Abstract
Memory diseases are the most important determinant of health care service use and quality of life among older individuals. Adverse effects of medication are common among older people, but this age group is underrepresented in clinical trials. Finnish statutory health care and prescription registers, together with personal identification numbers (PINs) and a tax-supported public health plan covering all citizens provide excellent opportunities for epidemiological research. We used routinely collected data from the Finnish health care system to establish the Medication use among persons with Alzheimer’s disease (MedAlz-2005) cohort. This cohort study will be used to assess medication use and its effects on health status and hospitalization among persons with Alzheimer’s disease (Ad). The cohort includes all community-dwelling persons who had a clinically verified diagnosis of Ad, resided in Finland, and were alive on December 31, 2005 and a matched comparison person for each affected individual. data on purchased prescription medicines (1995–2009), inpatient hospital admissions (1972–2009), outpatient visits (1995–2009), details on diagnosed cancers (1972–2009), and mortality (until October 2010) are available for the whole cohort. This paper describes how this data can be utilized in etiological research and the assessment of health care service use, drug utilization, and associated adverse outcomes in a particularly vulnerable group that is often underrepresented in clinical trials.
Background
Worldwide, 35.6 million people were estimated to have dementia in 2010, with Alzheimer’s disease (Ad) being the most common form of dementia.Citation1 The number of affected individuals is expected to rise to up to 115.4 million by 2050 due to changes in population structure.Citation1 Cognitive decline and dementia are the most important determinants of health care service use: health care costs of Ad or dementia patients are over three times higher than the costs of the aged population without these diseases.Citation2 In the UK, the treatment of dementia costs more than treatments for cancer and cardiovascular diseases combined.Citation3 In 2003, dementia contributed to 11.2% of all years lived with disability among those aged 60 years or older, which is more than the contributions of cancer, cardiovascular diseases, musculoskeletal disorders, or stroke.Citation4
Immense research effort has focused on modifiable risk factors and disease-modifying or -delaying therapies for Ad. However, the therapeutic advances have been modest, and only symptomatic treatment with acetylcholinesterase inhibitors and/or memantine is available. In addition, behavioral and psychological symptoms, eg, agitation, depression, apathy, delusions, hallucinations, and sleep disturbances are often treated with psychotropic drugs, such as antidepressants or antipsychotics, although evidence on the benefits of these treatments is inconsistent.Citation5 Further, aging leads to several pharmacokinetic and pharmacodynamic changes.Citation6,Citation7 Thus, the results from randomized controlled trials of psychotropic drugs with more restricted selections of patients are not likely to be generalizable to the actual user population.Citation8 In addition, the sample sizes needed to detect less common adverse effects would be unfeasibly large for randomized controlled trials and, thus, large population-based studies are needed to provide data on the effectiveness and possible adverse events related to medications in the actual user population. Persons with Ad are a particularly vulnerable group, and thus it is important to assess whether the risk–benefit profile, estimated in trials that have often excluded aged individuals, those with cognitive impairment, multiple comorbidities or concomitant medications, is directly applicable.
Finnish statutory health care and prescription registers, together with personal identification numbers (PINs) provide excellent opportunities for epidemiological research. Similar systems are used in other Nordic countries, but, in Finland and Denmark, the data are available from a longer period of time than in other Nordic countries (a national prescription register was introduced in 1994 in Finland and Denmark, but in 2004–2006 in other Nordic countries,Citation9 while hospital discharge data is available from 1967 in Finland and from 1977 in DenmarkCitation10). However, changes in Finnish PINs in the 1970s means that reliable automated linkage without individual checking and recoding of PINs is possible with data from 1972 onwards. Finland (population 5.4 million, gross domestic product €178,796 million in 2010) has a similar population structure to other developed countries: in 2012, 18.8% of the population was 65 years or older, and this percentage is expected to increase up to 26.9% by 2050.Citation11 For those born in 2011, life expectancy was 83.5 years for girls and 77.2 years for boys. All citizens/residents are covered by a tax-supported public healthcare plan and have unrestricted access to health services, independent of socioeconomic status.Citation9 Public health care, provided by municipalities, is organized according to a national framework, set by the Ministry of Social Affairs and Health. In addition to the public health care system, health care services are available from private providers.
The Medication use among persons with Alzheimer’s disease (MEDALZ-2005) cohort was set up to investigate medication use and health statuses of persons with Alzheimer’s disease on a nationwide level. The aim of this paper is to describe (1) how this routinely collected data from health care registers can be utilized in clinical epidemiology, and (2) the baseline characteristics of the MedAlz-2005 population.
Methods of the MEDALZ-2005 study
Study design
Our sample consists of two closed cohorts: (1) all 28,093 persons with a verified diagnosis of Ad residing in Finland (population 5.3 million, 97.8% Finnish citizens and 2.2% noncitizens) on December 31, 2005;Citation12 and (2) their matched comparison group with no diagnosed Ad. To be included in the sample, the participants needed to be (1) alive on 31 December, 2005, and (2) community-dwelling. Therefore, our sample does not include persons who were living in institutionalized settings at the beginning of the study.
Persons with Ad were identified from the Special Reimbursement RegisterCitation13 and a single comparison person matched for age (±1 year), sex, and region of residence (on the basis of university hospital district) per Ad case was identified from the Finnish Social Insurance Institution (SII) database, including all residents of Finland with a PIN, ie, all citizens and residents living in Finland for at least 2 years, but not those citizens who had been living abroad for more than 1 year.
Altogether, 145 comparison persons had temporarily been entitled to reimbursed Ad medication before 2006 and these persons, together with their matched pairs, have been excluded from the analyses. Altogether 2,015 persons converted to Ad during 2006–2009, and we have therefore modelled Ad as a time-dependent variable in the prospective studies. due to the advanced age of the population, it is likely that some of the participants were institutionalized during the follow-up, beginning on January 1, 2006.
Some public nursing homes provide residents’ medications and, for pharmacoepidemiological studies, it is no longer possible to reliably assess medication exposure from the prescription register after institutionalization. Thus, for pharmacoepidemiological studies, persons are censored at the date of institutionalization to facilities providing medication based on the date recorded in a special register maintained by the SII. Hospitalizations, together with diagnoses and operations, are still recorded in the hospital discharge register after institutionalization, so these persons can be included in studies assessing health status.
Diagnosis of AD
The Finnish special reimbursement register contains information on reimbursement due to specific chronic diseases such as diabetes, cardiovascular diseases, and Alzheimer’s disease. As per national guidelines for Ad treatment,Citation15 this register allows identification of every person with clinically diagnosed Ad, regardless of whether they purchased the Ad medication after being diagnosed. We are not aware of this kind of nationwide register with longitudinal data elsewhere, although Sweden recently set up the Swedish dementia Registry (SveDem), including 6,937 dementia patients diagnosed during 2007–2009.Citation14
The Finnish Current Care Guidelines recommend that all persons with Ad are treated with acetylcholinesterase inhibitors or with memantine unless there is a specific contraindication.Citation15 To be eligible for reimbursed Ad medication, the patient needs to have a verified diagnosis of Ad described in a medical statement submitted to the SII by a physician. The medical statement must state that the patient has: (1) symptoms consistent with Ad; (2) experienced a decrease in social capacity over a period of at least 3 months; (3) received a computed tomography/magnetic resonance imaging scan; (4) had possible alternative diagnoses excluded; and (5) received confirmation of the diagnosis by a registered neurologist or geriatrician.Citation15 Ad is diagnosed according to the National Institute of Neurological and Communicative disorders and Stroke and the Alzheimer’s disease and Related disorders Association (NINCDS-ADRDA [now known as the Alzheimer’s Association]) and The Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria for Alzheimer’s disease.Citation16,Citation17 The physician also needs to confirm whether the patient has other dementing diseases, such as multi-infarct dementia or Lewy body dementia. However, patients with these diseases are also entitled to reimbursed medicines if the symptoms are considered to be mainly caused by AD.Citation18
The special reimbursements for acetylcholinesterase inhibitors and memantine were introduced in 1999 and 2003, respectively, and the diagnostic criteria have been consistent. In the beginning, the reimbursement decisions were often made on a temporary basis, but permanent decisions were introduced in July 2003. earlier, only patients with mild or moderate Ad were entitled to reimbursed anti-dementia medication, but the reimbursement was not withdrawn if/when the patient developed severe ADs. People with severe Ad have been entitled to reimbursed AD medication since 2003. Thus, our study sample includes persons with all stages of AD. data on severity or stage of Alzheimer’s disease is not available from the registers, but the diagnosis date from the special reimbursement register can be used as a crude estimate on mild/moderate stages of AD. The Finnish public health system covers all residents regardless of age or income. Thus, one of the strengths of our cohort is that, unlike cohorts consisting of members of a particular private health care insurance scheme, MedAlz-2005 is not selected on the basis of socioeconomic position.
Data sources and variables collected
The data resource formation is described in and data provided by the individual registers are summarized in . The hospital discharge database includes all medical services and procedures billed on a fee-for-service basis, whether rendered in an outpatient, inpatient, or emergency department setting. Public health care service use is accurately described in these registers, as this is mandated by legislation. However, the same pressure does not apply to private health care providers. Thus, the accuracy of hospital discharge data depends on how much the study population utilizes private services. The mean age of our sample was 80.6 years and persons belonging to this age group almost exclusively use the services of the statutory health care system,Citation19 the hospital discharge register is representative of their health care service use. The hospital discharge register data were preprocessed, so we have the exact hospitalization periods, diagnoses, performed procedures, and cost per hospitalization period for each participant. The costs are calculated according to Finnish health care system unit costs.Citation20 diagnoses for each admission are made by the attending physician.
Figure 1 Cohort formation.
Abbreviations: AD, Alzheimer’s disease; DR, discharge register; MEDALZ-2005, MEDication use among persons with ALZheimer’s disease; NIHW, National Institute of Health and Welfare; PIN, personal identification number; SII, Social Insurance Institution.
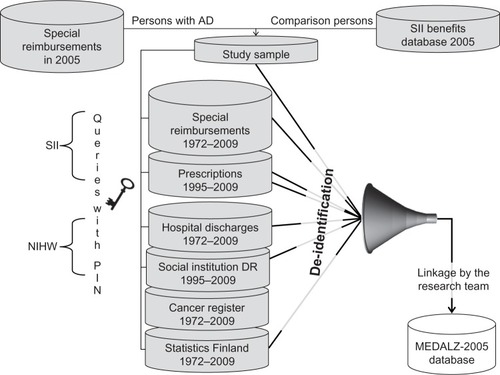
Table 1 MEDALZ-2005 data sources and their contents
Data on all reimbursed purchased prescription medicines, emollient creams, and clinical nutrients, regardless of the setting in which they were prescribed, are recorded in the prescription register. Over-the-counter medications and medicines used in the hospitals are not recorded in the register. Prescription data are converted to medication periods with start date, length, average dosage, and cost. The method of calculating medication-use periods from prescriptions is developed from previously used methods.Citation21–Citation23
The cancer register,Citation24 maintained by the Finnish Cancer Registry, is a continuously updated nationwide database on all primary cancers in Finland. Final coding is done by qualified secretaries and supervised by the Registry physician (a pathologist). Reporting of cancers has been mandated by law since 1961 (see for a detailed description of the contents of this register). In addition, we have data on discharges from social institutions as well as causes of death (). Mortality data were obtained from the SII. The SII registers are updated daily with mortality data using the Population Information System maintained by the Population Register Centre of Finland. data include each participant’s exact date of death.
Follow-up
Retrospective data from 1972 to 2005 are available. The individual-level data in Finnish health care registers are continuously updated. Currently, we have prospective data on hospital discharges, cancer register, prescriptions, and social institution discharges until 2009 and mortality data until September 2010. We are updating the database with data from 2009–2012 in 2013.
Research questions in the MEDALZ-2005 cohort
We are investigating four different research lines: (1) medication use, its persistence, and changes during follow-up; (2) health-related risk factors for Ad and clinically important changes in health status after Ad diagnosis; (3) medication use and its associations with adverse outcomes (mortality, hip fractures, gastrointestinal bleeds, and cardio- and cerebrovascular events) in persons with Ad; and (4) use of social and health care services.
Results
Baseline characteristics of the study population
The baseline status of the cohort is described in . Persons with Ad were somewhat more likely to have epilepsy or medically treated diabetes and less likely to have cardiovascular diseases or cancer, although the absolute differences in prevalence were small. The majority (over 60%) of Ad cases were diagnosed in 2003–2005, with over a quarter (25.8%) being diagnosed in 2005. The earliest diagnoses were from 1999.
Table 2 Description of the study population at baseline (December 31, 2005)
Medication use
In cross-sectional analyses of the 2005 data (), we found that antipsychotic use was nearly six times more common among community-dwelling persons with Ad (22.1%) in comparison to the matched population with no Ad (4.4%), and that most antipsychotics were prescribed by primary care physicians.Citation26 Further, use of older antiepileptic medicines (phenytoin, clonazepam, valproic acid, and oxcarbazepine) was more prevalent among persons with Ad,Citation27 although these persons are particularly vulnerable to adverse events associated with these medicines.Citation28 We also found that use of opioids was less common in persons with Ad, which highlights the challenges associated with diagnosing and treating pain in this population. Clinical manifestations of pain in older adults are often complex and multifactorial and the symptoms may be underreported by the patient, which may lead to undertreatment of pain.Citation29 In our study, the differences were mainly due to lower use of tramadol in people with AD. Tramadol is considered to have anticholinergic activity and it may interact with acetylcholinesterase inhibitors,Citation30 which may lead to limited tramadol prescribing in persons with AD. Persons with Ad may also be particularly susceptible to opioid-related adverse drug events, such as delirium,Citation31,Citation32 which can lead to avoidance of opioids. However, strong opioids and transdermal fentanyl patches were more commonly used among persons with Ad in our study.Citation33
Health status and mortality during follow-up
On average, persons with Ad were twice as likely to die during the follow-up in comparison to the matched population.Citation34 The risk difference was more pronounced at younger ages (<80 years old) and even a short duration of Ad (≤3 years) was associated with a significantly increased risk of death. The association persisted after adjusting for comorbidities, suggesting that Ad has an important and independent impact on mortality. Altogether, 18,395 (32.7%) persons (11,726 and 6,669 with and without Ad, respectively) died during 2006–2009.Citation34 Persons who died during follow-up were a mean age of 3.8 years older and were more likely to be men and have Alzheimer’s disease and other chronic comorbidities (). longer duration of Ad was also related to higher mortality.
Table 3 Differences between MEDALZ-2005 participants who died during 2006–2009 and survivors
Our further analyses of prospective data showed that persons with Ad had more incident hemorrhagic strokes than the age-matched population without Ad, although there were no differences in the incidence of ischemic strokes.Citation35 We also assessed the association between Ad and incident hip fracture.Citation36 Although there was some evidence of effect modification by age and sex, Ad was consistently associated with a doubling of the risk of incident hip fracture. The overall risk estimate was similar to that of previous observational studies,Citation37–Citation41 supporting the reliability and external validity of our data. The cohort contains persons with variable durations of Ad, which may have affected the results. To address the extent of bias introduced by different disease duration, we performed sensitivity analyses including only those Ad patients who were diagnosed in 2004–2005. This had no effect on the conclusions of our studies.
Retrospective analyses
In addition to prospective analyses, the study design allows retrospective analyses for possible Ad risk factors. diabetes requiring medical treatment, especially if diagnosed in midlife, was associated with higher risk of Ad in the Med-Alz-2005 cohort.Citation42 The estimates were in agreement with a previous meta-analysis from carefully phenotyped cohort studies.Citation43
Discussion
We have described how routinely collected health care data can be utilized in clinical epidemiology research to assess potential risk factors for Ad, describe differences in health status after Ad diagnosis, and assess drug utilization in a nationwide sample. We have also shown that our findings are similar to those that have been reported in previous cohort studies and meta-analyses,Citation37–Citation41,Citation43 supporting the external validity of our cohort. Our future plans are to assess the possible benefits and/or adverse outcomes related to medication use and compare the health care service use patterns between the Ad and non-Ad cohorts.
Strengths and weaknesses of this approach
The main prerequisite for using register-based data is the quality of the data, ie, the data need to have good coverage and validity. Studies assessing internal validity of Finnish administrative registersCitation44 and comparing register information with patient records or other information from the primary source have confirmed that the coverage and accuracy of Finnish register data are well-suited for epidemiological research.Citation45–Citation53 However, the prevalence of Ad is underestimated in the registers, especially in the earlier data, because only those persons seeking medical attention will be included. On the other hand, according to the Finnish Statistics on Medicines,Citation11 the number of new Ad diagnoses per year has increased, suggesting a better implementation of Ad diagnosis by heath care professionals. Another limitation is the lack of data on the severity of AD. On the other hand, due to explicit diagnostic criteria, positive predictive value, the Ad diagnosis is high. Some other chronic diseases, such as chronic obstructive pulmonary disease (COPD), will be underrepresented, as only the most severe cases will be captured while untreated and undiagnosed cases will be missing from the registers. Thus, the findings will be representative of the severe forms of, in this example, COPD and not necessarily generalizable to those with milder forms of the disease.
The main limitation of register-based data, which is not specific to our study, is the lack of information on genetic or lifestyle-related confounders, such as body composition and smoking or alcohol use. The latter variables are difficult to measure accurately in epidemiological studies in general, especially without biomarkers. We have access to complete medical histories of the cohort members from 1972 onwards, which can be used as a proxy measure for these confounders, eg, by constructing a comorbidity index ( and ). We also have data on health service use due to substance abuse, although this will likely capture only the most severe cases. Further, we currently lack the socioeconomic information for the cohort (eg, education, marital status, income), although we are planning to obtain this from Statistics Finland in future.
The prescription register does not include over-the-counter medicines or medication used in hospitals or nursing homes.Citation9 Further, only reimbursed medicines are recorded in the prescription register. It should also be noted that the prescription register contains only the dates of purchases and amount of purchased medications, which may not accurately represent the actual drug use. However, the validity of the prescription register in comparison with self-reported medication use has been confirmed.Citation54,Citation55 Further, the available information allows us to estimate use periods for each drug/drug group. This will provide more detailed estimation of the actual exposure period than a crude yes/no variable. Our data are well-suited for drug utilization and pharmacoepidemiological research. We have thorough records, with approximately 40 years of medical data and 15 years of prescription data, of all community-dwelling persons with a verified diagnosis of Ad residing in Finland. To our knowledge, similar longitudinal nationwide data does not exist elsewhere in the world. Thus, this project provides a unique opportunity to investigate the medication use and health status in a vulnerable population whose size will increase drastically in upcoming years.
Data access
MedAlz-2005 is a complex dataset and, although de-identified, the database includes sensitive personal information from over 50,000 individuals. We welcome suggestions for collaboration and note that we have agreed to comply with the regulations of database maintainers, ie, SII, National Institute of Health and Welfare, and Cancer Registry, all of which limit the use of data to strictly noncommercial purposes and restrict the data access to only those persons who are entitled to have access to the data according to accepted research proposals. Thus, new permissions for each individual accessing the data need to be filed. In addition, data provided by the National Institute of Health and Welfare, such as information on hospital discharges and social service use, cannot be sent abroad.
Conclusion
These routinely collected data enable the identification of all individuals with a clinically verified diagnosis of Ad as well as the assessment of their medication and health care service use. Register-based studies provide a feasible way for assessing medication use and associated outcomes in population groups that are underrepresented in randomized controlled trials.
Acknowledgments
We are grateful to the maintainers and data managers of Finnish health care registers for enabling this research effort.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationAlzheimer’s disease InternationalDementia: A Public Health PriorityGenevaWorld Health Organization2012
- Alzheimer’s AssociationThiesWBleilerL2011 Alzheimer’s disease facts and figuresAlzheimers Dement20117220824421414557
- Alzheimer’s Research TrustDementia 2010 The economic burden of dementia and associated research funding in the United KingdomCambridgeAlzheimer’s Research Trust2010
- World Health OrganizationNeurological Disorders: Public Health ChallengesGenevaWHO Press2007
- SeitzDPAdunuriNGillSSGruneirAHerrmannNRochonPAntidepressants for agitation and psychosis in dementiaCochrane Database Syst Rev2011162Cd00819121328305
- CusackBJPharmacokinetics in older personsAm J Geriatr Pharmacother20042427430215903286
- MangoniAAJacksonSHAge-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applicationsBr J Clin Pharmacol200457161414678335
- CherubiniAdel SignoreSOuslanderJSemlaTMichelJPFighting against age discrimination in clinical trialsJ Am Geriatr Soc20105891791179620863340
- FuruKWettermarkBAndersenMMartikainenJEAlmarsdottirABSørensenHTThe Nordic countries as a cohort for pharmacoepidemiological researchBasic Clin Pharmacol Toxicol20101062869419961477
- AndersenTFMadsenMJørgensenJMellemkjoerLOlsenJHThe Danish National Hospital Register. A valuable source of data for modern health sciencesDan Med Bull199946326326810421985
- Official Statistics of Finland (OSF): Population projectionHelsinkiStatistics Finland Available from: http://www.tilastokeskus.f/til/vaenn/index_en.htmlAccessed May 20, 2013
- Population structure [webpage on the Internet]HelsinkiStatistics Finland Available from: http://www.stat.f/til/vaerak/index_en.htmlAccessed May 20, 2013
- [Records and Information]. KELAN ATK-REKISTERIT JA NIIDEN SISÄlTÄMÄT TIEDOT REKISTEREITTÄIN Available from: http://uudistuva.kela.fi/in/internet/liite.nsf/net/310806125816mk/$file/atk.pdfAccessed July 2, 2013 Finnish
- ReligaDSpångbergKWimoAEdlundAKWinbladBEriksdotter-JönhagenMDementia diagnosis differs in men and women and depends on age and dementia severity: data from SveDem, the Swedish Dementia Quality RegistryDement Geriatr Cogn Disord2012332–3909522433665
- SuhonenJPirttiläTErkinjunttiTpdate on current care guidelines. The diagnosis and medical treatment of memory disordersDuodecim2010126182167216821072963
- McKhannGDrachmanDFolsteinMKatzmanRPriceDStadlanEMClinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of department of Health and Human Services Task Force on Alzheimer’s diseaseNeurology19843479399446610841
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders4th edWashington, DCAmerican Psychiatric Association1994
- Kela [homepage on the Internet] Available from: http://www.kela.fi/laake307Accessed July 2, 2013 Finnish
- HujanenTPeltolaMHäkkinenUPekurinenM[Healthcare costs of men and women according to age groups in 2006]. Miesten ja Naisten Terveysmenot Ikäryhmittäin 2006Report No: Stakesin Työpapereita 37/2008HelsinkiStakes2008 Finnish
- HujanenTKapiainenSTuominenUPekurinenM[Healthcare unit costs in Finland in 2006]. Terveydenhuollon Yksikkökustannukset Suomessa Vuonna 2006Report No: Stakesin Työpapereita 3/2008HelsinkiStakes2008 Finnish
- TiihonenJLönnqvistJWahlbeckK11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study)Lancet2009374969062062719595447
- PurhonenMKoponenHTiihonenJTanskanenAOutcome of patients after market withdrawal of thioridazine: a retrospective analysis in a nationwide cohortPharmacoepidemiol Drug Saf201221111227123122941581
- TiihonenJLönnqvistJWahlbeckKKlaukkaTTanskanenAHaukkaJAntidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohortArch Gen Psychiatry200663121358136717146010
- Finnish Cancer Registry [homepage on the Internet] Available from: http://www.cancer.fi/syoparekisteri/en/Accessed July 2, 2013
- National Institute for Health and WelfareKotihoidon asiakaslaskenta sosiaali- ja terveydenhuollossa 30.11 Available from: http://www.thl.fi/fi_FI/web/fi/tilastot/tietoa/rekisteriselosteet/kotihoidon_laskentaAccessed July 2, 2013 Finnish
- LaitinenMLBellJSLavikainenPLönnroosESulkavaRHartikainenSNationwide study of antipsychotic use among community-dwelling persons with Alzheimer’s disease in FinlandInt Psychogeriatr201123101623163121867581
- BellJSLönnroosEKoivistoAMUse of antiepileptic drugs among community-dwelling persons with Alzheimer’s disease in FinlandJ Alzheimers Dis201126223123721593561
- HommetCMondonKCamusVde ToffolBConstansTEpilepsy and dementia in the elderlyDement Geriatr Cogn Disord200825429330018311076
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older PersonsPharmacological management of persistent pain in older personsJ Am Geriatr Soc20095781331134619573219
- HanLAgostiniJVAlloreHGCumulative anticholinergic exposure is associated with poor memory and executive function in older menJ Am Geriatr Soc200856122203221019093918
- KünigGDätwylerSeschenASchreiter GasserUUnrecognised long-lasting tramadol-induced delirium in two elderly patients. A case reportPharmacopsychiatry200639519419916944413
- BrouquetACudennecTBenoistSImpaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgeryAnn Surg2010251475976520224380
- BellJSLaitinenMLLavikainenPLönnroosEUosukainenHHartikainenSUse of strong opioids among community-dwelling persons with and without Alzheimer’s disease in FinlandPain2011152354354721247697
- LönnroosEKyyrönenPBellJSvan der CammenTJHartikainenSRisk of death among persons with Alzheimer’s disease: a national register-based nested case-control studyJ Alzheimers Dis201333115716422914589
- TolppanenAMLavikainenPSolomonAKivipeltoMSoininenHHartikainenSIncidence of stroke in people with Alzheimer disease: A national register-based approachNeurology201380435335823284063
- TolppanenALavikainenPSoininenHHartikainenSIncident hip fractures among community dwelling persons with Alzheimer’s disease in a Finnish nationwide register-based cohortPloS One201383e5912423527106
- WellerISchatzkerJHip fractures and Alzheimer’s disease in elderly institutionalized CanadiansAnn Epidemiol200414531932415177270
- ZhaoYShenLJiHFAlzheimer’s disease and risk of hip fracture: a meta-analysis studyScientificWorldJournal2012201287217322629218
- ZhaoYKuoTCWeirSKramerMSAshASHealthcare costs and utilization for Medicare beneficiaries with Alzheimer’sBMC Health Serv Res2008810818498638
- BakerNLCookMNArrighiHMBullockRHip fracture risk and subsequent mortality among Alzheimer’s disease patients in the United Kingdom, 1988–2007Age Ageing2011401495421087990
- TrimpouPLandin-WilhelmsenKOdénARosengrenAWilhelmsenLMale risk factors for hip fracture-a 30-year follow-up study in 7,495 menOsteoporos Int201021340941619475474
- TolppanenAMLavikainenPSolomonAHistory of medically treated diabetes and risk of Alzheimer disease in a nationwide case-control studyDiabetes Care EpubAccessed January 22, 2013
- ProfennoLAPorsteinssonAPFaraoneSVMeta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disordersBiol Psychiatry201067650551219358976
- GisslerMShelleyJQuality of data on subsequent events in a routine Medical Birth RegisterMed Inform Internet Med2002271333812509121
- IsohanniMMäkikyröTMoringJA comparison of clinical and research DSM-III-R diagnoses of schizophrenia in a Finnish national birth cohort. Clinical and research diagnoses of schizophreniaSoc Psychiatry Psychiatr Epidemiol19973253033089257522
- TeppoLPukkalaELehtonenMData quality and quality control of a population-based cancer registry. experience in FinlandActa Oncol19943343653698018367
- TolonenHSalomaaVTorppaJSiveniusJImmonen-RäihäPLehtonenAFINSTROKE registerThe validation of the Finnish Hospital discharge Register and Causes of Death Register data on stroke diagnosesEur J Cardiovasc Prev Rehabil200714338038517568236
- MeretojaAKasteMRoineROTrends in treatment and outcome of stroke patients in Finland from 1999 to 2007. PERFECT Stroke, a nationwide register studyAnn Med201143Suppl 1S22S3021639714
- SundRNurmi-LüthjeILüthjePTanninenSNarinenAKeskimäkiIComparing properties of audit data and routinely collected register data in case of performance assessment of hip fracture treatment in FinlandMethods Inf Med200746555856617938779
- MähönenMSalomaaVTorppaJThe validity of the routine mortality statistics on coronary heart disease in Finland: comparison with the FINMONICA MI register data for the years 1983–1992. Finnish multinational MONItoring of trends and determinants in CArdiovascular diseaseJ Clin Epidemiol199952215716610201658
- PajunenPKoukkunenHKetonenMThe validity of the Finnish Hospital discharge Register and Causes of Death Register data on coronary heart diseaseEur J Cardiovasc Prev Rehabil200512213213715785298
- RapolaJMVirtamoJKorhonenPValidity of diagnoses of major coronary events in national registers of hospital diagnoses and deaths in FinlandEur J Epidemiol19971321331389084994
- SundRQuality of the Finnish Hospital discharge Register: a systematic reviewScand J Public Health201240650551522899561
- HaukkaJSuvisaariJTuulio-HenrikssonALönnqvistJHigh concordance between self-reported medication and official prescription database informationEur J Clin Pharmacol200763111069107417712552
- RikalaMHartikainenSSulkavaRKorhonenMJValidity of the Finnish Prescription Register for measuring psychotropic drug exposures among elderly finns: a population-based intervention studyDrugs Aging201027433734920359263