Abstract
Aim
To describe a new research tool, designed to reflect routine clinical practice and relying on population-based health care databases to detect clinical events in randomized clinical trials.
Background
Randomized clinical trials often focus on short-term efficacy and safety in a controlled environment. Trial follow-up may be linked with study-related investigations and differ from routine clinical practice. Because treatment and control in randomized trials differ from daily practice, trial results may have reduced general applicability and may be of limited value in clinical decision-making. Further, it is economically very costly to conduct randomized clinical trials.
Methods and results
Population-based health care databases collect data continuously and prospectively, and make it possible to monitor lifelong outcomes of cardiac interventions in large numbers of patients. This strengthens external validity by eliminating the effects of study-related monitoring or diagnostic tests. Further, follow-up data can be obtained at low expense. Importantly, data sources encompassing a complete population are likely to reflect clinical practice. Because population-based health care databases collect data for quality-control and administrative purposes unrelated to scientific investigations, certain biases, such as nonresponse bias, recall bias, and bias from losses to follow-up, can be avoided.
Conclusion
Event detection using population-based health care databases is a new research tool in interventional cardiology that may allow large, low-cost, randomized clinical trials to reflect daily clinical practice, covering a broad range of patients and end points with complete lifelong follow-up.
Introduction
In medicine, the randomized controlled clinical trial (RCT) is the gold standard in research design for assessing the level of evidence for new treatments.Citation1 Nevertheless, the clinical applicability of RCT results has shortcomings. RCTs often focus on short-term efficacy and safety in a controlled clinical environment among well-educated affluent patients.Citation2 In addition, the scientific investigation per se may influence the subject and the results of an investigation, also called the Hawthorne effect.Citation3 Important elements of study design are intensity of and methods for collection of follow-up data. These elements are likely to influence the completeness, validity, and number of events assessed, and may lead to biased estimates of the prognosis, because some study participants drop out over time.
In a population-based health care database, data are collected for quality-control or administrative purposes. This may reduce certain forms of bias, such as nonresponse bias, recall bias, and bias from loss to follow-up, which may influence prognostic estimates.Citation4 Also, there are considerable costs associated with conducting an RCT.
Based on our experience with head-to-head stent comparisons,Citation5–Citation8 we present a novel research tool in interventional cardiology: event detection using population-based health care databases. When event detection relies on population-based databases, conduct of RCTs does not influence everyday clinical practice. Complete and lifelong follow-up is possible, and a large-scale RCT may be performed at relatively low cost.
Population-based databases
Event detection using population-based databases requires access to existing health care databases, permission to merge different databases at the patient level, and algorithms to ensure anonymity for individual patients. It is essential that the database cover the target population over the entire study period and that patient migration out of the catchment area is minimal.Citation9
Clinical end points in coronary stent trials
Treatment with coronary stents is usually assessed using safety end points (all-cause death, cardiac death, and myocardial infarction [MI]) and efficacy end points (targetlesion or vessel revascularization [TLR or TVR] following percutaneous coronary intervention [PCI] or coronary bypass surgery).Citation10 It is well known that study-related follow-up procedures may affect the number of end points detected. In interventional cardiology, the Benestent studyCitation11 noted that TVR was found more often in patients undergoing systematic angiographic follow-up than in patients followed clinically. Thus, mandated angiographic monitoring increases event rates significantly, as the presence of visually assessed coronary artery stenosis leads to new revascularization procedures. Much effort has been made to avoid study-induced repeat revascularizations. In the Nordic Bifurcation studies,Citation12 clinical and angiographic monitoring were separated in time, with clinical follow-up preceding angiographic control. Other investigators have established strict rules for revascularization following angiographic detection of a new coronary artery narrowing.Citation13
Clinical study-related monitoring by means of outpatient visits or telephone contacts also is likely to lead to changes in patient compliance, increased detection of events, and registration of events that would remain undetected in everyday clinical practice.
Event detection using population databases in the Sort Out trials
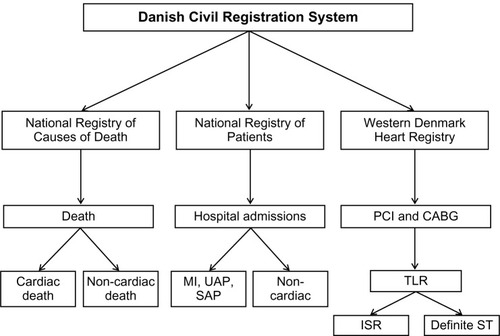
The Sort Out II–V trialsCitation5–Citation8 used existing national registries covering the entire population to detect events in the study cohorts. Thus, participants were exposed to the same clinical monitoring as nonstudy patients. Data on mortality (cardiac and noncardiac) were obtained from the Danish Civil Registration System and the National Registry of Causes of Death,Citation14,Citation15 hospital admission for myocardial infarction from the Danish National Registry of Patients,Citation16 and basic descriptive data, coronary angiography, repeat PCI, and coronary bypass surgery from the Western Denmark Heart Registry.Citation17 An important advantage of this study approach is the ability to describe baseline demographics and clinical outcomes in all patients treated with coronary stents during the study period, and not only those included in the RCT. Thus, important information is available regarding the general applicability, ie, the external validity, of study results.
In Denmark, all citizens have a personal civil registration number assigned at birth or upon immigration.Citation14 This unique personal identifier allows linkage of individual-level information across registries and databases. The Danish Civil Registration System is updated daily, and maintains records on date of birth, death, and current residence of all Danish citizens. The Danish National Registry of Patients contains information on all admissions and outpatient visits to the 52 Danish hospitals. For each hospital admission, the registry records dates of admission and discharge, surgical procedures performed, and up to 20 diagnoses classified according to the International Classification of Diseases (ICD), eighth revision, until the end of 1993, and tenth revision thereafter.Citation16 The Western Denmark Heart Registry contains detailed patient- and procedure-specific information on all coronary angiographies, coronary interventions and coronary bypass surgery performed at the three interventional and eight noninterventional cardiac centers in Western Denmark.Citation17 Information from the Western Denmark Heart Registry and the Danish National Registry of Patients has been validated in earlier studies, and the registries’ data completeness and validity are continuously monitored.Citation16,Citation18 The Sort Out trialsCitation5–Citation8 used the Danish Civil Registration System to assess all-cause mortality. The original death certificates were obtained from the National Registry of Causes of Death to classify deaths by underlying cause.
We defined new MIs as rehospitalization for MI after the index PCI, based on MI-related admissions and readmissions I (ICD-10 codes I21–I21.9) identified from the Danish National Patient Registry. Information on new diagnostic angiograms and new revascularizations performed with coronary bypass surgery or PCI was available from the Western Denmark Heart Registry. All possible end points were adjudicated by an independent end-point committee consisting of interventional and noninterventional cardiologists, who examined hospital files and reviewed diagnostic and therapeutic angiograms. The trial end points were all-cause death, cardiac death, noncardiac death, MI, TVR, TLR, and stent thrombosis, using Academic Research Consortium definitions.Citation10 The health care database-based event detection is shown schematically in .
Figure 1 Event detection using population-based health care databases. The Danish Civil Registration System allows linkage of individual-level information across registries.Citation14 It is updated daily, and maintains records on date of birth, death, and current residence of all Danish citizens. The National Registry of Causes of Death provides information on causes of death.Citation15 The National Registry of Patients contains information on all admissions and outpatient visits.Citation16 The Western Denmark Heart Registry provides detailed patient-and procedure-specific information on all coronary angiographies, coronary interventions, and coronary bypass surgery procedures performed in Western Denmark.Citation17
Randomization linked to a health care database
In the ongoing Swedish TASTE trial,Citation19,Citation20 the investigators have added a randomization module to their population-based PCI database, taking the integration of clinical databases and RCTs to a higher level. In TASTE, patients admitted with acute ST-elevation MI for primary PCI are randomized to thrombectomy or to conventional primary PCI. When initial procedural data are entered in the database and prespecified inclusion and exclusion criteria are fulfilled, the operator will have to consider randomization. This is an excellent example of a study design incorporating the essentials of an RCT in interventional cardiology. The study has a large sample size (>5,000 patients), a high inclusion rate, and a focused primary end point (1-month all-cause mortality).Citation19 Combining event detection using population-based health care databases with a randomization module may be a very efficient and cost-effective option when designing large scale RCTs with long term follow-up.
Discussion
Event detection using population-based registries in RCTs is a novel research tool that may be applied in a number of clinical settings, including coronary interventions.Citation21 This approach allows large RCTs to reflect daily clinical practice, to cover a broad range of patients, and to facilitate complete lifelong follow-up at low cost.
Drug-eluting stents were accepted for extensive clinical use based on randomized trials involving relatively few and selected patients, with angiographic follow-up data serving as the primary end point.Citation22–Citation24 Years later, detection of an increased risk of late stent thrombosis in patients treated with these stents raised questions about the scientific rigor of the device-approval process. Subsequently, large RCTs with few exclusion criteria and clinical end points were recommended to document acceptable safety and efficacy of new stents. Event detection using population-based databases may provide additional clinical support for this type of study. This method may make it possible, at low cost, to use an RCT design to study the safety and efficacy of medical devices in large cohorts of unselected patients, with a lifelong follow-up period and a clinical research setting identical to routine clinical practice.
Furthermore, combining different health care databases may allow assessment of variables not directly related to the RCT for generation of hypotheses for new prospective studies.Citation25,Citation26 Finally, combining event detection using population-based health care databases and a randomization module within the databaseCitation20 may be a very efficient and cost-effective option for large-scale long-term follow-up RCTs.
We do not know the extent to which event rates differ between clinical studies using population database-based event detection and those using conventional angiographic or other follow-up for event detection. It is likely that registration of death will be the same. With symptom-driven event detection, incidence of spontaneous MI may be slightly reduced and procedure-related MI further reduced due to fewer revascularizations. Because new revascularizations will be symptom-driven, it also is likely that rates of new revascularizations will be lower. However, the randomization process would balance possible under- or overreporting in the study groups.
The generalizability, or external validity, of study findings is a common challenge in clinical research, since information about nonstudy patients remains unknown or associated with a high degree of uncertainty. In this context, trials using population-based health care databases for event detection provide a significant advantage.Citation25,Citation26 Since all patients are registered in the existing databases, equally valid procedure and background information is available for both study patients and nonstudy patients.
Limitations
Databases have important limitations, which are frequently ignored, because researchers do not control methods of data collection. Therefore, a primary concern in health care database-based event detection is related to the quality of health care databases used. Here, a prerequisite is reliable population-based databases, which register all relevant data in a timely manner in a well-defined population. Additional requirements include the ability to identify all patients and link their data among different databases. Further, it is important to review and validate the information recorded in the databases continuously.Citation25
So far, our population-based health care database event detection has not been evaluated or approved by competent national health authorities. Further, we have no comparison of traditional event detection using clinical follow-up by phone call or outpatient visits and data registration by clinical research forms versus our proposed health care database-based event detection. Therefore, we may suggest a randomized comparison of the different forms of event detection to describe possible event-rate differences and a comparison of event rates and types of events detected by traditional event detection versus a health care database-based event detection in the same study cohort.
Ethical considerations
There are specific ethical aspects to using population-based health care database event detection in RCTs. First, the randomized patient should be informed how follow-up data will be obtained, and that, in principle, the event detection will be lifelong. Second, randomized and nonrandomized patients should be informed that treatment and outcome data will be registered in national health care databases and used to describe general treatment results and to assess the external validity of ongoing RCTs.
Conclusion
Event detection using population-based health care databases is a new research tool in interventional cardiology that may allow large RCTs to reflect daily clinical practice, to cover a broad range of patients, and to facilitate complete lifelong follow-up at low cost.
Disclosure
The authors report no conflicts of interest in this work.
References
- SilberSAlbertssonPAvilésFFGuidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of CardiologyEur Heart J200526880484715769784
- SchneeweissSAvornJA review of uses of health care utilization databases for epidemiologic research on therapeuticsJ Clin Epidemiol200558432333715862718
- FletcherRHFletcherSWTreatmentClinical Epidemiology: The EssentialsBaltimoreLippincott & Wilkins2005125145
- SorensenHTLashTLRothmanKJBeyond randomized controlled trials: a critical comparison of trials with nonrandomized studiesHepatology20064451075108217058242
- ChristiansenEHJensenLOThayssenPBiolimus-eluting biodegradable polymer-coated stent versus durable polymer-coated sirolimus-eluting stent in unselected patients receiving percutaneous coronary intervention (SORT OUT V): a randomised non-inferiority trialLancet2013381986766166923374649
- JensenLOThayssenPHansenHSRandomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV)Circulation2012125101246125522308301
- RasmussenKMaengMKaltoftAEfficacy and safety of zotarolimus-eluting and sirolimus-eluting coronary stents in routine clinical care (SORT OUT III): a randomised controlled superiority trialLancet201037597201090109920231034
- GalloeAMThuesenLKelbaekHComparison of paclitaxel- and sirolimus-eluting stents in everyday clinical practice: the SORT OUT II randomized trialJAMA2008299440941618230778
- FuruKWettermarkBAndersenMMartikainenJEAlmarsdottirABSørensenHTThe Nordic countries as a cohort for pharmacoepidemiological researchBasic Clin Pharmacol Toxicol20101062869419961477
- CutlipDEWindeckerSMehranRClinical end points in coronary stent trials: a case for standardized definitionsCirculation2007115172344235117470709
- RuygrokPNMelkertRMorelMADoes angiography six months after coronary intervention influence management and outcome? Benestent II InvestigatorsJ Am Coll Cardiol19993451507151110551700
- SteigenTKMaengMWisethRRandomized study on simple versus complex stenting of coronary artery bifurcation lesions: the Nordic bifurcation studyCirculation2006114181955196117060387
- KelbaekHThuesenLHelqvistSThe stenting coronary arteries in non-stress/benestent disease (SCANDSTENT) trialJ Am Coll Cardiol200647244945516412876
- FrankLEpidemiology. When an entire country is a cohortScience200028754622398239910766613
- JuelKHelweg-LarsenKThe Danish registers of causes of deathDan Med Bull199946435435710514943
- AndersenTFMadsenMJørgensenJMellemkjoerLOlsenJHThe Danish National Hospital Register. A valuable source of data for modern health sciencesDan Med Bull199946326326810421985
- JensenLOMaengMKaltoftAStent thrombosis, myocardial infarction, and death after drug-eluting and bare-metal stent coronary interventionsJ Am Coll Cardiol200750546347017662400
- MadsenMDavidsenMRasmussenSAbildstromSZOslerMThe validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registryJ Clin Epidemiol200356212413012654406
- FrobertOLagerqvistBGudnasonTThrombus aspiration in ST-elevation myocardial infarction in Scandinavia (TASTE trial). A multicenter, prospective, randomized, controlled clinical registry trial based on the Swedish angiography and angioplasty registry (SCAAR) platform. Study design and rationaleAm Heart J201016061042104821146656
- JamesSFrobertOLagerqvistBCardiovascular registries: a novel platform for randomised clinical trialsHeart201298181329133122591738
- KastratiASORT OUT V: A new episode in the DES warsLancet2013381986760961123374648
- MoriceMCSerruysPWSousaJEA randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularizationN Engl J Med2002346231773178012050336
- MosesJWLeonMBPopmaJJSirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary arteryN Engl J Med2003349141315132314523139
- StoneGWEllisSGCoxDAA polymer-based, paclitaxel-eluting stent in patients with coronary artery diseaseN Engl J Med2004350322123114724301
- SørensenHTBaronJARegistries and medical databasesOlsenJSaracciRTrichopoulosDTeaching Epidemiology: A Guide for Teachers in Epidemiology, Public Health and Clinical MedicineOxfordOxford University Press2009
- SajadiehAStormHHHansenJFVerapamil and risk of cancer in patients with coronary artery disease. DAVIT Study Group. Danish Verapamil Infarction TrialAm J Cardiol199983914191422A910235108