a Semen Sample in the FEPOS Cohort
Compared to semen parameters of 365 male cohort members 20–22 years of age in the Testicular Function Study nested in the Western Australian Pregnancy Cohort (Raine)Citation46 the median across all semen parameters were a little lower for FEPOS participants. A slightly lower median across all semen parameters was also found compared to those of young Danish men presenting for compulsory medical examination upon military conscription,Citation47 although the variation in FEPOS was smaller. The most pronounced differences were for semen volume and total sperm count which might be due to a slightly longer ejaculatory abstinence among conscripts than FEPOS participant (median 2.5 days versus 2 days). The longer abstinence time in conscripts can largely be ascribed to stricter requirements regarding abstinence (participants not complying were rescheduled) whereas all FEPOS participants were included even though they did not comply with abstinence time instructions. Compared to WHO’s lower reference limitsCitation43,Citation48 86% had a semen volume of ≥1.5 mL, 83% had a sperm concentration of ≥15 million/mL, 79% had a total sperm count of ≥39 million, 95% had ≥32% motile sperm, and 74% had ≥4% morphologically normal sperm. As the WHO reference limits were derived by studies of fertile men who recently became fathers it is to be expected that less than 95% of an unselected cohort like FEPOS have semen parameters above the reference limits.Citation46,Citation48–Citation50
Discussion
To date, only few birth cohorts have detailed maternal information and biological specimens and a sufficient follow-up period and population size that enables assessment of prenatal determinants of reproductive parameters in the offspring.Citation14,Citation51–Citation55 Of these, the Raine Testicular Function StudyCitation46,Citation54 compares best to the FEPOS cohort. With 1058 participants the FEPOS cohort is the largest population-based male-offspring cohort worldwide with wide-ranging information on maternal health, lifestyle, socioeconomic status, occupation, and a maternal blood sample analyzed for exposure biomarkers of several xenobiotic chemicals combined with biological markers of fertility in their sons collected after puberty. The detailed and prospectively collected prenatal information allow for comprehensive confounder control and reduces the risk of information bias.Citation56 Data collected from the mothers and children at different timepoints in the children’s lives by the DNBC and information on environmental exposures collected in the FEPOS cohort further enables studies on childhood and adolescence mediators. The possibility for linkage of the FEPOS cohort to the comprehensive Danish national health registers further increase the value of the FEPOS cohort.
The response rate of 19% is in line with response rates from previous semen studiesCitation56–Citation58 including the semen study among young Danish conscripts.Citation59 It is lower compared to the Raine Testicular Function Study of 46% (40% delivered a semen sample)Citation56 and the Danish study including sons of mothers from Healthy Habits for Two with a response rate of 49%.Citation23 The lower participation in FEPOS may be explained by the contact via E-boks which can be challenging for this younger age group, but the alternatives were too costly and possibly not more effective. Another reason for the lower participation could be the demanding recruitment process. To be in accordance with Danish ethical regulations, the recruitment process involved four steps entirely depending on action from the young men. This included verbal confirmation of participation, online informed consent, self-administered questionnaire, and online booking of clinical visit, between the initial invitation and final participation at the clinics. Future research studies assessing reproductive health among young men should focus on how to get hold of participants in a less demanding way.
Differential selection related to exposures, as well as semen quality has the potential to bias the risk estimates. In studies of fertility, selection bias is often caused by the higher propensity for participation by men concerned about their fertility, trying to father children or previously diagnosed with urogenital disorders or suspected infertility.Citation60 Participants in the FEPOS cohort were 18–19 years of age and presumably not yet concerned or aware of their fertility status, which minimizes the risk of self-selection bias. Moreover, a Danish study found that biomarkers of spermatogenesis, such as inhibin B level, did not differ between men who chose to participate in a semen study and men who did not.Citation59 The sons were intentionally not informed about the specific exposures of interest but only that the focus was fetal exposure. Therefore, the sons’ participation is unlikely to depend on exposure status. However, differential selection together with potential misclassification of exposure and outcomes should be considered in each specific hypothesis tested in this population. To minimize the risk of selection bias it is possible to use selection weightsCitation61 in the upcoming FEPOS studies.
Perspectives
The FEPOS cohort provides opportunity to study the “fetal origins of adult disease hypothesis” related to several suspected exposures.Citation18 Analyses of biological samples for several xenobiotic chemicals and questionnaire information from both the mother and son combined with linkage to nationwide Danish health registers provide countless research opportunities. The first two publications using the FEPOS cohort have just been published.Citation62,Citation63 The first study investigated fetal exposure to paternal smoking and semen quality in the adult son and found a lower semen concentration and total sperm count among sons paternally, but not maternally, exposed to smoking. Indication of a higher risk of small testicular volume among sons of smoking fathers, compared to sons of non-smoking fathers were also observed.Citation62 The second study investigated semen quality among young men taking protein supplements and found no association between use of protein supplements and semen parameters.Citation63
Data Sharing Statement
The FEPOS cohort is managed by researchers from multiple Danish institutions and is overseen by a scientific reference group consisting of researchers from the DNBC management group amongst others. The cohort is considered an open access resource for researchers with projects that fall within the policy and overall aim of the DNBC [https://www.dnbc.dk/access-to-dnbc-data]. The scientific management team reserves the right to prioritize ongoing projects and encourages external applicants to collaborate with Danish researchers including principal investigator of FEPOS, Sandra Søgaard Tøttenborg [[email protected]]. Further questions can be directed to the DNBC administrative office [[email protected]].
Author Contributions
Birgit Bjerre Høyer, Ina Olmer Specht, Jens Peter Bonde, Anne-Marie Nybo Andersen, Jørn Olsen, Christian Lindh and Aleksander Giwercman acquired funding. All authors made substantial contributions to conception and design. Data were acquired by Katia Keglberg Hærvig, Birgit Bjerre Høyer, Aleksander Giwercman, Cecilia Høst Ramlau-Hansen, Gunnar Toft, Jens Peter Bonde, Christian Lindh, and Sandra Søgaard Tøttenborg. The original draft was prepared by Katia Keglberg Hærvig. All authors contributed to data analysis and interpretation, revision of the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgments
We are grateful to all participants and to biomedical laboratory technologists Marianne Lipka Flensborg and Joan Dideriksen for running the clinics and collecting data. We also thank Josefine Rahbæk Larsen for assisting with recruitment and data entry, Cecilia Tingsmark for doing the morphology analysis, and Aleksandra Kondic for DNA fragmentation analyses.
The Danish National Birth Cohort was established with a significant grant from the Danish National Research Foundation. Additional support was obtained from the Danish Regional Committees, the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Health Foundation and other minor grants. The DNBC Biobank has been supported by the Novo Nordisk Foundation and the Lundbeck Foundation.
Disclosure
Prof. Dr. Aleksander Giwercman reports grants from Ferring Pharmaceuticals and personal fees from Besins Healthcare Nordic and Sandoz, outside the submitted work. The authors declare that they have no other possible conflicts of interest in this work.
Additional information
Funding
References
- Nielsen HS, Schmidt L, Andersen AN, et al. Forebyggelse af nedsat frugtbarhed [Prevention of infertility, in Danish]. 2016 1–152.
- Schmidt L. Infertility and assisted reproduction in Denmark epidemiology and psychosocial consequences. Dan Med Bull. 2006;53:390–417.17150146
- Louis JF, Thoma ME, Sørensen DN, et al. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology. 2014;1(5):741–748. doi:10.1111/j.2047-2927.2013.00110.x
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion [Internet]. Fertil Steril. 2015;103:e18–25.25597249
- Jørgensen N, Joensen UN, Jensen TK, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2(4):e000990. doi:10.1136/bmjopen-2012-000990
- Ramlau-Hansen CH, Thulstrup AM, Aggerholm AS, Jensen MS, Toft G, Bonde JP. Is smoking a risk factor for decreased semen quality ? A cross-sectional analysis. Hum Reprod. 2007;22(1):188–196. doi:10.1093/humrep/del36416966350
- Gabrielsen JS, Tanrikut C. Chronic exposures and male fertility: the impacts of environment, diet, and drug use on spermatogenesis. Andrology. 2016;4(4):648–661. doi:10.1111/andr.1219827230702
- Jensen TK, Gottschau M, Otto J, et al. Habitual alcohol consumption associated with reduced semen quality and changes in reproductive hormones; a cross-sectional study among 1221 young Danish men. BMJ Open. 2014;1–9.
- Villalta J, Ballesca JL, Nicolas JM, Osaba De MJM, Antunez E, Pimentel C. Testicular function in asymptomatic chronic alcoholics: relation to ethanol intake. Alcohol Clin Exp Res. 1997;21(1):128–133.9046385
- Gaskins AJ, Mendiola J, Afeiche M, Jørgensen N, Swan SH, Chavarro JE. Physical activity and television watching in relation to semen quality in young men. Br J Sports Med. 2015;49(4):265–270. doi:10.1136/bjsports-2012-09164423380634
- Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Louis GMB. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014;29(2):193–200. doi:10.1093/humrep/det42824306102
- Jensen TK, Andersson A, Skakkebæk NE, et al. Original contribution association of sleep disturbances with reduced semen quality: a cross- sectional study among 953 healthy young Danish men. Am J Epidemiol. 2013;177(10):1027–1037. doi:10.1093/aje/kws42023568594
- Zamkowska D, Karwacka A, Jurewicz J, Radwan M. Environmental exposure to non-persistent endocrine disrupting chemicals and semen quality: an overview of the current epidemiological evidence. Int J Occup Med Environ Health. 2018;31(4):377–414. doi:10.13075/ijomeh.1896.0119530160090
- Vested A, Ramlau-Hansen CH, Olsen SF, et al. Associations of in Utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect. 2013;121(4):453–458. doi:10.1289/ehp.120511823360585
- Wdowiak A, Skrzypek M, Stec M, Panasiuk L. Effect of ionizing radiation on the male reproductive system. Ann Agric Environ Med. 2019;26(2):210–216. doi:10.26444/aaem/10608531232047
- Jurewicz J, Dziewirska E, Radwan M, Hanke W. Air pollution from natural and anthropic sources and male fertility. Reprod Biol Endocrinol. 2018;16:109. doi:10.1186/s12958-018-0430-230579357
- Bonde JPE. Occupational causes of male infertility. Curr Opin Endocrinol Diabetes Obes. 2013;20(3):234–239. doi:10.1097/MED.0b013e32835f3d4b23422246
- Barker D, Osmond C. Infant mortality childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;327(8489):1077–1081. doi:10.1016/S0140-6736(86)91340-1
- Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–1396. doi:10.1016/0140-6736(93)90953-E8098802
- Skakkebæk NE, Rajpert-de Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects: opinion. Hum Reprod. 2001;16(5):972–978. doi:10.1093/humrep/16.5.97211331648
- Skakkebaek NE, Rajpert-de Meyts E, Buck Louis GM, et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev. 2015;96(1):55–97. doi:10.1152/physrev.00017.2015
- Kilcoyne KR, Mitchell RT. Assessing the impact of in-utero exposures: potential effects of paracetamol on male reproductive development. Arch Dis Child. 2017;102:1169–1175. doi:10.1136/archdischild-2016-31137428588045
- Ramlau-Hansen CH, Nohr EA, Thulstrup AM, Bonde JP, Storgaard L, Olsen J. Is maternal obesity related to semen quality in the male offspring ? A pilot study. Hum Reprod. 2007;22(10):2758–2762. doi:10.1093/humrep/dem21917704100
- Ramlau-Hansen CH, Thulstrup AM, Storgaard L, Toft G, Olsen J, Bonde JP. Is prenatal exposure to tobacco smoking a cause of poor semen quality? A follow-up study. Am J Epidemiol. 2007;165(12):1372–1379. doi:10.1093/aje/kwm03217369608
- Axelsson J, Rylander L, Rignell-Hydbom A, Silfver KÅ, Stenqvist A, Giwercman A. The impact of paternal and maternal smoking on semen quality of adolescent men. PLoS One. 2013;8(6):e66766. doi:10.1371/journal.pone.006676623840528
- Ramlau-Hansen CH, Toft G, Jensen MS, Strandberg-Larsen K, Hansen ML, Olsen J. Maternal alcohol consumption during pregnancy and semen quality in the male offspring: two decades of follow-up. Hum Reprod. 2010;25(9):2340–2345. doi:10.1093/humrep/deq14020587536
- Kilcoyne KR, Mitchell RT. Effect of environmental and pharmaceutical exposures on fetal testis development and function: a systematic review of human experimental data. Hum Reprod Update. 2019;25(4):397–421. doi:10.1093/humupd/dmz00430869130
- Bonde JP, Flachs EM, Rimborg S, et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: a systematic review and meta-analysis. Hum Reprod Update. 2016;23(1):104–125. doi:10.1093/humupd/dmw03627655588
- Nybo Andersen AM, Olsen J. The Danish national birth cohort: selected scientific contributions within perinatal epidemiology and future perspectives. Scand J Public Health. 2011;39(7):115–120. doi:10.1177/140349481140767421775368
- Olsen J, Melbye M, Olsen SF, et al. The Danish national birth cohort–its background, structure and aim. Scand J Public Health. 2001;29(4):300–307. doi:10.1177/1403494801029004020111775787
- Ebert JF, Huibers L, Christensen B, Christensen MB. Paper- or web-based questionnaire invitations as a method for data collection: cross-sectional comparative study of differences in response rate, completeness of data, and financial cost. J Med Internet Res. 2018;20(1):e24. doi:10.2196/jmir.835329362206
- Monitoring Smoking Habits in the Danish Population 2015 (Danskernes rygevaner 2015) [homepage on the Internet]. Danish Health Authority, Danish Lung Association, Danish Heart Foundation, Danish Cancer Society.Available from: https://www.sst.dk/da/udgivelser/2016/danskernes-rygevaner-2015. Accessed August 20, 2016
- Eskildsen A, Dalgaard VL, Nielsen KJ, et al. Cross-cultural adaptation and validation of the danish consensus version of the 10-item perceived stress scale. Scand J Work Environ Health. 2015;41(5):486–490. doi:10.5271/sjweh.351026111225
- Wise LA, Mckinnon C, Wesselink A, Rothman KJ, Hatch EE. Sleep and male fecundity in a north American preconception cohort study. Fertil Steril. 2016;106(3):e79. doi:10.1016/j.fertnstert.2016.07.231
- Jakobsen P & Bach E. Work Environment and Health in Denmark 2012 (Arbejdsmiljø og helbred i Danmark 2012). The National Research Center for the Working Environment (NFA). 2013. ISBN: 978-87-7904-253-7.
- Ramlau-Hansen CH, Thulstrup AM, Bonde JP, Ernst E. Is self-measuring of testicular volume by a Prader orchidometer a valid method? Fertil Steril. 2007;87(6):1480–1482. doi:10.1016/j.fertnstert.2006.11.03217296192
- Jensen MS, Nørgaard-pedersen B, Toft G, et al. Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environ Health Perspect. 2012;120(6):897–903. doi:10.1289/ehp.110452222398305
- Gustafsson P, Rylander L, Lindh CH, Jönsson BAG. Vitamin D status at birth and future risk of attention deficit/hyperactivity disorder (ADHD). PLoS One. 2015;10:1–9. doi:10.1371/journal.pone.0140164
- Lindh CH, Rylander L, Toft G, et al. Blood serum concentrations of perfluorinated compounds in men from Greenlandic Inuit and European populations. Chemosphere. 2012;88(11):1269–1275. doi:10.1016/j.chemosphere.2012.03.04922494529
- Gyllenhammar I, Glynn A, Jönsson BAG, et al. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs ? Environ Res. 2017;153:48–54. doi:10.1016/j.envres.2016.11.01227898309
- Kalliokoski O, Jellestad FK, Murison R. A systematic review of studies utilizing hair glucocorticoids as a measure of stress suggests the marker is more appropriate for quantifying short-term stressors. Sci Rep. 2019;9(1):1–14. doi:10.1038/s41598-019-48517-230626917
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Investig Med. 2007;30(5):183–192. doi:10.25011/cim.v30i5.2894
- WHO. Examination and Processing of Human Semen. V World Health; 2010.
- Aleksander G, Marcello S, Jaana L, Jens Peter EB. Quality assurance of semen analysis in multicenter studies Quality assurance of semen analysis in multicenter studies. Scand J Work Environ Health. 1999;25(1):23–25.10235402
- Palacios ER, Clavero A, Gonzalvo MC, et al. Acceptable variability in external quality assessment programmes for basic semen analysis. Hum Reprod. 2012;27(2):314–322. doi:10.1093/humrep/der41322144421
- Hart RJ, Doherty DA, McLachlan RI, et al. Testicular function in a birth cohort of young men. Human Reprod. 2015;30(12):2713–2724. doi:10.1093/humrep/dev244
- Priskorn L, Nordkap L, Bang AK, et al. Average sperm count remains unchanged despite reduction in maternal smoking: results from a large cross-sectional study with annual investigations over 21 years. Human Reprod. 2018;33(6):998–1008. doi:10.1093/humrep/dey090
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Human Reprod Update. 2009;16(3):231–245. doi:10.1093/humupd/dmp048
- Iwamoto T, Nozawa S, Mieno MN, et al. Semen quality of 1559 young men from four cities in Japan: a cross-sectional population-based study. BMJ Open. 2013;3:4–8. doi:10.1136/bmjopen-2012-002222
- Iwamoto T, Nozawa S, Yoshiike M, et al. Semen quality of fertile Japanese men: a cross-sectional population-based study of 792 men. BMJ Open. 2013;3:1–9. doi:10.1136/bmjopen-2012-002223
- Axelsson J, Rylander L, Rignell-Hydbom A, Lindh CH, Jönsson BAG, Giwercman A. Prenatal phthalate exposure and reproductive function in young men. Environ Res. 2015;138:264–270. doi:10.1016/j.envres.2015.02.02425743932
- Mocarelli P, Gerthoux PM, Needham LL, et al. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect. 2011;119(5):713–718. doi:10.1289/ehp.100213421262597
- Vested A, Ramlau-Hansen CH, Olsen SF, et al. In utero exposure to persistent organochlorine pollutants and reproductive health in the human male. Reproduction. 2014;148(6):635–646. doi:10.1530/REP-13-048825190505
- Straker L, Mountain J, Jacques A, et al. Cohort profile: the Western Australian pregnancy cohort (raine) study–generation 2. Int J Epidemiol. 2017;46(5):1384–1385j. doi:10.1093/ije/dyw30828064197
- Larsen PS, Kamper-jørgensen M, Adamson A, et al. Pregnancy and birth cohort resources in Europe: a large opportunity for aetiological child health research. Paediatr Perinat Epidemiol. 2013;27(4):393–414. doi:10.1111/ppe.1206023772942
- Jensen TK, Jørgensen N, Punab M, et al. Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1770 young men from the general population in five European countries. Am J Epidemiol. 2004;159(1):49–58. doi:10.1093/aje/kwh00214693659
- Cohn BA, Overstreet JW, Fogel RJ, Brazil CK, Baird DD, Cirillo PM. Epidemiologic studies of human semen quality: considerations for study design. Am J Epidemiol. 2002;155(7):664–671. doi:10.1093/aje/155.7.66411914194
- Stewart TM, Liu DY, Garrett C, Brown EH, Baker HWG. Recruitment bias in studies of semen and other factors affecting pregnancy rates in fertile men. Human Reprod. 2009;24(10):2401–2408. doi:10.1093/humrep/dep215
- Andersen AG, Jensen TK, Carlsen E, et al. High frequency of sub-optimal semen quality in an unselected population of young men. Human Reprod. 2000;15(2):366–372. doi:10.1093/humrep/15.2.366
- Eustache F, Auger J, Cabrol D, Jouannet P. Are volunteers delivering semen samples in fertility studies a biased population? Human Reprod. 2004;19(12):2831–2837. doi:10.1093/humrep/deh503
- Hernán MA, Hernández-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi:10.1097/01.ede.0000135174.63482.4315308962
- Hærvig KK, Høyer BB, Giwercman A, et al. Fetal exposure to paternal smoking and semen quality in the adult son [published online ahead of print, 2020 Mar 9]. Andrology. 2020.
- Tøttenborg S, Glazer C, Hærvig K, et al. Semen quality among young healthy men taking protein supplements [Accepted for publication]. Fertil Steril. 2020. doi:10.1016/j.fertnstert.2020.02.103