Abstract
Cryptococcal meningitis causes morbidity and mortality worldwide. The burden of disease is greatest in middle- and low-income countries with a high incidence of human immunodeficiency virus (HIV) infection. Patients taking immunosuppressive drugs and some immunocompetent hosts are also at risk. Treatment of cryptococcal meningitis consists of three phases: induction, consolidation, and maintenance. Effective induction therapy requires potent fungicidal drugs (amphotericin B and flucytosine), which are often unavailable in low-resource, high-endemicity settings. As a consequence, mortality is unacceptably high. Wider access to effective treatment is urgently required to improve outcomes. For human immunodeficiency virus-infected patients, judicious management of asymptomatic cryptococcal antigenemia and appropriately timed introduction of antiretroviral therapy are important.
Introduction
Cryptococcosis is an important infectious disease globally. The majority of illness is among patients with defective cell-mediated immunity. Human immunodeficiency virus (HIV) infection is the main risk factor, accounting for 95% of cases in middle- and low-income countries (MLICs)Citation1 and 80% of cases in high-income countries (HICs).Citation2 Individuals taking immunosuppressive drugs (eg, transplant recipients) constitute most of the remaining caseload, although immunocompetent hosts are susceptible in some settings.
The most common clinical presentation is cryptococcal meningitis (CM), with over 1 million cases and 600,000 deaths per year.Citation3 Nonmeningeal (eg, pulmonary and cutaneous) presentations also occur,Citation4 and bloodstream infection (cryptococcemia) may disseminate to multiple sites.Citation5
This review describes the epidemiology and management of cryptococcal disease. Worldwide distribution of the pathogen is outlined, incidence trends in patients with varying risk factors are assessed, and the prognostic implications of differing treatment protocols are highlighted.
Epidemiology of the infectious pathogen
Cryptococci are encapsulated saprophytic yeasts. Two species, transmitted by inhalation, are the principal human pathogens: Cryptococcus neoformans and Cryptococcus gattii.Citation6 C. neoformans was identified by Sanfelice in 1894,Citation7,Citation8 and may be divided into two subtypes on the basis of capsular agglutination assays.Citation9 C. neoformans var. grubii (capsular serotype D) is the most common, and causes 82% of cryptococcal disease worldwide. Var. neoformans (capsular serotype A) is responsible for 20%–30% of HIV-associated CM in northern Europe (notably France, Italy, and Denmark),Citation9–Citation11 but is less common in other global regions.Citation12,Citation13 Although both subtypes predominantly cause disease in immunocompromised individuals, several reports from the USCitation14 and AsiaCitation15 suggest that var. grubii cryptococcosis in patients with normal immune systems is more common than previously assumed. The environmental reservoir of both subtypes is avian guano, decaying organic matter, and soil.Citation8,Citation16
C. gattii is traditionally associated with illness in immunocompetent individualsCitation17–Citation19 from tropical and subtropical regions, including Thailand,Citation20 northern Australia,Citation21,Citation22 New Zealand, and Papua New Guinea.Citation23–Citation26 More recently, four molecular subtypes of C. gattii have been identified with distinct epidemiological characteristics that challenge this perspective.Citation6 Whilst VGI (var gattii I) is the main subtype in Australasia, an outbreak of disease attributable to VGII has been described in immunocompetent patients from British Columbia, Canada.Citation27–Citation29 Between 1999 and 2010, 218 cases were identified on Vancouver Island.Citation30,Citation31 In 2006, a further case was reported on Orcas Island, Washington, USA,Citation32 and C. gattii is now endemic throughout the Pacific Northwest of the US.Citation29 Sporadic disease has also been notified in other parts of North America, including Florida, North Carolina, Rhode Island, New Mexico, Michigan, Georgia, and Montana.Citation33 Additionally, C. gattii subtypes VGIII and -IV are more likely to be found in HIV-infected than immunocompetent patients. These strains may account for 2.4%–30% of HIV-associated cryptococcosis in some parts of Central and South AmericaCitation6 and southern Africa.Citation34–Citation37 The burden of human disease due to C. gattii is probably underrecognized, as many laboratories do not undertake detailed speciation of cryptococci.Citation6
The environmental reservoirs of C. gattii are incompletely understood. In Australia, India, and other Asian countries, it has been isolated in eucalyptus trees.Citation38 In British Columbia, it has been isolated from noneucalyptus tree species, soil, air, freshwater, and seawater.Citation29 Discovery of this organism in heterogeneous biogeoclimatic zones suggests that its ecological niche was previously underestimated or that its distribution is expanding.Citation39,Citation40 Possible explanations for a changing distribution include climate change or altered land-use practices, such as logging.
HIV as a risk factor
The largest influence on the epidemiology of cryptococcal disease over the last 30 years has been the evolution of the HIV pandemic. shows that a fivefold increase in the incidence of cryptococcosis in France from 1985 to 1993 was almost entirely due to burgeoning disease in HIV-infected patients, while the number of cases in HIV-uninfected patients remained stable.Citation41 Similar trends were observed in other HICs. In the UK, the number of annual cryptococcal case notifications rose from 13 (8% HIV-associated) in 1982 to 66 (83% HIV-associated) in 1991.Citation42 Of 517 cryptococcal infections in New York City in 1991, 96% were HIV-related.Citation43
Figure 1 Evolution of the incidence of cryptococcosis, by year of diagnosis in France (1985–2001), as reported to the National Reference Centre for Mycosis.
Abbreviation: HIV, human immunodeficiency virus.
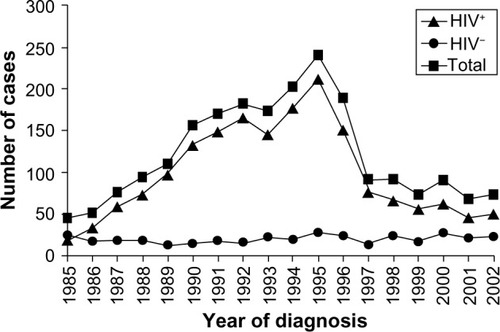
HIV-infected patients are mainly at risk of cryptococcosis when they become very immunosuppressed and their CD4 count drops below 100 cells/μL.Citation44,Citation45 Consequentially, indicates that after the development of effective combination antiretroviral therapy (ART) in 1997, the upsurge in new cases of cryptococcal disease from HICs was reversed and incidence began to decline.Citation46–Citation48 From 1997–2001, France saw a 46% decrease in cases,Citation41 from 1996 to 2007, incidence per 1,000 persons in the UK fell from three to 0.2,Citation49 and from 1992 to 2000, incidence per 1,000 persons in Atlanta, Georgia, USA fell from 66 to seven.Citation50
shows that disease trends in MLICs have been much worse. It is well recognized that sub-Saharan Africa has been the global region most heavily affected by HIV, with an estimated 2.6 million new infections per year at the peak of the epidemic in 1997.Citation51 Contemporaneously, in the 1990s, CM became the leading cause of adult meningitis in many African countries, including Malawi (27% of cases),Citation52 Zimbabwe (45% of cases),Citation53,Citation54 and South Africa (31% of cases).Citation55 Delayed and incomplete access to ART meant that unlike Europe and North America, the incidence of CM did not recede after the turn of the millennium; a tertiary referral hospital in Botswana described C. neoformans growth from 15% of all cerebrospinal fluid (CSF) samples submitted for analysis in 2003,Citation56 and a report from Cape Town, South Africa noted that CM still accounted for 31% of all inpatient days in new ART patients in 2007.Citation57 A similar persistent elevation of the cryptococcal disease burden since the 1990s has been described from MLICs in Southeast AsiaCitation56,Citation58 and Latin America.Citation59
Figure 2 Global incidence and mortality from cryptococcal meningitis among United Nations global regions from 1997 to 2007.
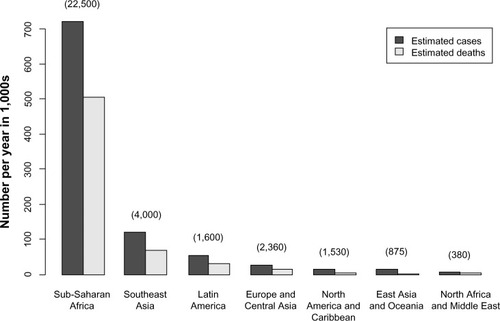
In addition to disparities in incidence between HICs and MLICs, there are well-documented differences in outcomes ().Citation3 A combination of earlier access to ART and availability of fungicidal drugs has contributed to falling mortality in HICs,Citation2,Citation60,Citation61 while the death rate in MLICs has been relentlessly high.Citation62 Analysis of pooled data from case series, surveillance reports, and clinical trials has estimated that the 90-day case-fatality rate from HIV-associated CM in East Asia, Oceania, Western Europe and the US is 9%, compared with 55% in other parts of Asia and South America and 70% in sub-Saharan Africa.Citation3 Even acknowledging that some North American studies exclude the sickest patients,Citation63 and some studies in MLICs show better case-fatality rates than others,Citation64–Citation67 these results are unacceptable.
Other risk factors
Although HIV is the largest driver of cryptococcal disease, it is important to acknowledge other factors. Prior to the HIV era in the UK, the incidence of cryptococcosis per 1,000 persons increased from 1.4 in 1963–1968 to 7.4 in 1973–1978.Citation68 This was predominantly attributable to disease in patients on immunosuppressive medications. Use of potent immunosuppressants (eg, corticosteroids, calcineurin inhibitors,Citation69 cytotoxic agents,Citation70 and monoclonal antibodiesCitation71,Citation72) for transplant conditioning or treatment of cancer and inflammatory conditions has continued to expand in HICs over the last 30 years, sustaining a small but important minority of cryptococcal illness in settings where HIV-related cases are in decline.Citation73
Cryptococcosis disease occurs after 2.8%–8% of solid-organ transplants, and is the third-commonest invasive fungal infection in this setting, after Candida and Aspergillus.Citation69,Citation74 In a retrospective review of US data from 1996 to 2010, kidney-transplant recipients were most often affected, followed by liver, heart, lung, and pancreas recipients.Citation5 The median time to diagnosis after solid-organ transplantation is 20 months, and the etiology is normally reactivation of latent disease.Citation5,Citation74,Citation75 Symptoms may emerge sooner after lung or liver transplants, perhaps because the required level of postoperative immunosuppression is higher.Citation69 The overall likelihood of cryptococcal disease does not vary between patients using tacrolimus, cyclosporine, or azathioprine as the primary immunosuppressive agent, but patients who are coadministered high-dose corticosteroids may be at higher risk.Citation5
Infrequently, cryptococcal infection is acquired from donor tissue. This is particularly suspected if disease occurs within 30 days of transplantation, at surgical graft sites, or in multiple organ recipients of a single donor.Citation75–Citation77 Screening of donors is not routinely performed, but should be undertaken if the donor has unexplained pulmonary lesions, undiagnosed neurological illness, or unexplained fever with relevant comorbid risk factors.Citation76,Citation77
Cryptococcal disease is rare following hematopoietic stem cell transplant or corneal tissue transplant.Citation78 Data from a consortium of US transplant centers (Transplant-Associated Infection Surveillance Network) revealed an incidence of only 0.6% in hematopoietic stem cell-transplant recipients between 2001 and 2006.Citation79,Citation80 For unknown reasons, the risk is higher in autologous than allogenic transplants.Citation78
Non-HIV-infected, nontransplant recipients with cryptococcosis are a heterogeneous group. Except for C. gattii outbreaks in immunocompetent hosts, most patients have immune dysfunction related to the pathophysiology or treatment of an underlying autoimmune disease, malignancy, or innate immunological disorder. It is difficult to generalize about these cases, but in HICs they tend to experience higher mortality than their HIV-infected counterparts. The reasons for this include the effects of underlying illness and late diagnosis, because the pathogen was not initially suspected.Citation5,Citation73,Citation81
Clinical presentation
Epidemiological variables, including the nature of immunosuppressive risk factors and pathogen species, influence the presentation of cryptococcal disease. CM is the leading presentation overall,Citation19,Citation41,Citation42,Citation78 but nonmeningeal manifestations are proportionally more frequent in non-HIV-infected individuals.Citation5,Citation42,Citation73,Citation82 Transplant-associated cryptococcosis is often limited to the lungs,Citation83,Citation84 with disseminated or neurological disease in 52%–61% of cases.Citation69,Citation84
Presentation of C. gattii infection in immunocompetent hosts varies according to molecular subtype. In Australia, where the majority of disease is due to VGI, CM is most common,Citation6,Citation85 but in North America, VGII disease presents with respiratory symptoms in 76%, neurological symptoms in 7.8%, and both respiratory and neurological symptoms in 10.1% of cases.Citation19,Citation24,Citation31
The commonest features of CM are subacute headache and confusion. Intracranial pressure (ICP) is often elevated, and may cause cranial nerve palsies or seizures. Classical features of “meningism” (eg, neck stiffness) occur in less than 20% of patients.Citation86 Altered mental state is associated with higher mortality.Citation5,Citation50,Citation87
Neurological infection may be complicated by mass lesions (cryptococcomas). This is more common with C. gattii than C. neoformans. Clinical sequelae of cryptococcomas include hydrocephalus and blindness.Citation88,Citation89 Some patients require neurosurgical intervention.Citation17,Citation18
Forty percent of patients with CM have ocular involvement, including papilledema and uveitis with multifocal chorioretinitis.Citation90,Citation91 Immune-mediated optic nerve dysfunction and blindness have been particularly reported amongst C. gattii patients from Papua New Guinea.Citation54,Citation89,Citation92 The spectrum of pulmonary illness ranges from asymptomatic colonization to severe, progressive pneumonia and cryptococcomas in the lungs. Skin lesions often contain the infecting organism. In severely immunocompromised individuals, disseminated disease (involvement of two or more sites) may present as fever and rash before other symptoms and signs appear.
Investigations
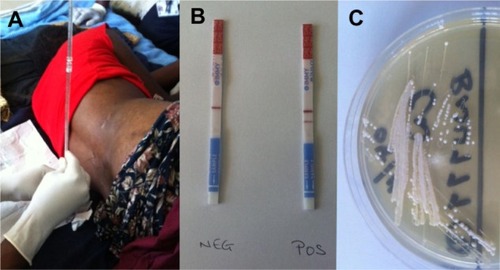
Confrmation of CM requires lumbar puncture (LP) and examination of CSF, as shown in . Lack of LP equipment may result in underestimation of the disease burden in MLICs.Citation93 Typical CSF features include a raised opening pressure (reflecting elevated ICP), lymphocytic pleocytosis, and evidence of inflammation. However, CSF may be normal in 10%–17% of patients,Citation54,Citation94 especially in HIV-endemic populations.Citation5 Identification of the infecting organism is traditionally done by light microscopy after India ink staining, but this method is user-dependent with variable sensitivity. Detection of cryptococcal antigen (CrAg) by a latex-agglutination testCitation63 or lateral flow immunoassay (LFA) is better (). The lateral flow immunoassay is cheaper than latex agglutination,Citation95,Citation96 and may be applied to urine samples when CSF is unobtainable.Citation97,Citation98 Fungal culture of CSF on Sabouraud media is required to isolate the organism for antimicrobial susceptibility testing. Characteristic colonies grow after 36 hours ().
Figure 3 (A–C) Diagnosis of cryptococcal meningitis. (A) Lumbar puncture being performed on a human immunodeficiency virus-infected patient with suspected meningitis in Malawi. (B) Lateral flow immunoassay test strips for cryptococcal antigen detection (the strip on the left shows a negative result, indicated by a single horizontal “control” band in the center; the strip on the right shows a positive result, indicated by adjacent horizontal “control” and “test” bands). (C) Cryptococcus neoformans growing on Sabouraud media. Images kindly supplied by Kate Gaskell, College of Medicine, University of Malawi, and Brigitte Denis, Malawi Liverpool wellcome Trust Clinical Research Programme.
Radiology has little role in the diagnosis of CM, but computed tomography and magnetic resonance imaging scans are necessary to detect complications (eg, cryptococcomas and noncommunicating hydrocephalus). These modalities are generally unavailable in MLICs.
Nonmeningeal cryptococcosis may be confirmed by tissue sampling for microbiological analysis. Cryptococcemia is identified from fungal blood cultures or CrAg detection in serum samples. Asymptomatic antigenemia (a positive serum CrAg in the absence of clinical disease) has been described in HIV-infected patients, and may predict impending CM. Screening and treatment for asymptomatic antigenemia will be discussed later.
Treatment and prognosis
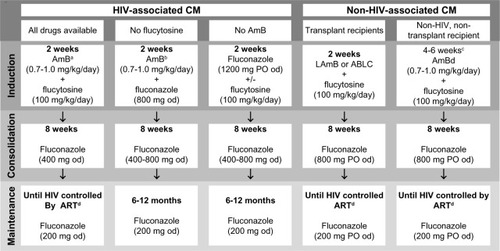
The Infectious Diseases Society of America (IDSA)Citation4 and World Health Organization (WHO)Citation99 have recently issued updated treatment guidelines to reflect advances over the last decade, but routine practice in MLICs continues to be impeded by poor drug availability. summarizes current recommendations.
Figure 4 Treatment options for cryptococcal meningitis (CM), summarized from infectious Diseases Society of America and world Health Organization guidelines.
Abbreviations: HIV, human immunodeficiency virus; AmB, amphotericin B; LAmB, liposomal amphotericin B (3–6 mg/kg/day); ABLC, amphotericin B lipid complex (5 mg/kg/day); od, once daily; PO, per os (by mouth); ART, antiretroviral therapy.
Induction and consolidation antifungal therapy for HIV-associated CM
CM treatment consists of three phases: induction, consolidation, and maintenance. IDSA and WHO guidelines emphasize the importance of potent fungicidal drugs during induction therapy, because the rate of fungal clearance from the CSF during the first 2 weeks, known as early fungicidal activity (EFA),Citation100 predicts 10-week survival,Citation101 and CSF sterilization by 14 days predicts long-term prognosis.Citation63
Amphotericin B (AmB), the drug with the greatest EFA, is administered intravenously for 14 days during induction therapy whenever possible. Its activity is concentration-dependent,Citation102 but the required dose (0.7–1.0 mg/kg) of the commonest preparation (AmB deoxycholate [AmBd])Citation33,Citation63–Citation65 can be nephrotoxic.Citation103,Citation104 A solution in HICs is the use of lipid drug formulations with fewer renal side effects, including liposomal AmB (LAmB; 3–6 mg/kg/day) and AmB lipid complex (ABLC, 5 mg/kg/day).Citation105–Citation107 The cost of these (over US$1,000 per dayCitation108) is prohibitive for MLICs, so alternative strategies, including preemptive hydration and electrolyte supplementation, have been developed to minimize the toxicity of AmBd.Citation99 Studies in Kampala,Citation62 Cape Town,Citation65 and BangkokCitation109 support this approach.
Even a 2-week course of AmBd (US$12–$15 per day) is too expensive for some LICs.Citation110 In this scenario, data from South Africa and Malawi have demonstrated that 5–7 days of AmBd is better than treatment that is restricted to less fungicidal drugs.Citation64,Citation111 AmBd-treated patients who survive the first 6 months have a subsequent 5-year survival rate of 88%.Citation112 This advocates for investment in early AmBd to achieve satisfactory long-term outcomes.
Flucytosine should accompany AmB during induction therapy at an intravenous or oral dose of 100 mg/kg/day.Citation100,Citation113–Citation115 Omission of this agent has been associated with higher rates of mortality,Citation113 treatment failure,Citation116 and late relapse.Citation117 Toxic cytopenias can occur during flucytosine therapy, and so regular full blood counts and therapeutic drug monitoring (TDM) are advised by IDSA guidelines.Citation4,Citation57,Citation118 TDM is arduous, and the absolute need for it in MLICs was questioned by a recent study in Vietnam that did not use TDM but demonstrated safe and effective use of AmBd-flucytosine.Citation113
Despite its value, flucytosine remains unlicensed in most African and Asian countries,Citation114,Citation119,Citation120 so alternative agents have been considered for combination with AmB. The obvious contender is fluconazole, which is freely distributed in MLICs (http://www.pfizer.com/responsibility/global_health/ diflucan_partnership_program), but is fungistatic rather than fungicidal in the normal human dose range. Definitive evidence to support AmB-fluconazole induction therapy is lacking, but reports from several countries describe rapid EFA when intravenous AmBd (0.7–1 mg/kg/day) is prescribed alongside oral fluconazole (800–1,200 mg/day).Citation109,Citation113,Citation121,Citation122 A meta-analysis of various induction strategies in resource-poor settings has described AmBd-fluconazole as cost-effective.Citation123 The WHO recommends this approach when flucytosine cannot be obtained.Citation99
After a 14-day induction phase, treatment proceeds with a consolidation phase of 400 mg once daily (od) for a further 8 weeks. This dose may be increased to 800 mg when gold-standard induction therapy with AmB-flucytosine is unavailable.
Gaps in drug provision leave fluconazole as the only agent for induction and consolidation therapy in many high-burden countries, contributing to significant variation in prognosis. The response to this requires discussion. Until recently, the routine dose of fluconazole for CM monotherapy was 200–400 mg od, and 8-week mortality was 78%–90%.Citation86,Citation124 In one South African study from 2006, median patient survival on induction- and consolidation-phase fluconazole (400 mg od) was only 76 days.Citation125
Such poor outcomes prompted dose escalation. Clinical studies in Uganda and Malawi based on 800 mg od induction and 400 mg od consolidation therapy reported improved 10-week mortality rates of 58%–60%.Citation87,Citation126 A further induction-phase dose increase to 1,200 mg was associated with faster EFACitation126 but did not improve survival,Citation126,Citation127 and a pharmacokinetic–pharmacodynamic bridging study from a mouse model indicated that 1,200 mg od will fail to achieve fungal stasis in the CSF of 33% of patients.Citation128 An exploratory clinical trial has suggested that induction with 1,600–2,000 mg of fluconazole would be more efficacious,Citation126,Citation129 but additional evaluation of these doses is required. WHO guidelines currently advocate that where fluconazole monotherapy is the only option, 1,200 mg od should be used for 2-week induction therapy followed by a consolidation phase of 800 mg od for 8 weeks.Citation99
A final option when intravenous drug administration is not feasible is a fully oral induction phase of fluconazole and flucytosine. Trials in Malawi have confirmed that fluconazole (1,200 mg od) and flucytosine (100 mg/kg/day) achieve faster EFA and lower 10-week mortality than fluconazole alone,Citation70,Citation111,Citation127 suggesting that fluconazole monotherapy should be augmented by induction-phase flucytosine wherever possible.
Overall, reduced availability of fungicidal drugs continues to compromise outcomes in high-burden countries. An ongoing multicenter clinical trial (Advancing Cryptococcal meningitis Treatment in Africa [ACTA]Citation130) in sub-Saharan Africa hopes to confirm the shortest, simplest effective regimens, but will require backup by universal provision of medicines for routine care.
Maintenance antifungal therapy for HIV-associated CM
After consolidation therapy, secondary prophylaxis with fluconazole (200 mg od) minimizes the risk of CM relapse.Citation131 Alternative, less effective maintenance regimens include oral itraconazole (200 mg od)Citation117 and intravenous AmBd (1 mg/kg/once weekly).Citation132
The required duration of maintenance therapy has recently been examined, particularly as the 1-year default rate from secondary prophylaxis in some African settings exceeds 90%.Citation133,Citation134 In the pre-ART era, lifelong fluconazole was recommended after a presentation with CM, but it now appears that late relapse is unlikely during successful ART.Citation135–Citation138 International guidelines state that immune restoration by ART permits discontinuation of maintenance therapy (). However, evidence to support cessation of secondary prophylaxis is weaker when induction/consolidation therapy is not fungicidal (eg, fluconazole monotherapy), and isolated CM relapses have been described in patients on ART with CD4 counts up to 495 cells/μL.Citation139
Differences in antifungal therapy for non-HIV-associated CM
Treatment of non-HIV-associated CM varies from that described above, dependent on characteristics of the host and pathogen (). Patients developing CM after solid-organ transplant often take nephrotoxic immunosuppressants (tacrolimus, cyclosporine or sirolimus) to prevent graft rejection, and 25% of transplant recipients have renal dysfunction at CM diagnosis. Therefore, kidney-friendly liposomal preparations of AmB (eg, LAmB or ABLC) are recommended during induction therapy.Citation4 It is uncertain whether immunosuppressive therapy should be stopped during CM treatment; this may accelerate eradication of the pathogen, but poses a risk of proinflammatory immune reconstitution syndrome or transplant rejection.Citation140 Although clear evidence is lacking, some authors report good outcomes with a staged reduction in immunosuppressive therapy alongside antifungal drugs.Citation141
Secondary fluconazole prophylaxis in transplant recipients may stop after 6–12 months, as the late cryptococcal relapse rate is only 1%–3%.Citation142 There is no standard regimen for non-HIV, nontransplant patients with CM. Some authors advocate a longer (4–6 weeks) induction phase of AmB/flucytosine (), while others favor a standard 2-week induction phase. Consolidation and maintenance therapy are identical to transplant recipients. C. gattii infection should be treated with the same drugs as C. neoformans, but the response to therapy may be slower, due to higher azole minimum inhibitory concentrationsCitation143,Citation144 and poor drug penetration of cryptococcomas.Citation17,Citation18,Citation85
Asymptomatic antigenemia and primary prophylaxis
Between 4% and 20% of patients with newly identified HIV infection and a CD4 count,150 cells/μL have a positive serum CrAg test in the absence of clinical cryptococcosis.Citation145–Citation147 Asymptomatic antigenemia predicts impending CM,Citation148 and is associated with increased mortality.Citation149 Serum CrAg screening of new HIV patients is being implemented in South Africa,Citation150 prompting debate on the use of preemptive therapy for those at highest risk.
Treatment of asymptomatic antigenemia is not recommended in HICs,Citation4 but some studies support fluconazole therapy for serum CrAg-positive patients in MLICs.Citation151–Citation153 A management algorithm is shown in .Citation154 However, deployment of preventive screening and therapy strategies is operationally difficult, and an implementation study in Kenya achieved effective fluconazole administration for only 52% of eligible patients.Citation155 The benefit of presumptive therapy for asymptomatic antigenemia will depend on the logistics of integrating this strategy into routine practice at ART clinics.
Figure 5 A screening and management strategy for asymptomatic antigenemia.
Abbreviations: HIV, human immunodeficiency virus; CrAg, cryptococcal antigen; LP, lumbar puncture; CM, cryptococcal meningitis; od, once daily; ART, antiretroviral therapy.
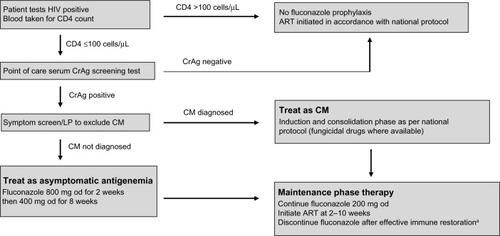
An alternative means of CM prevention is primary prophylaxis with fluconazole for all patients with CD4 counts <100 cells/μL, irrespective of serum CrAg testing. Studies of this approach in ThailandCitation156 and UgandaCitation157 demonstrated that fluconazole 200 mg once daily reduced the incidence of CM, but there was no reduction in overall mortality and rates of fluconazole-resistant Candida albicans infection increased.Citation158 The cost-effectiveness of no preventive treatment, therapy for asymptomatic antigenemia, or primary prophylaxis is likely to vary between regions, based on the underlying epidemiology of cryptococcal infection and availability of ART.
Additional therapeutic considerations
Outcomes from CM may be improved by reduction of raised ICP during early therapy, careful management of immune reconstitution inflammatory syndrome (IRIS) and appropriately timed ART initiation for HIV-infected patients.
Reducing raised intracranial pressure
Increased ICP (>25 cm/H2O) is associated with greater CSF fungal burdenCitation159 and higher mortalityCitation160 Regular CSF drainage by serial LPs is recommended.Citation115,Citation161–Citation164 Insertion of a temporary CSF-drainage catheterCitation165 or ventriculoperitoneal shunt may also be used.Citation166 Drug therapy (eg, acetazolamide or corticosteroids) to reduce CSF pressure or prevent blindness in CM patients is not beneficial, and acetazolamide may cause harm.Citation88,Citation160,Citation167
Managing immune reconstitution inflammatory syndromes
IRIS in CM patients occurs when host immune recovery triggers inflammatory reactions to persistent fungal antigens. There are two main forms: unmasking and paradoxical ().Citation168
Figure 6 Types of immune restoration inflammatory syndrome (IRIS) in cryptococcal disease.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; CM, cryptococcal meningitis.
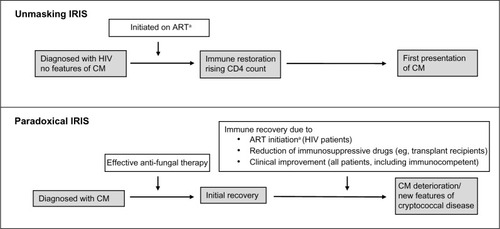
Unmasking IRIS occurs in HIV-infected patients when subclinical cryptococcal disease emerges after ART is commenced. This might be prevented by careful pre-ART screening. However, late ART is generally associated with lower survival,Citation169 and patients who are already on HIV therapy when they present with CM tend to have better outcomes,Citation64,Citation170 so excessive delays should be avoided.
Paradoxical IRIS is best described in HIV-associated CM patients who initially respond to antifungal drugs and then deteriorate within 12 months of starting ART. Studies from South Africa and Ethiopia estimate an incidence of 7%–33%.Citation171–Citation173 Paradoxical IRIS also affects solid organ-transplant patients when antirejection medications are interrupted during CM therapy,Citation141 and apparently healthy hosts may suffer from reactivation of immune defenses that were previously overwhelmed by high fungal burden.
Paradoxical IRIS in the central nervous system has a mortality of up to 36%.Citation174,Citation175 Risk factors include severe disease at presentation and slow fungal elimination.Citation176,Citation177 The benefit of anti-inflammatory drugs (eg, corticosteroidsCitation100) is unproven.Citation178 Highly fungicidal induction-phase therapy and rapid CSF steri lization is the best way to minimize unwanted reactions.Citation179,Citation180
Combining anticryptococcal and anti-HIV medications
In HIV-associated CM, IRIS is more likely during immune reconstitution from lower baseline CD4 counts, and is influenced by the timing of ART introduction. Balancing the danger of early mortality from advanced immunosuppression against that of accelerated immune recovery is difficult, and best practice remains to be established. A retrospective study of mortality after ART initiation in Thailand at time points 1–12 months into CM therapy did not show any association between timing of ART and outcome.Citation181 summarizes subsequent prospective trials.Citation135,Citation136,Citation182–Citation185 The most convincing data come from a recent study in Uganda and South Africa, which was terminated early because ART initiation within the first 28 days of CM treatment led to a higher risk of IRIS and death.Citation185 Introduction of ART 4–10 weeks after starting antifungal treatment is currently considered the safest approach.
Table 1 Prospective open-label randomized trials to assess optimal timing of antiretroviral therapy (ART) initiation in human immunodefciency virus (HIV)-infected patients with cryptococcal meningitis (CM)
Conclusion
Advanced HIV infection continues to drive cryptococcal disease worldwide. Patients on immunosuppressive drugs and some immunocompetent hosts are also at risk. Although treatment with potent drug combinations provides effective cure, poor availability of fungicidal drugs in MLICs results in high case-fatality rates. Expanded provision of fungicidal treatment is urgently required. Ongoing research on management of asymptomatic antigenemia and optimal timing of ART initiation is important to improve the prognosis of HIV-associated CM.
Disclosure
The authors report no conflicts of interest in this work.
References
- LealALFaganelloJFuentefriaAMBoldoJTBassanesiMCVainsteinMHEpidemiological profile of cryptococcal meningitis patients in Rio Grande do Sul, BrazilMycopathologia2008166717518443922
- PyrgosVSeitzAESteinerCAPrevotsDRWilliamsonPREpidemiology of cryptococcal meningitis in the US: 1997–2009PLoS One20138e5626923457543
- ParkBJWannemuehlerKAMarstonBJGovenderNPappasPGChillerTMEstimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDSAIDS20092352553019182676
- PerfectJRDismukesWEDromerFClinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of AmericaClin Infect Dis20105029132220047480
- BrizendineKDBaddleyJWPappasPGPredictors of mortality and differences in clinical features among patients with cryptococcosis according to immune statusPLoS One20138e6043123555970
- ByrnesEJ3rdBartlettKHPerfectJRHeitmanJCryptococcus gattii: an emerging fungal pathogen infecting humans and animalsMicrobes Infect20111389590721684347
- SanfeliceFSull’azione patogena dei bastomiceti [On the action of pathogenic bastomiceti]Ann Isto Igiene R Univ Roma18955239262 Italian
- SanfeliceFContributo alle morfologia e biologia dei blastomiceti che si sviluppano nei succhi di alcuni frutti [Contribution to the morphology and biology of blastomiceti developing in some fruit juices]Ann Isto Igiene R Univ Roma18944463495 Italian
- FranzotSPSalkinifCasadevallACryptococcus neoformans var grubii: separate varietal status for Cryptococcus neoformans serotype A isolatesJ Clin Microbiol1999378388409986871
- DromerFMathoulinSDupontBLetenneurLRoninOIndividual and environmental factors associated with infection due to Cryptococcus neoformans serotype D. French Cryptococcosis Study GroupClin Infect Dis19962391968816135
- TortoranoAMVivianiMARigoniALCogliatiMRoverselliAPaganoAPrevalence of serotype D in Cryptococcus neoformans isolates from HIV positive and HIV negative patients in ItalyMycoses1997402973029476513
- AntinoriSNew Insights into HIV/AIDS-associated cryptococcosisISRN AIDS2013201347136324052889
- Kwon-ChungKJBennettJEEpidemiologic differences between the two varieties of Cryptococcus neoformansAm J Epidemiol19841201231306377880
- PappasPGPerfectJRCloudGACryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapyClin Infect Dis20013369069911477526
- LuiGLeeNIpMCryptococcosis in apparently immunocompetent patientsQJM20069914315116504989
- VelagapudiRHsuehYPGeunes-BoyerSWrightJRHeitmanJSpores as infectious propagules of Cryptococcus neoformansInfect Immun2009774345435519620339
- MitchellDHSorrellTCAllworthAMCryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcomeClin Infect Dis1995206116167756484
- SpeedBDuntDClinical and host differences between infections with the two varieties of Cryptococcus neoformansClin Infect Dis1995212834 discussion 5–67578756
- ChenSCSlavinMAHeathCHClinical manifestations of Cryptococcus gattii infection: determinants of neurological sequelae and deathClin Infect Dis20125578979822670042
- SukroongreungSNilakulCRuangsomboonOChuakulWEampokalapBSerotypes of Cryptococcus neoformans isolated from patients prior to and during the AIDS era in ThailandMycopathologia199613575789063001
- FisherDBurrowJLoDCurrieBCryptococcus neoformans in tropical northern Australia: predominantly variant gattii with good outcomesAust N Z J Med1993236786828141697
- EllisDHCryptococcus neoformans var gattii in AustraliaJ Clin Microbiol1987254304313546370
- SeatonRAThe management of cryptococcal meningitis in Papua New GuineaP N G Med J19963967739522853
- SeatonRAHamiltonAJHayRJWarrellDAExposure to Cryptococcus neoformans var gattii – a seroepidemiological studyTrans R Soc Trop Med Hyg1996905085128944257
- LaurensonIFLallooDGNaraqiSCryptococcus neoformans in Papua New Guinea: a common pathogen but an elusive sourceJ Med Vet Mycol1997354374409467113
- LaurensonIFTrevettAJLallooDGMeningitis caused by Cryptococcus neoformans var gattii and var. neoformans in Papua New GuineaTrans R Soc Trop Med Hyg19969057608730314
- KiddSEHagenFTscharkeRLA rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada)Proc Natl Acad Sci U S A2004101172581726315572442
- FraserJAGilesSSWeninkECSame-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreakNature20054371360136416222245
- DattaKBartlettKHBaerRSpread of Cryptococcus gattii into Pacific Northwest region of the United StatesEmerg Infect Dis2009151185119119757550
- Centers for Disease Control and PreventionEmergence of Cryptococcus gattii – Pacific Northwest, 2004–2010MMWR Morb Mortal Wkly Rep20105986586820651641
- BartlettKHChengPYDuncanCA decade of experience: Cryptococcus gattii in British ColumbiaMycopathologia201217331131921960040
- UptonAFraserJAKiddSEFirst contemporary case of human infection with Cryptococcus gattii in Puget Sound: evidence for spread of the Vancouver Island outbreakJ Clin Microbiol2007453086308817596366
- BrandtMEHutwagnerLCKlugLAMolecular subtype distribution of Cryptococcus neoformans in four areas of the United States. Cryptococcal Disease Active Surveillance GroupJ Clin Microbiol1996349129178815107
- KarstaedtASCrewe-BrownHHDromerFCryptococcal meningitis caused by Cryptococcus neoformans var gattii, serotype C, in AIDS patients in Soweto, South AfricaMed Mycol20024071111860015
- McCarthyKMMorganJWannemuehlerKAPopulation-based surveillance for cryptococcosis in an antiretroviral-naive South African province with a high HIV seroprevalenceAIDS2006202199220617086060
- SteeleKTThakurRNthobatsangRSteenhoffAPBissonGPIn-hospital mortality of HIV-infected cryptococcal meningitis patients with C. gattii and C. neoformans infection in Gaborone, BotswanaMed Mycol2010481112111520438294
- LitvintsevaAPThakurRRellerLBMitchellTGPrevalence of clinical isolates of Cryptococcus gattii serotype C among patients with AIDS in sub-Saharan AfricaJ Infect Dis200519288889216088839
- PfeifferTJEllisDHEnvironmental isolation of Cryptococcus neoformans var gattii from Eucalyptus tereticornisJ Med Vet Mycol1992304074081469544
- BartlettKHKiddSEKronstadJWThe emergence of Cryptococcus gattii in British Columbia and the Pacific NorthwestCurr Infect Dis Rep200810586518377817
- MacDougallLKiddSEGalanisESpread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USAEmerg Infect Dis200713425017370514
- DromerFMathoulin-PelissierSFontanetARoninODupontBLortholaryOEpidemiology of HIV-associated cryptococcosis in France (1985–2001): comparison of the pre- and post-HAART erasAIDS20041855556215090810
- KnightFRMackenzieDWEvansBGPorterKBarrettNJWhiteGCIncreasing incidence of cryptococcosis in the United KingdomJ Infect1993271851918228302
- CurrieBPCasadevallAEstimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York CityClin Infect Dis199419102910337888529
- JarvisJNHarrisonTSHIV-associated cryptococcal meningitisAIDS2007212119212918090038
- KisengePRHawkinsATMaroVPLow CD4 count plus coma predicts cryptococcal meningitis in TanzaniaBMC Infect Dis200773917493266
- van EldenLJWalenkampAMLipovskyMMDeclining number of patients with cryptococcosis in the Netherlands in the era of highly active antiretroviral therapyAIDS2000142787278811125898
- MocroftAVellaSBenfieldTLChanging patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study GroupLancet1998352172517309848347
- PalellaFJJrDelaneyKMMoormanACDeclining morbidity and mortality among patients with advanced human immunodefciency virus infection. HIV Outpatient Study InvestigatorsN Engl J Med19983388538609516219
- GarveyLWinstonAWalshJHIV-associated central nervous system diseases in the recent combination antiretroviral therapy eraEur J Neurol20111852753421159073
- MirzaSAPhelanMRimlandDThe changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992–2000Clin Infect Dis20033678979412627365
- Joint United Nations Programme on HIV/AIDSWorld AIDS Day Report – 2011GenevaUNAIDS2011 Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/jc2216_worldaidsday_report_2011_en.pdfAccessed February 13, 2014
- GordonSBWalshALChapondaMBacterial meningitis in Malawian adults: pneumococcal disease is common, severe, and seasonalClin Infect Dis200031535710913396
- HeydermanRSGangaidzoITHakimJGCryptococcal meningitis in human immunodeficiency virus-infected patients in Harare, ZimbabweClin Infect Dis1998262842899502443
- HakimJGGangaidzoITHeydermanRSImpact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patientsAIDS2000141401140710930155
- SchutteCMVan der MeydenCHMagaziDSThe impact of HIV on meningitis as seen at a South African academic hospital (1994 to 1998)Infection2000283710697783
- BissonGPLukesJThakurRMtoniIMacGregorRRCryptococcus and lymphocytic meningitis in BotswanaS Afr Med J20089872472519113056
- HarlingGOrrellCWoodRHealthcare utilization of patients accessing an African national treatment programBMC Health Serv Res200778017555564
- NissapatornVLessons learned about opportunistic infections in Southeast AsiaSoutheast Asian J Trop Med Public Health20083962564119058599
- LeimannBCKoifmanRJCryptococcal meningitis in Rio de Janeiro State, Brazil, 1994–2004Cad Saude Publica2008242582259219009138
- LortholaryOPoizatGZellerVLong-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapyAIDS2006202183219117086058
- AntinoriSRidolfoAFasanMAIDS-associated cryptococcosis: a comparison of epidemiology, clinical features and outcome in the pre- and post-HAART eras. Experience of a single centre in ItalyHIV Med20091061119125961
- KambuguAMeyaDBRheinJOutcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapyClin Infect Dis2008461694170118433339
- van der HorstCMSaagMSCloudGATreatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials GroupN Engl J Med199733715219203426
- BicanicTMeintjesGWoodRFungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazoleClin Infect Dis200745768017554704
- BicanicTWoodRMeintjesGHigh-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trialClin Infect Dis20084712313018505387
- PitisuttithumPTansuphasawadikulSSimpsonAJHowePAWhiteNJA prospective study of AIDS-associated cryptococcal meningitis in Thailand treated with high-dose amphotericin BJ Infect20014322623311869059
- SloanDJDedicoatMJLallooDGTreatment of cryptococcal meningitis in resource limited settingsCurr Opin Infect Dis20092245546319587589
- HayRJMackenzieDWCampbellCKPhilpotCMCryptococcosis in the United Kingdom and the Irish Republic: an analysis of 69 casesJ Infect1980213227185913
- HusainSWagenerMMSinghNCryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcomeEmerg Infect Dis2001737538111384512
- SlavinMAChenSCCryptococcosis, lymphoproliferative disorders and modern day chemotherapy regimensLeuk Lymphoma20135444945023035788
- HageCAWoodKLWiner-MuramHTWilsonSJSarosiGKnoxKSPulmonary cryptococcosis after initiation of anti-tumor necrosis factor-alpha therapyChest20031242395239714665529
- NathDSKandaswamyRGruessnerRSutherlandDEDunnDLHumarAFungal infections in transplant recipients receiving alemtuzumabTransplant Proc20053793493615848579
- BrattonEWEl HusseiniNChastainCAComparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplantPLoS One20127e4358222937064
- NeofytosDFishmanJAHornDEpidemiology and outcome of invasive fungal infections in solid organ transplant recipientsTranspl Infect Dis20101222022920113459
- SunHYAlexanderBDLortholaryOUnrecognized pretransplant and donor-derived cryptococcal disease in organ transplant recipientsClin Infect Dis2010511062106920879857
- BaddleyJWSchainDCGupteAATransmission of Cryptococcus neoformans by organ transplantationClin Infect Dis201152e94e9821220771
- SinghNHuprikarSBurdetteSDMorrisMIBlairJEWheatLJDonor-derived fungal infections in organ transplant recipients: guidelines of the American Society of Transplantation, infectious diseases community of practiceAm J Transplant2012122414242822694672
- SunHYWagenerMMSinghNCryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trendsClin Infect Dis2009481566157619402789
- PappasPGAlexanderBDAndesDRInvasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET)Clin Infect Dis2010501101111120218876
- KontoyiannisDPMarrKAParkBJProspective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) databaseClin Infect Dis2010501091110020218877
- NguyenMHHusainSClancyCJOutcomes of central nervous system cryptococcosis vary with host immune function: results from a multi-center, prospective studyJ Infect20106141942620732350
- DromerFMathoulinSDupontBLaporteAEpidemiology of cryptococcosis in France: a 9-year survey (1985–1993). French Cryptococcosis Study GroupClin Infect Dis19962382908816134
- SinghNAlexanderBDLortholaryOCryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortalityJ Infect Dis200719575676417262720
- ShaariahWMoradZSuleimanABCryptococcosis in renal transplant recipientsTransplant Proc199224189818991412904
- ChenSSorrellTNimmoGEpidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study GroupClin Infect Dis20003149950810987712
- MwabaPMwansaJChintuCClinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditionsPostgrad Med J20017776977311723315
- RotheCSloanDJGoodsonPA prospective longitudinal study of the clinical outcomes from cryptococcal meningitis following treatment induction with 800 mg oral fluconazole in Blantyre, MalawiPLoS One20138e6731123840659
- SeatonRAVermaNNaraqiSWembriJPWarrellDAThe effect of corticosteroids on visual loss in Cryptococcus neoformans var gattii meningitisTrans R Soc Trop Med Hyg19979150529093628
- SeatonRAVermaNNaraqiSWembriJPWarrellDAVisual loss in immunocompetent patients with Cryptococcus neoformans var gattii meningitisTrans R Soc Trop Med Hyg19979144499093627
- LesserRLSimonRMLeonHSiegelNCryptococcal meningitis and internal ophthalmoplegiaAm J Ophthalmol197987682687443340
- KestelynPTaelmanHBogaertsJOphthalmic manifestations of infections with Cryptococcus neoformans in patients with the acquired immunodefciency syndromeAm J Ophthalmol19931167217278250075
- LallooDFisherDNaraqiSCryptococcal meningitis (C. neoformans var gattii) leading to blindness in previously healthy Melanesian adults in Papua New GuineaQ J Med1994873433498041866
- TrachtenbergJDKambuguADMcKellarMThe medical management of central nervous system infections in Uganda and the potential impact of an algorithm-based approach to improve outcomesInt J Infect Dis20071152453017512773
- MoosaMYCoovadiaYMCryptococcal meningitis in Durban, South Africa: a comparison of clinical features, laboratory findings, and outcome for human immunodeficiency virus (HIV)-positive and HIV-negative patientsClin Infect Dis1997241311349114135
- KabandaTSiednerMJKlausnerJDMuzooraCBoulwareDRPoint-of-care diagnosis and prognostication of cryptococcal meningitis with the cryptococcal antigen lateral flow assay on cerebrospinal fluidClin Infect Dis20145811311624065327
- RugemalilaJMaroVPKapandaGNdaroAJJarvisJNCryptococcal antigen prevalence in HIV-infected Tanzanians: a cross-sectional study and evaluation of a point-of-care lateral flow assayTrop Med Int Health2013181075107923937699
- McMullanBJHallidayCSorrellTCClinical utility of the cryptococcal antigen lateral flow assay in a diagnostic mycology laboratoryPLoS One20127e4954123166705
- JarvisJNPercivalABaumanSEvaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitisClin Infect Dis2011531019102321940419
- World Health OrganizationRapid Advice: Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and ChildrenGenevaWHO2011 Available from: http://whqlibdoc.who.int/publications/2011/9789241502979_eng.pdfAccessed February 13, 2014
- BrouwerAERajanuwongAChierakulWCombination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trialLancet20043631764176715172774
- BicanicTMuzooraCBrouwerAEIndependent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patientsClin Infect Dis20094970270919613840
- AndesDPharmacokinetics and pharmacodynamics of antifungalsInfect Dis Clin North Am20062067969716984875
- DenningDWHopeWWTherapy for fungal diseases: opportunities and prioritiesTrends Microbiol20101819520420207544
- CornelyOAMaertensJBresnikMLiposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial)Clin Infect Dis2007441289129717443465
- HamillRJSobelJDEl-SadrWComparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safetyClin Infect Dis20105122523220536366
- LeendersACReissPPortegiesPLiposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitisAIDS199711146314719342068
- ChenSCCryptococcosis in Australasia and the treatment of cryptococcal and other fungal infections with liposomal amphotericin BJ Antimicrob Chemother200249Suppl 1576111801583
- BicanicTOgdenDWhitneyLLoyseAJarvisJBritish HIV Association opportunistic infection guidelines: in defence of amphotericin B deoxycholateHIV Med20121363663723051823
- PappasPGChetchotisakdPLarsenRAA phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV-associated cryptococcal meningitisClin Infect Dis2009481775178319441980
- RajasinghamRMeyaDBBoulwareDRIntegrating cryptococcal antigen screening and pre-emptive treatment into routine HIV careJ Acquir Immune Defic Syndr201259e85e9122410867
- JacksonATNussbaumJCPhulusaJA phase II randomized controlled trial adding oral flucytosine to high-dose fluconazole, with short-course amphotericin B, for cryptococcal meningitisAIDS2012261363137022526517
- ButlerEKBoulwareDRBohjanenPRMeyaDBLong term 5-year survival of persons with cryptococcal meningitis or asymptomatic subclinical antigenemia in UgandaPLoS One20127e5129123251485
- DayJNChauTTLallooDGCombination antifungal therapy for cryptococcal meningitisN Engl J Med20133682522252323802521
- LoyseADromerFDayJLortholaryOHarrisonTSFlucytosine and cryptococcosis: time to urgently address the worldwide accessibility of a 50-year-old antifungalJ Antimicrob Chemother2013682435244423788479
- O’ConnorLLivermoreJSharpADPharmacodynamics of liposomal amphotericin B and flucytosine for cryptococcal meningoencephalitis: safe and effective regimens for immunocompromised patientsJ Infect Dis201320835136123599314
- DromerFBernede-BauduinCGuillemotDLortholaryOMajor role for amphotericin B-flucytosine combination in severe cryptococcosisPLoS One20083e287018682846
- SaagMSCloudGAGraybillJRA comparison of itraconazole versus fluconazole as maintenance therapy for AIDS-associated cryptococcal meningitis. National Institute of Allergy and Infectious Diseases Mycoses Study GroupClin Infect Dis19992829129610064246
- AndesDPascualAMarchettiOAntifungal therapeutic drug monitoring: established and emerging indicationsAntimicrob Agents Chemother200953243418955533
- BicanicTWoodRBekkerLGDarderMMeintjesGHarrisonTSAntiretroviral roll-out, antifungal roll-back: access to treatment for cryptococcal meningitisLancet Infect Dis2005553053116122672
- LoyseAThangarajHEasterbrookPCryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countriesLancet Infect Dis20131362963723735626
- MuzooraCKKabandaTOrtuGShort course amphotericin B with high dose fluconazole for HIV-associated cryptococcal meningitisJ Infect201264768122079502
- LoyseAWilsonDMeintjesGComparison of the early fungicidal activity of high-dose fluconazole, voriconazole, and flucytosine as second-line drugs given in combination with amphotericin B for the treatment of HIV-associated cryptococcal meningitisClin Infect Dis20125412112822052885
- RajasinghamRRolfesMABirkenkampKEMeyaDBBoulwareDRCryptococcal meningitis treatment strategies in resource-limited settings: a cost-effectiveness analysisPLoS Med20129e100131623055838
- Mayanja-KizzaHOishiKMitaraiSCombination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDSClin Infect Dis199826136213669636863
- SchaarsCFMeintjesGAMorroniCPostFAMaartensGOutcome of AIDS-associated cryptococcal meningitis initially treated with 200 mg/day or 400 mg/day of fluconazoleBMC Infect Dis2006611816846523
- LongleyNMuzooraCTaseeraKDose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern UgandaClin Infect Dis2008471556156118990067
- NussbaumJCJacksonANamarikaDCombination flucytosine and high-dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in MalawiClin Infect Dis20105033834420038244
- SudanALivermoreJHowardSJPharmacokinetics and pharmacodynamics of fluconazole for cryptococcal meningoencephalitis: implications for antifungal therapy and in vitro susceptibility breakpointsAntimicrob Agents Chemother2013572793280023571544
- MilefchikELealMAHaubrichRFluconazole alone or combined with flucytosine for the treatment of AIDS-associated cryptococcal meningitisMed Mycol20084639339518415850
- St George’s University of London (UK)Advancing Cryptococcal meningitis Treatment for Africa: Oral fluconazole plus flucytosine or one week amphotericin B-based therapy vs two weeks amphotericin B-based therapy for initial treatment of HIV-associated cryptococcal meningitis Available from: http://www.controlled-trials.com/ISRCTN45035509. ISRCTN identifier: ISRCTN45035509Accessed February 28, 2014
- BozzetteSALarsenRAChiuJA placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodefciency syndrome. California Collaborative Treatment GroupN Engl J Med19913245805841992319
- PowderlyWGSaagMSCloudGAA controlled trial of fluconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. The NIAID AIDS Clinical Trials Group and Mycoses Study GroupN Engl J Med19923267937981538722
- CollettGParrishAFluconazole donation and outcomes assessment in cryptococcal meningitisS Afr Med J20079717517617440660
- JarvisJNMeintjesGWilliamsZRebeKHarrisonTSSymptomatic relapse of HIV-associated cryptococcal meningitis in South Africa: the role of inadequate secondary prophylaxisS Afr Med J201010037838220526411
- MussiniCPezzottiPMiroJMDiscontinuation of maintenance therapy for cryptococcal meningitis in patients with AIDS treated with highly active antiretroviral therapy: an international observational studyClin Infect Dis20043856557114765351
- VibhagoolASungkanuparphSMootsikapunPDiscontinuation of secondary prophylaxis for cryptococcal meningitis in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy: a prospective, multicenter, randomized studyClin Infect Dis2003361329133112746781
- MartínezEGarcía-ViejoMAMarcosMADiscontinuation of secondary prophylaxis for cryptococcal meningitis in HIV-infected patients responding to highly active antiretroviral therapyAIDS2000142615261711101078
- RollotFBossiPTubianaRDiscontinuation of secondary prophylaxis against cryptococcosis in patients with AIDS receiving highly active antiretroviral therapyAIDS2001151448144911504971
- SeddonJMangeyaNMillerRFCorbettELFerrandRARecurrence of cryptococcal meningitis in HIV-infected patients following immune reconstitutionInt J STD AIDS20092027427519304977
- SinghNLortholaryOAlexanderBDAn immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipientsClin Infect Dis2005401756176115909263
- SinghNPerfectJRImmune reconstitution syndrome associated with opportunistic mycosesLancet Infect Dis2007739540117521592
- SinghNLortholaryOAlexanderBDAntifungal management practices and evolution of infection in organ transplant recipients with Cryptococcus neoformans infectionTransplantation2005801033103916278582
- Morera-LópezYTorres-RodríguezJMJiménez-CabelloTBaró-TomásTCryptococcus gattii: in vitro susceptibility to the new antifungal albaconazole versus fluconazole and voriconazoleMed Mycol20054350551016320494
- ChongHSDaggRMalikRChenSCarterDIn vitro susceptibility of the yeast pathogen cryptococcus to fluconazole and other azoles varies with molecular genotypeJ Clin Microbiol2010484115412020844209
- SmithRMNguyenTAHaHTPrevalence of cryptococcal antigenemia and cost-effectiveness of a cryptococcal antigen screening program – VietnamPLoS One20138e6221323626792
- BeyeneTWoldeamanuelYAsratDAyanaGBoulwareDRComparison of cryptococcal antigenemia between antiretroviral naive and antiretroviral experienced HIV positive patients at two hospitals in EthiopiaPLoS One20138e7558524124498
- AlemuASKempkerRRTennaAHigh prevalence of cryptococcal antigenemia among HIV-infected patients receiving antiretroviral therapy in EthiopiaPLoS One20138e5837723469276
- FrenchNGrayKWateraCCryptococcal infection in a cohort of HIV-1-infected Ugandan adultsAIDS2002161031103811953469
- LiechtyCASolbergPWereWAsymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural UgandaTrop Med Int Health20071292993517697087
- GovenderNPChettyVRoyMPhased implementation of screening for cryptococcal disease in South AfricaS Afr Med J201210291491723498036
- JarvisJNHarrisonTSLawnSDMeintjesGWoodRClearySCost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South AfricaPLoS One20138e6928823894442
- JarvisJNLawnSDVogtMBanganiNWoodRHarrisonTSScreening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South AfricaClin Infect Dis20094885686219222372
- MeyaDBManabeYCCastelnuovoBCost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count ≤100 cells/microL who start HIV therapy in resource-limited settingsClin Infect Dis20105144845520597693
- JarvisJNGovenderNChillerTCryptococcal antigen screening and preemptive therapy in patients initiating antiretroviral therapy in resource-limited settings: a proposed algorithm for clinical implementationJ Int Assoc Physicians AIDS Care (Chic)20121137437923015379
- MeyerACKendiCKPennerJAThe impact of routine cryptococcal antigen screening on survival among HIV-infected individuals with advanced immunosuppression in KenyaTrop Med Int Health20131849550323368667
- ChangLWPhippsWTKennedyGERutherfordGWAntifungal interventions for the primary prevention of cryptococcal disease in adults with HIVCochrane Database Syst Rev2005CD00477316034947
- Parkes-RatanshiRWakehamKLevinJPrimary prophylaxis of cryptococcal disease with fluconazole in HIV-positive Ugandan adults: a double-blind, randomised, placebo-controlled trialLancet Infect Dis20111193394121982529
- ApisarnthanarakAMundyLMThe impact of primary prophylaxis for cryptococcosis on fluconazole resistance in Candida speciesJ Acquir Immune Defc Syndr200847644645
- BicanicTBrouwerAEMeintjesGRelationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar puncturesAIDS20092370170619279443
- GraybillJRSobelJSaagMDiagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment GroupsClin Infect Dis200030475410619732
- PappasPGManaging cryptococcal meningitis is about handling the pressureClin Infect Dis20054048048215668875
- DammertPBustamanteBTiconaETreatment of cryptococcal meningitis in Peruvian AIDS patients using amphotericin B and fluconazoleJ Infect20085726026518707764
- de VediaLArechavalaACalderonMIRelevance of intracranial hypertension control in the management of Cryptococcus neoformans meningitis related to AIDSInfection2013411073107724122543
- WijewardanaIJarvisJNMeintjesGHarrisonTSBicanicTLarge volume lumbar punctures in cryptococcal meningitis clear cryptococcal antigen as well as lowering pressureJ Infect20116348448621930156
- ManosuthiWSungkanuparphSChottanapundSTemporary external lumbar drainage for reducing elevated intracranial pressure in HIV-infected patients with cryptococcal meningitisInt J STD AIDS20081926827118482948
- WoodworthGFMcGirtMJWilliamsMARigamontiDThe use of ventriculoperitoneal shunts for uncontrollable intracranial hypertension without ventriculomegally secondary to HIV-associated cryptococcal meningitisSurg Neurol200563529531 discussion 531–53215936373
- NewtonPNThai leHTipNQA randomized, double-blind, placebo-controlled trial of acetazolamide for the treatment of elevated intracranial pressure in cryptococcal meningitisClin Infect Dis20023576977212203177
- HaddowLJColebundersRMeintjesGCryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitionsLancet Infect Dis20101079180221029993
- LawnSDHarriesADAnglaretXMyerLWoodREarly mortality among adults accessing antiretroviral treatment programmes in sub-Saharan AfricaAIDS2008221897190818784453
- BissonGPNthobatsongRThakurRThe use of HAART is associated with decreased risk of death during initial treatment of cryptococcal meningitis in adults in BotswanaJ Acquir Immune Defc Syndr200849227229
- KlotzSAAziz MohammedAGirmai WoldemichaelMWorku MitkuMHandrichMImmune reconstitution inflammatory syndrome in a resource-poor settingJ Int Assoc Physicians AIDS Care (Chic)2009812212719258527
- LawnSDBekkerLGMyerLOrrellCWoodRCryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programmeAIDS2005192050205216260920
- MurdochDMVenterWDFeldmanCVan RieAIncidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective studyAIDS20082260161018317001
- FrenchMAHIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisalClin Infect Dis20094810110719025493
- LongleyNHarrisonTSJarvisJNCryptococcal immune reconstitution inflammatory syndromeCurr Opin Infect Dis201326263423242412
- LortholaryOFontanetAMemainNMartinASitbonKDromerFIncidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in FranceAIDS2005191043104915958835
- BicanicTMeintjesGRebeKImmune reconstitution infammatory syndrome in HIV-associated cryptococcal meningitis: a prospective studyJ Acquir Immune Defic Syndr20095113013419365271
- LeshoEEvidence base for using corticosteroids to treat HIV-associated immune reconstitution syndromeExpert Rev Anti Infect Ther2006446947816771623
- ChangCDorasamayAElliottJHIV+ patients with CM who attain CSF sterility pre-cART commencement experience improved outcomes in the first 24 weeksPoster presented at: 19th Conference on Retroviruses and Opportunistic InfectionsMarch 5–8, 2012Seattle, WA
- JacksonAvan der HorstCNew insights in the prevention, diagnosis, and treatment of cryptococcal meningitisCurr HIV/AIDS Rep2012926727722763808
- ManosuthiWChottanapundSSungkanuparphSMortality rate of early versus deferred initiation of antiretroviral therapy in HIV-1-infected patients with cryptococcal meningitisJ Acquir Immune Defic Syndr20084850850918614933
- ZolopaAAndersenJPowderlyWEarly antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trialPLoS One20094e557519440326
- BissonGPMolefiMBellamySEarly versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitisClin Infect Dis2013561165117323362285
- MakadzangeATNdhlovuCETakarindaKEarly versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-Saharan AfricaClin Infect Dis2010501532153820415574
- BoulwareDMeyaDMuzooraCART initiation within the first 2 weeks of cryptococcal meningitis is associated with higher mortality: a multisite randomized trialPoster presented at: 20th Conference on Retroviruses and Opportunistic InfectionsMarch 3–6, 2013Atlanta, GA
