Abstract
The human bacterial pathogen Neisseria meningitidis remains a serious worldwide health threat, but progress is being made toward the control of meningococcal infections. This review summarizes current knowledge of the global epidemiology and the pathophysiology of meningococcal disease, as well as recent advances in prevention by new vaccines. Meningococcal disease patterns and incidence can vary dramatically, both geographically and over time in populations, influenced by differences in invasive meningococcal capsular serogroups and specific genotypes designated as ST clonal complexes. Serogroup A (ST-5, ST-7), B (ST-41/44, ST-32, ST-18, ST-269, ST-8, ST-35), C (ST-11), Y (ST-23, ST-167), W-135 (ST-11) and X (ST-181) meningococci currently cause almost all invasive disease. Serogroups B, C, and Y are responsible for the majority of cases in Europe, the Americas, and Oceania; serogroup A has been associated with the highest incidence (up to 1000 per 100,000 cases) and large outbreaks of meningococcal disease in sub-Saharan Africa and previously Asia; and serogroups W-135 and X have emerged to cause major disease outbreaks in sub-Saharan Africa. Significant declines in meningococcal disease have occurred in the last decade in many developed countries. In part, the decline is related to the introduction of new meningococcal vaccines. Serogroup C polysaccharide-protein conjugate vaccines were introduced over a decade ago, first in the UK in a mass vaccination campaign, and are now widely used; multivalent meningococcal conjugate vaccines containing serogroups A, C, W-135, and/or Y were first used for adolescents in the US in 2005 and have now expanded indications for infants and young children, and a new serogroup A conjugate vaccine has recently been introduced in sub-Saharan Africa. The effectiveness of these conjugate vaccines has been enhanced by the prevention of person-to-person transmission and herd immunity. In addition, progress has been made in serogroup B-specific vaccines based on conserved proteins and outer membrane vesicles. However, continued global surveillance is essential in understanding and predicting the dynamic changes in the epidemiology and biological basis of meningococcal disease and to influence the recommendations for current and future vaccines or other prevention strategies.
Introduction
Human infections caused by meningococcus (Neisseria meningitidis) remain a serious health problem, infecting 500,000 to 1.2 million people and killing between 50,000 and 135,000 per year worldwide.Citation1 Infections due to N. meningitidis can present as a spectrum of clinical illness, with meningitis and septicemia being the most common, but also including pneumonia, septic arthritis, pericarditis, conjunctivitis, and urethritis.Citation2 Meningococcal meningitis (infection of the subarachnoid space involving the meninges and the central nervous system) often presents with fever, rash, meningeal signs (headache, stiff neck), and altered mental status. Deafness or other cranial nerve loss and long-term cognitive disability can also be a consequence.Citation2 Meningococcal septicemia is a fulminant infection (sometimes < 24 hours) with initial symptoms that are nonspecific (fever, muscle aches) and is difficult to diagnose before the onset of a maculopapular, petechial, or purpuric rash. Septicemia can result in rapid onset of hypotension, multiorgan dysfunction shock, peripheral ischemia, limb loss, and death. Overall mortality for invasive meningococcal disease is approximately 10% of infected individuals,Citation3 but is up to 40% in cases of septicemia.
The diagnosis of meningococcal meningitis is confirmed by cerebrospinal fluid pleocytosis, Gram stain, polymerase chain reaction, culture of cerebrospinal fluid, or cultures of blood or skin lesions.Citation2 Diagnosis of other invasive meningococcal disease is based on blood or skin lesion culture or Gram stain, polymerase chain reaction, and culture of normally sterile sites, such as synovial or pericardial fluid. In addition to polymerase chain reaction, rapid tests used to identify N. meningitidis based on latex agglutination or chromatography immunodetection have been used.Citation4 Early antibiotic treatment with a third-generation cephalosporin, penicillin, or meropenam to stop proliferation of N. meningitidis immediately is the primary goal of treating infected individuals.Citation4 Due to the potential for contact and epidemic spread, rapid onset, high case-fatality rate, and neurologic sequelae of meningococcal disease, a single case elicits an immediate public health response. Chemoprophylaxis with rifampin, ciprofloxacin, ceftriaxone, or azithromycin to eradicate nasopharyngeal carriage of the meningococcus is recommended for close contacts of patients to protect susceptible individuals and prevent further transmission.Citation5 Current prevention measures also include immunization with meningococcal polysaccharide conjugate vaccines directed at one to four meningococcal serogroups, ie, A, C, Y, and W-135.Citation5 Serogroup B vaccines based on outer membrane vesicles have been used to control serogroup B outbreaks.Citation6–Citation8
Changing global epidemiology
Meningococci are classified according to serologic typing based on the biochemical composition of the capsular polysaccharide. In total, 13 serogroups of N. meningitidis have been reported, with A, B, C, E, H, I/K, L, W-135, X, Y and Z confirmed genetically.Citation1 However, six serogroups (A, B, C, W-135, X, and Y) cause almost all worldwide life-threatening disease.Citation2 Genomic typing (eg, multilocus sequence typing) and whole genome comparisons have unlocked a broader understanding of the global epidemiology of meningococcal disease. With multilocus sequence typing, meningococcal isolates are classified into different sequence types based on polymorphisms in seven housekeeping genes considered not to be under selective pressure.Citation9
The incidence of meningococcal disease is cyclical in nature, having peaks and troughs every 5–8 years in some epidemiologic settings.Citation10 However, disease patterns and incidence vary in populations geographically and over time among the different invasive meningococcal serogroups and sequence type (ST) clonal complexes.
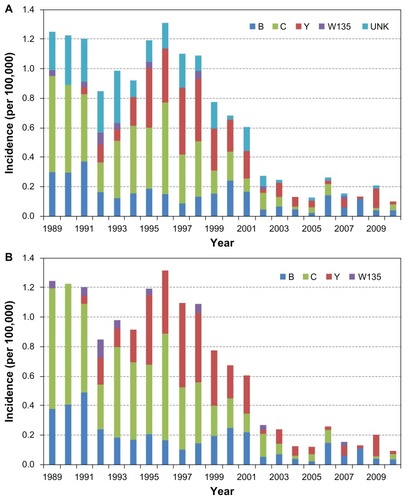
In the US, active bacterial core surveillance is a prospective laboratory and population-based surveillance system that tracks invasive bacterial pathogens including N. meningitidisCitation11,Citation12 (). The incidence of meningococcal disease in the US in the last quarter century peaked at roughly 1.7 per 100,000 in the mid 1990s and since has continually declined to 0.35 per 100,000 in 2007.Citation11,Citation12 The distribution of serogroups causing disease has also shifted.Citation13 Serogroup C accounted for the majority of cases in the first decade of surveillance. However, the incidence of serogroup C diminished significantly after 1999, before the introduction of meningococcal conjugate vaccines.Citation10 In comparison, the rates of serogroup B was consistent between 1992 and 2001, but have also declined since 2001. Serogroup Y cases emerged in the mid 1990s and the disease incidence peaked in 1997. Although decreases in serogroup Y incidence have occurred since 2001 with the overall decline in the incidence of meningococcal disease, serogroup Y continues to cause disease in the population (). Almost all of the serogroup C and Y meningococcal disease was caused by the ST-11 complex and ST-23 clonal complexes, respectively,Citation14 suggesting that closely related strains circulate in the community and cause sporadic disease.
Figure 1 (A) Active bacterial core surveillance of the incidence of meningococcal disease by serogroups, 1988–2010, Metropolitan Atlanta, GA. (B) Cases with unknown serogroups have been shown to be genetically serogroups B, C, and Y,Citation102 and thus were imputed by proportionally categorizing into those serogroups.
Serogroup A is associated with the highest incidences of meningococcal disease. In sub-Saharan countries of Africa, extending from Senegal in the west to Ethiopia in the east, known as the African meningitis belt, there have been large periodic epidemics of serogroup A meningococcal disease occurring every 8–10 years since 1905, with rates of disease that can exceed 1000 cases per 100,000.Citation10 In the last two decades, two ST clonal complexes, ST-5 and ST-7, have been responsible for African meningitis belt outbreaks due to serogroup A.Citation15 The patterns in the region are linked to environmental factors, such as climatic changes (dry season, winds of the Harmattan), coinfection, crowding, and specific population susceptibility.Citation16–Citation18 In the US as well as other industrialized countries, outbreaks of serogroup A disease occurred with similar periodicity during the first part of the 20th century, but disappeared after World War II for unknown reasons. Even though it is now virtually nonexistent in the US and most developed countries, serogroup A meningococcal disease remains a public health threat in sub-Saharan Africa and other areas of the developing world. Over the last two decades, great strides have been made in development of meningococcal conjugate vaccines with the recent successful introduction of a new serogroup A meningococcal conjugate vaccine, MenAfriVac™ (Serum Institute of India Ltd., Pune, India), in sub-Saharan Africa (see “New vaccines for prevention” section).
Serogroup B meningococcal disease is generally associated with a lower incidence of disease compared with serogroups A and C. However, serogroup B is an important cause of sporadic disease and of prolonged outbreaks in developed countries. In the US, the incidence of serogroup B meningococcal disease has fluctuated, but currently is contributing to 30%–40% of all meningococcal disease.Citation1 In the Pacific Northwest (Oregon and parts of Washington State), serogroup B (clonal complex ST-32) caused a prolonged outbreak in 1993–2007.Citation19 Curiously, serogroup B disease is now rare in sub-Saharan Africa. There is greater genetic diversity and thus antigenic diversity in serogroup B strains that cause sporadic serogroup B disease compared with other serogroups, with ST-41/44, ST-32, ST-18, ST-269, ST-35, and ST-8 causing the majority of serogroup B cases, and a number of other STs are found in collections of serogroup B isolates.Citation20 Novel serogroup B strains are occurring worldwide and the diversity of clonal complexes causing serogroup B presents a challenge to control through vaccination. Also, a number of ST-11 isolates, a clonal complex usually associated with serogroup C, express the serogroup B capsule.Citation20 As described below, meningococcal “capsule-switching”Citation21 due to transformation and recombination at the cps locus allows escape of vaccine-induced or natural protective immunity. This escape mechanism has raised concerns about serogroup replacement as a threat to the effectiveness of meningococcal conjugate vaccines. Serogroup B strains are of special concern due to the absence of a vaccine for the routine prevention of serogroup B disease. The serogroup B capsule is poorly immunogenic due to identity with human antigensCitation22 and is not a component of meningococcal conjugate vaccines. However, outer membrane vesicles have been developed for control of serogroup B clonal outbreaks.Citation6,Citation23 An outer membrane vesicle vaccine helped control a serogroup B outbreak in New Zealand in 2004 and a serogroup B outer membrane protein-containing vaccine, VA-MenGOC-BC® (Finlay Institute, Habana, Cuba), has been extensively used (over 55 million doses) in Cuba and Latin America.Citation7,Citation23–Citation26 New approaches based on conserved proteins are in late-stage clinical trials.Citation27–Citation31
The other serogroup that accounts for a majority of meningococcal disease cases throughout the world is serogroup C. In the US, serogroup C disease is responsible partly for endemic disease as well as clusters of local outbreaks, accounting for approximately 30% of overall disease.Citation11,Citation14 Increases in serogroup C meningococcal disease were seen in the 1980s and 1990s worldwide, attributed to the spread of a hypervirulent ST-11 complex/ET-37 complex clone.Citation10 The meningococcal serogroup C conjugate vaccine was first introduced in the UK in 1999 to address the growing incidence of disease. The incidence of meningococcal disease decreased by one half from 1999 to 2006 in Europe (following the introduction of serogroup C conjugate vaccines), but has subsequently stabilized.Citation32 As noted above, since the turn of this century, there has been a significant decline in overall meningococcal disease as well as serogroup C disease in the US.Citation12
With periodic exceptions, other serogroups account for less than 10% of all meningococcal disease. As noted, in parts of the US, serogroup Y emerged in the early 1990s and increased in incidence until the mid to late 1990s. Serogroup Y increased to almost 50% of cases in the mid 1990s, but accounted only for 2% of all meningococcal infections in the early 1990s.Citation33 In 1998, a carriage study examining nasopharyngeal specimens from 1818 high school students from hypersporadic counties in the metropolitan area of Atlanta, GA, found the rate of carriage to be 7.7% and of these, 48% were serogroup Y.Citation34 However, in 2006–2007, a similar carriage study in high school students found a much lower proportion of serogroup Y carriage.Citation35 The lower frequency of serogroup Y and overall meningococcal carriage was reflected in a decrease in invasive meningococcal cases between 1998 and 2007.Citation12 Serogroup Y disease has also been reported in South America, South Africa, Europe, and Israel.Citation36,Citation37 Most of the serogroup Y disease increase is associated with ST-23 and ST-167 clonal complexes.Citation36,Citation38 Serogroup X (ST-181) has caused localized outbreaks in certain African countries, including Kenya, Niger, and Ghana,Citation39–Citation43 but is rarely seen as a cause of disease outside of Africa. Serogroup W-135 has emerged in the last two decades as a cause of epidemic outbreaks in Hajj pilgrimages and in the African meningitis belt.Citation44,Citation45 Outbreaks caused by the spread of W-135 (ST-11) strains closely related to ST-11 serogroup C strains are believed to be in part attributable to capsule switching.Citation46
Pathophysiology and natural history
N. meningitidis, a Gram-negative β-proteobacterium of the family Neisseriaceae, is an exclusive pathogen in humans, carried asymptomatically in the nasopharynx by 5%–10% of adults in nonepidemic periods. It is an aerobic diplococcus and can be either structurally encapsulated or not encapsulated. Capsule polysaccharide expression of the bacteria plays a key role in meningococcal pathogenesis. N. meningitidis strains that cause invasive disease are almost always encapsulated, which helps survival of the bacteria during invasive disease and promotes transmission as well as protection from antibodies and phagocytic cells.Citation47 With the exception of the serogroup A and X capsules, meningococcal capsular polysaccharides associated with invasive disease are composed of or contain sialic acid units. Serogroups B and C are (α2→8)-linked and (α2→9)-linked polysialic acid, respectively,Citation48 while serogroups Y and W-135 are alternating units of D-glucose or D-galactose and sialic acid, respectively.Citation49 The serogroup A capsule is composed of (α1→6)-linked N-acetyl-mannosamine-1- phosphate,Citation50 while serogroup X expresses (α1→4)-linked N-acetyl-D-glucosamine 1-phosphate.Citation51 Other properties of the meningococcus that contribute to its virulence are expression of surface adhesive proteins such as pili that allow movement (twitching motility) and binding to and passage into epithelial cells, meningococcal endotoxin or lipo-oligosaccharide that binds to TLR-4 and produces acute vascular and cerebrospinal fluid inflammation, and proteins that bind iron as an important growth factor during colonization.Citation1
N. meningitidis has a dynamic biology in that it undergoes frequent (up to 10−3/cell) antigenic variability and escape from vaccine-induced or natural protective immunity.Citation52,Citation53 Genetic mechanisms of variability include horizontal gene transfer of DNA sequences,Citation54 phase variation through a slipped strand mispairing mechanism,Citation55–Citation57 and transposition of mobile elementsCitation58 that create surface structure variability of the organism, gene conversion, and antigenic variation via homologous recombinationCitation21,Citation59–Citation62 and regulation by the two-component regulatory system.Citation63 Capsular switching occurs through horizontal gene transfer, allowing the bacteria to exchange serogroup specific capsule biosynthesis genes and thus change its capsular phenotype.Citation21,Citation54,Citation64–Citation68 This mechanism is detected by identifying strains that are genetically related, such as by multilocus sequence typing. Capsular switching is a potential concern for vaccines that do not include protection against all meningococcal serogroups. However, no meaningful increase in meningococcal disease due to other serogroups occurred after the introduction of serogroup C conjugate vaccines in the UK.Citation69
Understanding meningococcal carriage and humanto- human transmission is a key to the understanding of meningococcal epidemiology. Hosted only by humans, transmission of the meningococcus occurs usually through large respiratory droplets from asymptomatic human carriers or individuals who are ill with upper respiratory symptoms. Meningococcal disease occurs usually 1–10 days after acquisition. Asymptomatic nasopharyngeal carriage can be another outcome of transmission and acquisition. Carriage can last for days to several months and is found in 3%–25% of human populations in cross-sectional studies.Citation70 Why the meningococcus causes invasive disease in a few individuals while colonizing the nasopharynx of many has been a fundamental question in meningococcal biology and pathogenesis. The absence of protective humoral bactericidal antibodies is a major host risk factor of invasive meningococcal disease.Citation71 Infants and very young children are at highest risk of developing meningococcal disease before serum bactericidal antibodies develop and after maternal antibodies have waned. Additional factors for abnormal bactericidal activity in human sera include congenital or acquired immunoglobulin deficiencies and complement deficiencies.Citation72–Citation78
Other individual risk factors for meningococcal disease that have been found are active and passive smoking,Citation79–Citation82 concurrent respiratory infections,Citation83 and crowding.Citation84,Citation85 The transmission of the meningococcus is clearly elevated in close contactCitation84,Citation86 and crowded living conditions (eg, barracks, dorms, pilgrimages).Citation87,Citation88 While the majority of invasive meningococcal cases are sporadic, a total of 69 outbreaks were identified in the US between mid 1994 and mid 2002, most of which were serogroup C outbreaks occurring in both the community and in institutional settings, such as nursing homes, schools, and colleges.Citation89 In addition, climatic conditions change the risk of invasive meningococcal disease. While meningococcal disease occurs year round, the majority of cases occur during the winter and early spring.Citation11 In the meningitis belt in sub-Saharan Africa, epidemics usually occur in the dry season between March and April when it is hot, arid, and dusty, and last until the rainy season.Citation16–Citation18
New vaccines for prevention
An ideal vaccine for prevention of meningococcal disease would be effective against the invasive serogroups of meningococci and would elicit long-lasting immunity in all age groups, especially infants, children, and adolescents. Meningococcal polysaccharide vaccines for A, C, Y, and W-135 have been available since the 1970s and 1980s. However, they have been or are being replaced in routine use by polysaccharide-protein conjugate vaccines. Meningococcal polysaccharides alone elicit a poor immunologic response in infants and toddlers and the serum antibody response is generally short-lived, and even in older children and adults the protective response lasts approximately 5 years. In addition, the serogroup C and A polysaccharide vaccines have been shown to induce a hyporesponsive state to repeated meningococcal C or A polysaccharide administration.Citation90
The immunogenicity of the meningococcal polysaccharide (shift from a T-independent to a T-dependent immune response) can be greatly improved by conjugation with a protein carrier (diphtheria or tetanus toxoid). Meningococcal serogroup C conjugate vaccines were first introduced in 1999 in the UK. They produced a dramatic decrease in the number of deaths and cases of invasive serogroup C meningococcal disease, as well as a 66% reduction in the carriage of N. meningitidis.Citation69 The remarkable herd immunity effect of these conjugate vaccines through preventing acquisition and transmission accounts for about 50% of their effectiveness.Citation69,Citation91,Citation92 Following the success of the meningococcal group C conjugate vaccine in circumventing the problems of the plain polysaccharide vaccine in the UK,Citation69 tetravalent conjugate vaccines incorporating capsular groups A, C, Y, and W-135, and recently a bivalent C, Y conjugate, covalently linked to tetanus or diphtheria toxoid, have been developed and are in clinical use.Citation93 In May 2005, the US Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommended the first quadrivalent meningococcal conjugate vaccine for routine use in all US adolescents for protection against serogroups A, C, W-135, and Y.Citation5 While the first quadrivalent conjugate vaccines were shown to have very high efficiency at generating protective bactericidal antibody titers, the overall population impact of the vaccine in the US was difficult to determine due to the falling incidence of disease and the initial low vaccine uptake.Citation94 Other tetravalent and bivalent meningococcal conjugate vaccines are now licensedCitation95 and the current age range of licensure approval is 2–55 years, with licensure for young children and infants approved by the US Food and Drug Administration for the C, Y, Haemophilus influenzae type b conjugate.
Even though the generalized use of the quadrivalent conjugate vaccine would be ideal, the cost of the vaccine has made these vaccines unaffordable for routine use in developing countries. The Meningitis Vaccine Project, a partnership between the World Health Organization and the Program for Appropriate Technology in Health, aims to eliminate epidemics of invasive meningococcal disease in sub-Saharan Africa and has developed a serogroup A-specific vaccine at an affordable price (<50 cents per dose).Citation96,Citation97 The vaccine, named MenAfriVac, was prequalified by the World Health Organization in June 2010.Citation98 Mass vaccination campaigns began in December 2010 in Burkina Faso and are underway in other parts of the meningitis belt.Citation98,Citation99 This is an important public health priority to reduce the incidence of serogroup A meningococcal disease in this region, which has been so devastated by epidemics due to this serogroup.
Serogroup B polysaccharide of N. meningitidis is not included in the quadrivalent meningococcal conjugate vaccines due to the structural homology between the capsular polysaccharide and human antigens, including the human neural cell adhesion molecule.Citation22,Citation100 Efforts to develop a serogroup B-specific vaccine have used outer membrane vesicles and/or targeted relatively conserved and antigenic meningococcal outer membrane proteins. Progress is being made in the development of these vaccines.Citation101
Conclusion
The human bacterial pathogen Neisseria meningitidis remains a serious worldwide health threat, but progress is being made toward the control of meningococcal infections. The incidence of meningococcal disease has decreased in developed countries in the last decade due at least in part to the new meningococcal polysaccharide-protein conjugate vaccines’ effect against serogroups C, Y, W-135; and a serogroup A meningococcal conjugate vaccine is now being introduced into sub-Saharan Africa, a region with the highest rates of meningococcal disease. However, meningococcal disease is characterized by fluctuations in incidence and shifts in serogroups and genotypes. The basis for the dynamic epidemiology of meningococcal disease is not completely understood. Continued surveillance is essential in detecting, understanding, and predicting the changes in the epidemiology of meningococcal disease. Active surveillance for serogroup-specific and genotype-specific invasive disease allows for close monitoring of trends in meningococcal disease over time. A detailed understanding of meningococcal disease in communities will affect recommendations for current or future vaccines or other prevention strategies. Meningococcal vaccines that are effective and affordable against invasive strains, elicit long-lasting immunity in all age groups, and provide significant herd immunity will allow the development of strategies for the global elimination of meningococcal disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- RouphaelNGStephensDSNeisseria meningitidis: biology, microbiology, and epidemiologyMethods Mol Biol201279912021993636
- RosensteinNEPerkinsBAStephensDSPopovicTHughesJMMeningococcal diseaseN Engl J Med2001344181378138811333996
- GoldacreMJRobertsSEYeatesDCase fatality rates for meningococcal disease in an English population, 1963–1998: database studyBMJ2003327741559659712969927
- TunkelARHartmanBJKaplanSLPractice guidelines for the management of bacterial meningitisClin Infect Dis20043991267128415494903
- BilukhaOORosensteinNPrevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Recomm Rep200554RR-712115917737
- OsterPLennonDO’HallahanJMeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strainVaccine20052317–182191219615755593
- RodriguezAPDickinsonFBalyAMartinezRThe epidemiological impact of antimeningococcal B vaccination in CubaMem Inst Oswaldo Cruz199994443344010445998
- GallowayYStehr-GreenPMcNicholasAO’HallahanJUse of an observational cohort study to estimate the effectiveness of the New Zealand group B meningococcal vaccine in children aged under 5 yearsInt J Epidemiol200938241341818988650
- BrehonyCJolleyKAMaidenMCMultilocus sequence typing for global surveillance of meningococcal diseaseFEMS Microbiol Rev2007311152617168997
- HarrisonLHTrotterCLRamsayMEGlobal epidemiology of meningococcal diseaseVaccine200927Suppl 2B51B6319477562
- RosensteinNEPerkinsBAStephensDSThe changing epidemiology of meningococcal disease in the United States, 1992–1996J Infect Dis199918061894190110558946
- CohnACMacNeilJRHarrisonLHChanges in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal diseaseClin Infect Dis201050218419120001736
- HarrisonLHEpidemiological profile of meningococcal disease in the United StatesClin Infect Dis201050Suppl 2S37S4420144015
- HarrisonLHShuttKASchminkSEPopulation structure and capsular switching of invasive Neisseria meningitidis isolates in the pre-meningococcal conjugate vaccine era – United States, 2000–2005J Infect Dis201020181208122420199241
- CaugantDANicolasPMolecular surveillance of meningococcal meningitis in AfricaVaccine200725Suppl 1A8A1117521785
- MolesworthAMCuevasLEConnorSJMorseAPThomsonMCEnvironmental risk and meningitis epidemics in AfricaEmerg Infect Dis20039101287129314609465
- GreenwoodBMBradleyAKWallRAMeningococcal disease and season in sub-Saharan AfricaLancet1985284598298302864546
- SultanBLabadiKGueganJFJanicotSClimate drives the meningitis epidemics onset in west AfricaPLoS Med200521e615696216
- DiermayerMHedbergKHoeslyFEpidemic serogroup B meningococcal disease in Oregon: the evolving epidemiology of the ET-5 strainJAMA1999281161493149710227318
- RaclozVNLuizSJThe elusive meningococcal meningitis serogroup: a systematic review of serogroup B epidemiologyBMC Infect Dis20101017520565757
- SwartleyJSMarfinAAEdupugantiSCapsule switching of Neisseria meningitidisProc Natl Acad Sci U S A19979412712768990198
- TroyFA2ndPolysialylation: from bacteria to brainsGlycobiology1992215231550990
- SotolongoFCampaCCasanuevaVCuban meningococcal BC vaccine: experiences and contributions from 20 years of applicationMEDICC Rev200891162221483377
- de MoraesJCPerkinsBACamargoMCProtective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, BrazilLancet19923408827107410781357461
- MilagresLGRamosSRSacchiCTImmune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacyInfect Immun19946210441944247927704
- NoronhaCPStruchinerCJHalloranMEAssessment of the direct effectiveness of BC meningococcal vaccine in Rio de Janeiro, Brazil: a case-control studyInt J Epidemiol1995245105010578557439
- RichmondPCMarshallHSNissenMDSafety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trialLancet Infect Dis201212859760722569484
- SantolayaMEO’RyanMLValenzuelaMTImmunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled studyLancet2012379981661762422260988
- GossgerNSnapeMDYuLMImmunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trialJAMA2012307657358222318278
- CohnACMessonnierNEInching toward a serogroup B meningococcal vaccine for infantsJAMA2012307661461522318284
- StephensDSPrevention of serogroup B meningococcal diseaseLancet2012379981659259422260987
- Control ECfDPaAnnual epidemiological report 2011–Reporting on 2009 surveillance data and 2010 epidemic intelligence dataEuro Surveill201116452001222114980
- JacksonLAWengerJDLaboratory-based surveillance for meningococcal disease in selected areas, United States, 1989–1991MMWR CDC Surveill Summ199342221308510639
- KellermanSEMcCombsKRayMGenotype-specific carriage of Neisseria meningitidis in Georgia counties with hyper- and hyposporadic rates of meningococcal diseaseJ Infect Dis20021861404812089660
- ClarkTASternEPondoTThe effect of quadrivalent (A, C, Y, W-135) meningococcal conjugate vaccine on serogroup-specific carriage of Neisseria meningitidisPresented at the 16th International Pathogenic Neissera conferenceRotterdam, The NetherlandsSeptember 7–12, 2008
- AbadRAgudeloCIBrandileoneMCMolecular characterization of invasive serogroup Y Neisseria meningitidis strains isolated in the Latin America regionJ Infect200959210411419576638
- WhitneyAMCoulsonGBvon GottbergAGenotypic comparison of invasive Neisseria meningitidis serogroup Y isolates from the United States, South Africa, and Israel, isolated from 1999 through 2002J Clin Microbiol20094792787279319571028
- TsangRSHendersonAMCameronMLGenetic and antigenic analysis of invasive serogroup Y Neisseria meningitidis isolates collected from 1999 to 2003 in CanadaJ Clin Microbiol20074561753175817442798
- MateruSCoxHSIsaakidisPSerogroup X in meningococcal disease, Western KenyaEmerg Infect Dis200713694494517582900
- GagneuxSWirthTHodgsonAClonal groupings in serogroup X Neisseria meningitidisEmerg Infect Dis20028546246611996679
- GagneuxSPHodgsonASmithTAProspective study of a serogroup X Neisseria meningitidis outbreak in northern GhanaJ Infect Dis2002185561862611865418
- BoisierPNicolasPDjiboSMeningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in NigerClin Infect Dis200744565766317278055
- DelrieuIYaroSTamekloeTAEmergence of epidemic Neisseria meningitidis serogroup X meningitis in Togo and Burkina FasoPLoS One201165e1951321625480
- AguileraJFPerrocheauAMeffreCHahneSGroupWWOutbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000Emerg Infect Dis20028876176712141959
- RaghunathanPLJonesJDTiendrebeogoSRPredictors of immunity after a major serogroup W-135 meningococcal disease epidemic, Burkina Faso, 2002J Infect Dis2006193560761616453255
- MayerLWReevesMWAl-HamdanNOutbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electophoretic type-37 complexJ Infect Dis2002185111596160512023765
- StephensDSGreenwoodBBrandtzaegPEpidemic meningitis, meningococcaemia, and Neisseria meningitidisLancet200736995802196221017604802
- LiuTYGotschlichECDunneFTJonssenEKStudies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharideJ Biol Chem197124615470347124327325
- BhattacharjeeAKJenningsHJKennyCPMartinASmithICStructural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W-135, and BO1Can J Biochem197654118814976
- LiuTYGotschlichECJonssenEKWysockiJRStudies on the meningococcal polysaccharides. I. Composition and chemical properties of the group A polysaccharideJ Biol Chem19712469284928584995120
- BundleDRJenningsHJKennyCPStudies on the group-specific polysaccharide of Neisseria meningitidis serogroup X and an improved procedure for its isolationJ Biol Chem197424915479748014211095
- DavidsenTTonjumTMeningococcal genome dynamicsNat Rev Microbiol200641112216357857
- HillDJGriffithsNJBorodinaEVirjiMCellular and molecular biology of Neisseria meningitidis colonization and invasive diseaseClin Sci (Lond)2010118954756420132098
- KrizPGiorginiDMusilekMLarribeMTahaMKMicroevolution through DNA exchange among strains of Neisseria meningitidis isolated during an outbreak in the Czech RepublicRes Microbiol1999150427328010376489
- van der EndeAHopmanCTDankertJDeletion of porA by recombination between clusters of repetitive extragenic palindromic sequences in Neisseria meningitidisInfect Immun19996762928293410338501
- BerringtonAWTanYCSrikhantaYPhase variation in meningococcal lipooligosaccharide biosynthesis genesFEMS Immunol Med Microbiol200234426727512443826
- HammerschmidtSMullerASillmannHCapsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal diseaseMol Microbiol1996206121112208809773
- HammerschmidtSHilseRvan PuttenJPModulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic elementEMBO J19961511921988598202
- CahoonLASeifertHSFocusing homologous recombination: pilin antigenic variation in the pathogenic NeisseriaMol Microbiol20118151136114321812841
- HelmRASeifertHSFrequency and rate of pilin antigenic variation of Neisseria meningitidisJ Bacteriol2010192143822382320472803
- AndrewsTDGojoboriTStrong positive selection and recombination drive the antigenic variation of the PilE protein of the human pathogen Neisseria meningitidisGenetics20041661253215020403
- BudroniSSienaEHotoppJCNeisseria meningitidis is structured in clades associated with restriction modification systems that modulate homologous recombinationProc Natl Acad Sci U S A2011108114494449921368196
- TzengYLDattaAAmbroseKDThe MisR/MisS two-component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitidisJ Biol Chem2004279350533506215173178
- AlcalaBArreazaLSalcedoCCapsule switching among C:2b:P1.2,5 meningococcal epidemic strains after mass immunization campaign, SpainEmerg Infect Dis20028121512151412498676
- SimoesMJCunhaMAlmeidaFFurtadoCBrumLMolecular surveillance of Neisseria meningitidis capsular switching in Portugal, 2002–2006Epidemiol Infect2009137216116518667108
- StefanelliPFazioCNeriASofiaTMastrantonioPFirst report of capsule replacement among electrophoretic type 37 Neisseria meningitidis strains in ItalyJ Clin Microbiol200341125783578614662983
- KerteszDACoulthartMBRyanJAJohnsonWMAshtonFESerogroup B, electrophoretic type 15 Neisseria meningitidis in CanadaJ Infect Dis19981776175417579607865
- TsangRSLawDKTylerSDPotential capsule switching from serogroup Y to B: The characterization of three such Neisseria meningitidis isolates causing invasive meningococcal disease in CanadaCan J Infect Dis Med Microbiol200516317117418159539
- BalmerPBorrowRMillerEImpact of meningococcal C conjugate vaccine in the UKJ Med Microbiol200251971772212358061
- StephensDSUncloaking the meningococcus: dynamics of carriage and diseaseLancet1999353915794194210459897
- GoldschneiderIGotschlichECArtensteinMSHuman immunity to the meningococcus. I. The role of humoral antibodiesJ Exp Med19691296130713264977280
- BishofNAWelchTRBeischelLSC4B deficiency: a risk factor for bacteremia with encapsulated organismsJ Infect Dis199016212482502355198
- HogasenKMichaelsenTMellbyeOJBjuneGLow prevalence of complement deficiencies among patients with meningococcal disease in NorwayScand J Immunol19933744874898469932
- WestendorpRGLangermansJAde BelCERelease of tumor necrosis factor: an innate host characteristic that may contribute to the outcome of meningococcal diseaseJ Infect Dis19951714105710607706790
- NadelSNewportMJBooyRLevinMVariation in the tumor necrosis factor-alpha gene promoter region may be associated with death from meningococcal diseaseJ Infect Dis199617448788808843235
- WestendorpRGLangermansJAHuizingaTWGenetic influence on cytokine production and fatal meningococcal diseaseLancet199734990461701739111542
- VermontCLde GrootRHazelzetJABench-to-bedside review: genetic influences on meningococcal diseaseCrit Care200261606511940267
- HaralambousEDollySOHibberdMLFactor H, a regulator of complement activity, is a major determinant of meningococcal disease susceptibility in UK Caucasian patientsScand J Infect Dis200638976477116938729
- HanebergBTonjumTRodahlKGedde-DahlTWFactors preceding the onset of meningococcal disease, with special emphasis on passive smoking, symptoms of ill healthNIPH Ann1983621691736676682
- CoenPGTullyJStuartJMIs it exposure to cigarette smoke or to smokers which increases the risk of meningococcal disease in teenagers?Int J Epidemiol200635233033616394119
- FischerMHedbergKCardosiPTobacco smoke as a risk factor for meningococcal diseasePediatr Infect Dis J199716109799839380476
- YusufHRRochatRWBaughmanWSMaternal cigarette smoking and invasive meningococcal disease: a cohort study among young children in metropolitan Atlanta, 1989–1996Am J Public Health199989571271710224983
- CartwrightKAJonesDMSmithAJInfluenza A and meningococcal diseaseLancet199133887665545571678811
- BakerMMcNicholasAGarrettNHousehold crowding a major risk factor for epidemic meningococcal disease in Auckland childrenPediatr Infect Dis J2000191098399011055601
- DeutchSLabouriauRSchonheyederHCCrowding as a risk factor of meningococcal disease in Danish preschool children: a nationwide population-based case-control studyScand J Infect Dis2004361202315000554
- OlcenPKjellanderJDanielssonDLindquistBLEpidemiology of Neisseria meningitidis; prevalence and symptoms from the upper respiratory tract in family members to patients with meningococcal diseaseScand J Infect Dis19811321051096797052
- HudsonPJVogtRLHeunEMEvidence for school transmission of Neisseria meningitidis during a Vermont outbreakPediatr Infect Dis1986522132173081880
- NealKRNguyen-Van-TamJSJeffreyNChanging carriage rate of Neisseria meningitidis among university students during the first week of term: cross sectional studyBMJ2000320723884684910731181
- BrooksRWoodsCWBenjaminDKJrRosensteinNEIncreased case-fatality rate associated with outbreaks of Neisseria meningitidis infection, compared with sporadic meningococcal disease, in the United States, 1994–2002Clin Infect Dis2006431495416758417
- MacDonaldNEHalperinSALawBJInduction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trialJAMA199828019168516899832000
- StephensDSProtecting the herd: the remarkable effectiveness of the bacterial meningitis polysaccharide-protein conjugate vaccines in altering transmission dynamicsTrans Am Clin Climatol Assoc201112211512321686214
- MaidenMCIbarz-PavonABUrwinRImpact of meningococcal serogroup C conjugate vaccines on carriage and herd immunityJ Infect Dis2008197573774318271745
- HarrisonLHMohanNKirkpatrickPMeningococcal group A, C, Y and W-135 conjugate vaccineNat Rev Drug Discov20109642943020514064
- PichicheroMEMeningococcal conjugate vaccinesExpert Opin Biol Ther20055111475148916255651
- HalperinSAGuptaAJeanfreauRComparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2–10 years of ageVaccine201028507865787220943209
- Marc LaForceFRavenscroftNDjingareyMVivianiSEpidemic meningitis due to Group A Neisseria meningitidis in the African meningitis belt: a persistent problem with an imminent solutionVaccine200927Suppl 2B13B1919477559
- BishaiDMChampionCSteeleMEThompsonLProduct development partnerships hit their stride: lessons from developing a meningitis vaccine for AfricaHealth Aff (Millwood)20113061058106421653957
- FraschCPreziosiMPLaforceFMDevelopment of a group A meningococcal conjugate vaccine, MenAfriVac (TM)Hum Vaccin Immunother201286 [Epub ahead of print.]
- DjingareyMHBarryRBonkoungouMEffectively introducing a new meningococcal A conjugate vaccine in Africa: the Burkina Faso experienceVaccine201230Suppl 2B40B4522607898
- McCoyRDVimrERTroyFACMP-NeuNAc:poly-alpha-2,8-sialosyl sialyltransferase and the biosynthesis of polysialosyl units in neural cell adhesion moleculesJ Biol Chem19852602312695126994044605
- HolstJStrategies for development of universal vaccines against meningococcal serogroup B disease: the most promising options and the challenges evaluating themHum Vaccin20073629029417712231
- Dolan-LivengoodJMMillerYKMartinLEUrwinRStephensDSGenetic basis for nongroupable Neisseria meningitidisJ Infect Dis2003187101616162812721942