Abstract
Background
A number of studies have shown poorer survival among cancer patients with comorbidity. Several mechanisms may underlie this finding. In this review we summarize the current literature on the association between patient comorbidity and cancer prognosis. Prognostic factors examined include tumor biology, diagnosis, treatment, clinical quality, and adherence.
Methods
All English-language articles published during 2002–2012 on the association between comorbidity and survival among patients with colon cancer, breast cancer, and lung cancer were identified from PubMed, MEDLINE and Embase. Titles and abstracts were reviewed to identify eligible studies and their main results were then extracted.
Results
Our search yielded more than 2,500 articles related to comorbidity and cancer, but few investigated the prognostic impact of comorbidity as a primary aim. Most studies found that cancer patients with comorbidity had poorer survival than those without comorbidity, with 5-year mortality hazard ratios ranging from 1.1 to 5.8. Few studies examined the influence of specific chronic conditions. In general, comorbidity does not appear to be associated with more aggressive types of cancer or other differences in tumor biology. Presence of specific severe comorbidities or psychiatric disorders were found to be associated with delayed cancer diagnosis in some studies, while chronic diseases requiring regular medical visits were associated with earlier cancer detection in others. Another finding was that patients with comorbidity do not receive standard cancer treatments such as surgery, chemotherapy, and radiation therapy as often as patients without comorbidity, and their chance of completing a course of cancer treatment is lower. Postoperative complications and mortality are higher in patients with comorbidity. It is unclear from the literature whether the apparent undertreatment reflects appropriate consideration of greater toxicity risk, poorer clinical quality, patient preferences, or poor adherence among patients with comorbidity.
Conclusion
Despite increasing recognition of the importance of comorbid illnesses among cancer patients, major challenges remain. Both treatment effectiveness and compliance appear compromised among cancer patients with comorbidity. Data on clinical quality is limited.
Introduction
It is essential to personalized medicine to understand how patient characteristics such as age and coexisting diseases (comorbidity) affect cancer detection, treatment, and outcome. With more than 60% of cancer patients diagnosed at age 65 or older in high-income countries,Citation1 many patients have comorbidities that complicate the decision-making process. Because the elderly and persons with comorbidity are often underrepresented in clinical trials,Citation2,Citation3 information regarding treatment effectiveness is often extrapolated from studies of younger patients without comorbidity.
Several studies have shown poorer survival among cancer patients with comorbidity,Citation4–Citation11 but the underlying mechanisms remain unclear. In recent decades 5-year survival rates have improved among cancer patients without comorbidity, but not among patients with severe comorbidity.Citation4–Citation6 An understanding of how comorbidity affects survival in patients with cancer is needed to guide clinical practice. We therefore reviewed the literature on the association between comorbidity and survival among patients with three of the most commonly diagnosed cancers: colon cancer, breast cancer, and lung cancer.
Methods
Definition and measurement of comorbidity
Comorbidity has been defined as “any additional clinical entity that has existed or that may occur during the clinical course of a patient with an index disease under study.”Citation12,Citation13 The term “multimorbidity” is often used interchangeably with “comorbidity” but has a slightly different meaning. Multimorbidity refers to the coexistence of ≥2 illnesses without identifying an index disease.Citation14 Comorbidity must also be distinguished from complications that arise as a consequence of the cancer or its treatment. A number of studies have examined the prognostic impact of patients’ “performance status” at the time of cancer diagnosis. Performance status is a measure of a cancer patient’s well-being defined as the amount of normal daily activity the patient can maintain.Citation15–Citation17 However, performance status is affected by cancer, complications of cancer, and comorbid conditions.Citation18 Therefore, measures of comorbidity must be distinguished from measures of performance status.
Data on comorbid diseases are available from different data sources, such as medical records, administrative databases, physical examinations, and self-reports using questionnaires.Citation19–Citation21 Comorbidity can be assessed by counting the number of coexisting diseases diagnosed in a cancer patient or by using a comorbidity index that combines the number and severity of the diseases. The most widely used index is the Charlson Comorbidity Index (CCI). The original Charlson measure was constructed to predict 1-year mortality in 559 medical patients as well as 10-year mortality rates for death attributable to comorbid diseases among 685 breast cancer patients.Citation22 The index is based on 19 distinct medical disease categories. Each condition has a weight assigned from 1 to 6, derived from the relative risk estimates obtained from a regression model. The CCI score is the sum of weights for all prevalent conditions. The score can theoretically range from 0–33 but was collapsed into categories of 0, 1–2, 3–4, and ≥5 in its initial presentation.Citation22,Citation23
Literature search
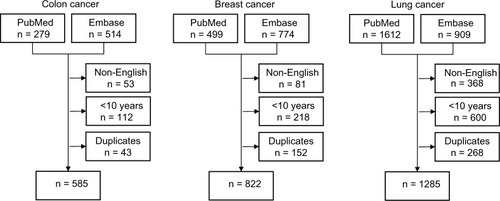
We searched PubMed, MEDLINE and Embase to identify and summarize existing information on the association between comorbidity and cancer survival in patients with colon cancer, breast cancer, and lung cancer. We used the following keywords to identify potentially useful articles: “comorbidity,” “multimorbidity,” and “coexisting diseases.” In addition, we searched for articles on the following prevalent comorbid diseases: diabetes, cardiovascular diseases, chronic pulmonary disease, and dementia. Furthermore, we queried the databases using the terms “colon cancer,” “breast cancer,” “lung cancer,” and “comorbidity” combined with such terms as “pathogenesis,” “histology,” “differentiation,” “stage,” “diagnosis,” “centralized treatment,” “specialized treatment,” “patient volume,” and “surgeon volume.” We limited our search to English-language articles published within the last 10 years. All searches were performed at the end of November 2012. Our PubMed search strategy is described in detail in .
Overall, we identified 2,692 potentially eligible articles (). The first author (MS) reviewed the titles and abstracts and removed articles not relevant to comorbidity and cancer survival. The information summarized in this review was gleaned from the remaining articles and prior publications cited by these articles.
Results
Prevalence of comorbidity among cancer patients
As shown in , comorbidity is common in patients with colon cancer (14%–68%), breast cancer (20%–35%), and lung cancer (26%–81%). A recent Danish population-based cohort study found that elderly patients with colorectal and lung cancer had a higher prevalence of comorbidity than an age- and sex-matched comparison cohort from the general population, as measured by CCI scores (CCI score of 1 or 2: 12.3% vs 9.6% and CCI score of ≥3: 5.6% vs 4.0%).Citation24 The probable reason for these findings is that known risk factors for colorectal or lung cancer, such as smoking, obesity, and physical inactivity, are also common risk factors for non-cancer diseases such as ischemic heart disease.
Table 1 Results of selected studies on the association between comorbidity and treatment
As average life expectancy increases in Western countries, the proportion of elderly cancer patients also is expected to increase.Citation25 Because the prevalence of comorbidity increases with age, the number of cancer patients with comorbidity will increase concomitantly. This is indicated in a US study of 49,646 women aged 67 years or older with breast cancer in the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked data set. Among these patients, 23% of women aged 85–89 years and 11.2% of women aged 67–69 years had severe comorbidity.Citation26 In a study from The Netherlands, 53% of patients aged 60–74 years were found to have at least one comorbidity, and this proportion increased to 63% in patients aged 75 years and older.Citation24
Impact of comorbidity on cancer survival
Most observational studies have found that cancer patients with comorbidities have poorer survival than patients without comorbidities.Citation4–Citation11,Citation24,Citation28–Citation30 Cohort studies with 5–7 years of follow-up have reported 1.1- to 5.8-fold higher mortality for breast cancer patients with any comorbidity compared to patients with no comorbidity.Citation4,Citation20,Citation26,Citation31,Citation32 Similarly, studies of patients with colon cancer have reported 1.2- to 4.8-fold higher 5-year mortality for patients with comorbidity versus without comorbidity.Citation5,Citation8,Citation9,Citation25,Citation32,Citation34 Correspondingly, mortality in patients with lung cancer is 1.1 to 1.5 times higher for patients with comorbidity in studies with 1–5 years of follow-up.Citation7,Citation29,Citation34,Citation35 Not surprisingly, if survival among patients with a particular type of cancer is generally very poor, the additional effect of comorbid diseases on mortality on a relative scale is small.Citation34,Citation36,Citation37 Thus, the relatively lower prognostic impact of comorbidity among lung cancer patients is probably due to a 1-year mortality rate above 70% even among otherwise healthy patients.Citation24
Stage at diagnosis influences decisions about the appropriate course of treatment and is strongly associated with cancer survival. Thus, stage-specific analyses may provide more insight into the association between comorbidity and cancer survival. Among 62,591 women diagnosed with early-stage breast cancer in Denmark during 1990–2008, the adjusted hazard ratios (HRs) for all-cause mortality were 1.45 (95% confidence interval [CI]: 1.40–1.51) for patients with low comorbidity, 1.52 (95% CI: 1.45–1.60) for patients with moderate comorbidity, and 2.21 (95% CI: 2.08–2.35) for patients with severe comorbidity, compared to patients without comorbidity. Median follow-up time was 8.2 years.Citation6 Recently, Patnaik et alCitation28 used the SEER-Medicare linked data set to determine the effect of 13 distinct comorbid conditions on survival and all-cause mortality among 64,034 breast cancer patients aged 66 years or older from 1992 to 2000. Patients with any of the 13 comorbidities had lower rates of 5-year survival than patients with no comorbidities. In addition, stage I cancer patients with serious comorbid conditions had survival rates similar to stage II cancer patients without comorbidities. Thus, patients with early-stage cancers and significant comorbidities had outcomes comparable to patients with later-stage tumors.
A key question is whether the higher mortality observed in cancer patients with comorbidity stems from their comorbidity or whether their cancer-specific mortality is elevated. In a recent Danish cohort study of 6,325 patients aged ≥70 years with breast, lung, colorectal, prostate, or ovarian cancer, 5-year all-cause mortality increased with higher levels of comorbidity.Citation24 For 5-year cancer-specific mortality, however, comorbidity was associated with increased rates only in patients with lung cancer (5-year HR for CCI score ≥3 vs CCI score of 0 = 1.29 [95% CI: 1.03–1.60]). For patients with breast cancer, the 5-year HR for CCI score ≥3 vs CCI score of 0 was 0.48 (95% CI: 0.21–1.07), and for patients with colorectal cancer, the 5-year HR for CCI score ≥3 vs CCI score of 0 was 1.00 (95% CI: 0.76–1.33). In contrast, Land et alCitation6 recently found an association between comorbidity and cancer-specific mortality in women with breast cancer (HR for CCI score ≥3 vs CCI score of 0 = 1.79 [95% CI: 1.66–1.93]). Median follow-up time in the study was 8.2 years. Berglund et alCitation38 found a similar association in women with early-stage breast cancer (stage I: HR for CCI score ≥2 vs CCI score of 0 = 1.47 [95% CI: 1.11–1.94]), but not in women with more advanced cancer (stage IIB: HR for CCI score ≥2 vs CCI score of 0 = 0.83 [95% CI: 0.63–1.10]). Several other studies have found an association between increasing levels of comorbidity and higher cancer-related mortality among patients with colon, breast, or lung cancer.Citation8,Citation9,Citation25,Citation39–Citation41 However, there is considerable uncertainty in defining whether death was due to the cancer or to other causes (including comorbidity), and the validity of cause-of-death data may be questioned.Citation42–Citation44
Effect of comorbidity on survival
Comorbidity can affect cancer survival through its impact on such factors as cancer detection, treatment, and adherence.Citation45 In the following sections, we focus on the potential role of comorbidity on different points from cancer detection through diagnosis and treatment.
Impact of comorbidity on cancer morphology, histology, differentiation, and proliferation status
It is plausible that comorbidity is associated with differences in morphology, histology, differentiation, and proliferation status. Cancer risk is elevated in patients with obesity; in patients with diabetes and resulting insulin resistance and chronic hyperinsulinemia;Citation46–Citation49 and in patients with inherited, acquired (eg, from HIV/AIDS), or drug-induced (eg, from treatment with steroids or biologics) immunosuppression.Citation50,Citation51 Some of these risk factors also may be associated with rate of cancer growth and cancer grade/differentiation and thus with prognosis. Conversely, drugs such as nonsteroidal anti-inflammatory agents,Citation52,Citation53 aspirin,Citation54,Citation55 statins,Citation56 and long-term antibiotics used to treat comorbidity-associated infectionsCitation57 may decrease cancer incidence,Citation52–Citation55 progression,Citation53,Citation56 and risk of recurrence and improve cancer prognosis.Citation58–Citation61
As shown in , the proportion of squamous cell carcinoma in lung cancer patients with comorbidity has been found to be 6%–11% higher than in patients without comorbidity.Citation10,Citation11 Chlebowski et alCitation62 found a slightly higher proportion of ductal breast cancer (69% vs 65%) and a slightly lower proportion of estrogen-receptor-positive breast cancer (74% vs 78%) and progesterone-receptor-positive breast cancer (61% vs 64%) among diabetic compared with nondiabetic breast cancer patients (). Kaplan et alCitation63 also found a higher incidence of ductal breast cancer among diabetics compared with nondiabetics (89% vs 82%). In contrast, Land et alCitation6 found no differences in histology or receptor status according to level of comorbidity. However, few studies provided data on tumor biology by comorbidity level.
Table 2 Results of selected studies on the association between comorbidity and cancer characteristics
Comorbidity and other patient characteristics
Age is closely related to comorbidity and is also a strong predictor of mortality in cancer patients. Thus, older age could potentially explain the prognostic impact of comorbidity. However, the association between comorbidity and cancer survival persists even after adjusting for age. The association also remains after adjusting for other prognostic factors, such as cancer stage and treatment.Citation64 It is also plausible that age may modify the relationship between comorbidity and cancer survival if clinicians tend to focus more on comorbidity in older than in younger patients when deciding on type of cancer treatment.Citation65 Sex may also play a role, as several studies have indicated that women with lung cancer have a better prognosis than men with lung cancer.Citation66–Citation68 The underlying reasons are debated and remain unresolved. In addition, converging evidence from epidemiological studies conducted in a variety of settings have documented racial and socioeconomic disparities in cancer survival.Citation67–Citation74 Multiple factors may contribute to these disparities, but comorbidity seems to play an important role.Citation72–Citation77 In a US cohort study of 906 women with breast cancer, Tammemagi et alCitation78 found an HR for all-cause mortality of 1.14 (95% CI: 0.92–1.40) for blacks compared to whites after adjusting for age, tumor stage, estrogen receptor status, surgery, chemotherapy, and radiation therapy. After further adjustment for comorbidity, the HR decreased to 1.02 (95% CI: 0.83–1.27). The two most important comorbidities explaining the disparities were diabetes and hypertension.Citation78 A Danish cohort study conducted by Dalton et alCitation79 found an interaction between income and comorbidity, resulting in 15% lower survival within 10 years after primary surgery for early-stage breast cancer among women of low socioeconomic status with comorbid conditions (~65%) compared to more affluent women with similar comorbid conditions (~80%). This suggests a differential effect of comorbidity on risk of dying of early-stage breast cancer by socioeconomic group.Citation75
Impact of comorbidity on stage at diagnosis
It is often argued that comorbidities may be associated with late-stage cancer diagnosis because they may mask early cancer symptoms. Dementia,Citation80,Citation81 alcohol consumption,Citation82,Citation83 and major depressionCitation84 have been associated with late-stage diagnosis of colon cancer and breast cancer. However, as shown in , several studies have found a higher prevalence of comorbidity in patients diagnosed with early-stage lung cancer, breast cancer, and colorectal cancer. Earlier cancer diagnosis in patients with comorbidities is plausible because these patients are more likely to require frequent medical care, and hence to receive closer clinical monitoring, than persons without comorbidities. Nonetheless, the association between comorbidity and earlier diagnosis seems to depend on the specific comorbid condition. Fleming et alCitation85 found that women with cardiovascular disease, musculoskeletal disease, gastrointestinal disease, osteoarthritis, and genitourinary disease had a 7%–24% lower risk of being diagnosed with advanced breast cancer (). In contrast, women with diabetes, renal disease, other endocrine disorders, psychiatric disease, osteoporosis, hematologic disease, obesity, and AIDS had an 11 %–20% higher risk of being diagnosed with advanced disease ().Citation85 Similarly, Yasmeen et alCitation76 found that presence of certain comorbidities (eg, arthritis, depression, diabetes, stable coronary artery disease) was associated with higher utilization of screening mammograms and greater likelihood of diagnosis of localized disease (odds ratio [OR] = 0.8,95% CI: 0.7–0.9), while a group of other comorbidities judged to be more serious (including severe heart failure, cardiac arrhythmias, and end-stage pulmonary disease) was associated with less screening mammography and later stage at diagnosis (OR =1.3, 95% CI: 1.2–1.4) ().Citation76 Studies relating comorbidity to breast cancer screening have had mixed results, showing either increased or decreased risk of late-stage disease according to comorbidity burden.Citation76,Citation86
Table 3 Results of selected studies on the association between comorbidity and cancer stage at diagnosis
Impact of comorbidity on choice of treatment
As shown in , surgical management steadily declines with increasing comorbidity regardless of cancer site and disease stage. Berglund et alCitation38 found that the OR of no surgery was 1.88 (95%o CI: 1.65–2.14) among breast cancer patients with a CCI score of 1, and 3.01 (95% CI: 2.67–3.41) among those with a CCI score ≥2, compared with patients without comorbidity. In a population-based cohort study conducted in Northern Denmark, Iversen et alCitation5 found that 83.8%o of colon cancer patients with a CCI score of 0 undergo surgical resection, compared with 77.7%o of patients with CCI scores of 1 or 2 and 63.2%o of patients with a CCI score ≥3. Similarly, other studies have reported 25%–58%o lower odds of surgical resection in lung cancer patients with severe comorbidity compared with patients without comorbidityCitation11,Citation87,Citation88 An increased risk of complications among patients with comorbidities who undergo surgical resection for colon cancer (adjusted OR for body mass index of 30–19 = 1.26 [95% CI: 1.05–1.49]; for chronic obstructive pulmonary disease [COPD] = 1.84 [95% CI: 1.49–2.27]; and for high ASA physical classification score = 1.65 [95%o CI: 1.26–2.16]),Citation89 for breast cancer (6% with low comorbidity had complications vs 10 % with moderate comorbidity),Citation32 and for lung cancer (adjusted OR for a CCI score of 1 = 1.38 [95% CI: 1.15–1.66] and for a CCI score ≥2 = 1.83 [95% CI: 1.50–2.23]),Citation90 compared with patients without comorbidity (). Other studies have reported 2- to 4-fold higher 30-day postoperative mortality rates in colon cancer patients with comorbidity compared to patients without comorbidities.Citation89,Citation91
Patients with comorbidities are less likely to receive any adjuvant chemotherapy,Citation92–Citation99 more likely to receive a reduced dose,Citation10,Citation100,Citation101 and more likely not to complete chemotherapy treatment when initiatedCitation101,Citation102–Citation104 (). While some studies report that patients with comorbidity are less likely to be referred to a medical oncologist,Citation95,Citation105 a US cohort study of 4,765 colon cancer patients found that patients with comorbidity who underwent resection consulted an oncologist more frequently than patients without comorbidity (adjusted OR for consultation among patients with a CCI score of 1 = 1.25 [95% CI: 0.98–1.59] and among patients with a CCI score ≥2 = 1.61 [95% CI: 1.17 to 2.20]).Citation94 However, in another US study, colon cancer patients with comorbidity were less likely to receive chemotherapy, whether or not they consulted an oncologist ().Citation95 Presence of comorbidity has also been associated with increased time from cancer detection to surgical resection or initiation of chemotherapy or radiotherapy.Citation88,Citation95,Citation104,Citation106,Citation107 The reasons for this remain unknown.
There are few data on the impact of comorbidity on risk of complications after chemotherapy and radiation therapy. Grønberg et alCitation10 found that lung cancer patients with severe comorbidity were more likely than lung cancer patients without comorbidity to develop thrombocytopenia (46% vs 36%) or febrile neutropenia (12% vs 5%) or to die of neutropenic infection (3% vs 0.%) following chemotherapy treatment. Conversely, Gross et alCitation108 found that risk of hospitalization attributable to chemotherapy treatment was lower among colon cancer patients with COPD, chronic heart failure, or diabetes, compared with patients without these conditions ().
Impact of comorbidity on health care-related factors
Treatment in specialized medical centers or by a high-volume surgeon has been associated with improved treatment and survival.Citation109–Citation114 However, there are very few studies on the prognostic impact of receiving high-volume-cancer-center care and highly specialized treatment in relation to comorbidity. A US study of 211,084 patients with lung, breast, colorectal, and prostate cancer found that patients treated at National Cancer Institute-designated cancer centers had lower mortality than patients treated at volume-matched hospitals across all levels of comorbidity (3-year mortality for specialized vs nonspecialized treatment: adjusted OR for CCI score of 0 = 0.89 [95% CI: 0.85–0.98]; adjusted OR for CCI score of 1 or 2 = 0.87 [95% CI: 0.80–0.95]; adjusted OR for CCI score ≥3 = 0.83 [95% CI: 0.74–1.00]).Citation111 Furthermore, while some studies have shown a social gradient in access to specialized cancer care,Citation114,Citation115 few studies have examined potential disparities in access to specialized care among patients with comorbidities.
To better understand the observed underutilization of treatment by age and comorbidity, a number of studies have explored physician and patient perspectives regarding the decision to use adjuvant chemotherapy.Citation105,Citation116–Citation119 It has been found that 24%–70% of cancer patients with comorbidity are not treated according to guidelines.Citation105,Citation118,Citation120–Citation122 In a US national survey of surgeons and medical oncologists caring for patients with colorectal cancer, physicians agreed with guidelines recommending adjuvant chemotherapy for young, otherwise healthy patients with stage III colon cancer, but differed widely on recommendations for patients with comorbid illnesses.Citation119 Comorbidity is the most frequent reason for nonreceipt of cancer treatment cited in the medical charts of patients with lung (68% of nontreated patients) and colorectal (47% of nontreated patients) cancer.Citation117,Citation118 To some extent, this finding probably reflects concern about toxicity in patients with comorbidity. Among patients with lung cancer, Gironés et alCitation123 recently showed that withholding treatment was associated with factors such as poor health, advanced age, depression, and dementia, but not related to symptoms at diagnosis or cancer stage.
Physicians’ motivations and treatment barriers are also influenced by age, race, and education level. Studies have shown that duration of consultations and amount of information provided to patients increases with higher education levels.Citation124–Citation126 While patient perceptions and preferences play a role in treatment decisions and outcomes, the treating physician’s recommendation has been found to be a major determinant of patients’ preferences for chemotherapy.Citation127,Citation128 It remains unclear whether patient preferences differ according to level of comorbidity.
Influence of comorbidity on treatment regimen completion
Patients with comorbidities may be compromised in their ability to comply with treatment regimens or to tolerate their side effects. In a US cohort study of 3,733 colon cancer patients aged ≥65 years with records in the linked SEER-Medicare dataset during 1995–1999, comorbidity was associated with lower odds of completing adjuvant chemotherapy (adjusted ORs were 0.75 [95% CI: 0.60–0.97] for patients with one comorbidity and 0.62 [95% CI: 0.46–0.84] for patients with >1 comorbidity) compared with patients without comorbidity.Citation129 Several other studies have also shown that comorbidity is associated with decreased likelihood of completing chemotherapy treatment among patients with colon,Citation1,Citation102,Citation108 breast,Citation100 and lung cancerCitation10 (). However, none of these studies examined whether failure to complete chemotherapy was related to poorer adherence or to level of side effects. Many studies of women with early-stage breast cancer, based on pharmacy, medical, and health insurance data, have reported high rates of discontinuation of adjuvant tamoxifen, ranging from 35%–51% during study periods of 3.5–5 years.Citation130–Citation133 Patient refusal reportedly accounts for a third of occurrences of treatment underuse,Citation134 and comorbidity has been identified as a predictor of discontinuation and nonadherence to regimens of tamoxifen and aromatase inhibitors.Citation132,Citation133 However, a very recent German cohort study of 12,412 women with breast cancer, among whom 7,312 were treated with tamoxifen, demonstrated lower rates of tamoxifen discontinuation among patients with diabetes (adjusted HR = 0.81 [95% CI: 0.75–0.86]) and depression (adjusted HR = 0.92 [95% CI: 0.87–0.97]).Citation135
Methodological considerations
Several methodological concerns must be considered when evaluating the summary evidence from the studies reviewed above. This review is not a systematic review. A research librarian assisted our searches, but we did not use explicit predefined criteria to select the articles included. Thus, our study selection was subjective and we may have missed relevant papers. The studies included were heterogeneous and included vastly different patient populations (–). Moreover, many were designed as predictive studies and included a wide range of potential prognostic factors besides comorbidity in regression models. Some studies also included variables such as patient performance status (activities of daily living),Citation136 which may constitute an intermediate variable in the causal path from comorbidity to cancer survival. Adjusting for patient performance status thus may weaken the prognostic impact of comorbidity. A further challenge in summarizing the effect of comorbidity on cancer survival was inconsistent definitions of comorbidity. Comorbidity was measured in different ways in the studies under review, referring either to one specific disease or aggregation of several diseases using an index. Moreover, indices varied from general comorbidity measures to disease-specific measures. Most studies aggregated comorbidity into a comorbidity index (most frequently the CCI) () with little consideration of how specific conditions affected outcomes. Although shown repeatedly to be a valid prognostic predictor, the CCI itself is based on simple assumptions about mortality risk when various conditions co-occur. In addition, most studies collapsed the CCI score of above a certain threshold into a single open-ended category (eg, 0, 1–2, and ≥3) to improve comprehension and the statistical efficacy of the analysis (the prevalence of patients with high CCI scores is low in most study populations). The effect of the combined category is a weighted average of the individual scores.Citation23 Analyses based on individual comorbid diseases would avoid these assumptions but are difficult to conduct, as they require much larger cohorts to identify subgroups with specific conditions of sufficient size.Citation137 It must also be noted that virtually none of the studies under review examined the impact of duration and/or severity of comorbidity on cancer prognosis.
Most studies in our review were based on analyses of population-based cancer registry data linked with administrative data. Such data are generally adequate for determining prevalence of comorbidity and survival outcomes, but generally provide limited information on treatment delivery or patient tolerance for treatment regimens. Furthermore, studies relying on such databases may miss important comorbidities, underestimate their severity, or fail to address confounding factors such as smoking and other lifestyle factors. Thus, to improve research on comorbidity, studies should include information from different data sources (ie, administrative data, chart review, prescription records, and records of general practitioners) to provide more information on level and severity of comorbidity.
Conclusion
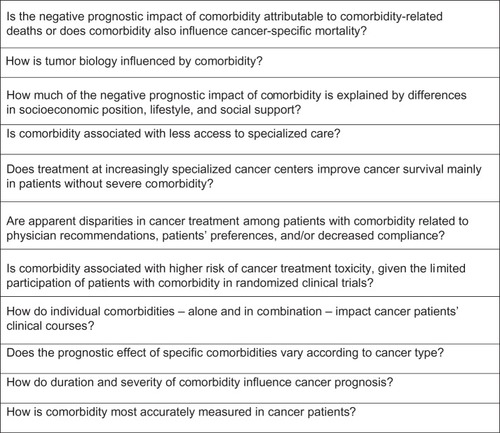
Despite increasing recognition of the impact of comorbid illnesses on the prognosis of cancer patients, challenges remain. A large number of studies reported suboptimal treatment among patients with comorbidity across tumor sites and stages of disease. However, because most studies examined diagnosis, treatment, physician and/or patient preferences, but not all factors, it is unclear whether suboptimal cancer treatment reflects appropriate consideration of increased risk of toxicity due to comorbid illness, patient preferences, lower quality of clinical care, or poor adherence. Consequently, a number of questions remain unanswered about the relationship between comorbidity and cancer outcome (). To adequately address these questions, studies are needed that elucidate whether comorbidity in general or only specific diseases or disease combinations are associated with poorer survival. Thus, studies with a more specific focus should be undertaken, including those that address the impact of an individual comorbidity on treatment provided to a homogenous population of cancer patients (ie, with comparable stage and tumor type).
Acknowledgments
This review was conducted as part of the Aarhus University Disease Epidemiology and Outcomes (AUDEO) Program at the Department of Clinical Epidemiology, Aarhus University Hospital. This study received financial support from the Danish Cancer Society and the Department of Clinical Epidemiology’s research foundation.
Supplementary material
Table S1 PubMed search strategy
Disclosure
The authors report no conflicts of interest in this work.
References
- YancikRRiesLACancer in older persons: an international issue in an aging worldSemin Oncol200431212813615112144
- HutchinsLFUngerJMCrowleyJJColtmanCAJrAlbainKSUnderrepresentation of patients 65 years of age or older in cancer-treatment trialsN Engl J Med1999341272061206710615079
- BiganzoliLWildiersHOakmanCManagement of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA)Lancet Oncol2012134e148e16022469125
- Cronin-FentonDPNørgaardMJacobsenJComorbidity and survival of Danish breast cancer patients from 1995 to 2005Br J Cancer20079691462146817406360
- IversenLHNørgaardMJacobsenJLaurbergSSørensenHTThe impact of comorbidity on survival of Danish colorectal cancer patients from 1995 to 2006 – a population-based cohort studyDis Colon Rectum2009521717819273959
- LandLHDaltonSOJensenMBEwertzMImpact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990–2008Breast Cancer Res Treat201213131013102022002567
- AsmisTRDingKSeymourLNational Cancer Institute of Canada Clinical Trials GroupAge and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trialsJ Clin Oncol2008261545918165640
- RoxburghCMcDonaldASalmondJAdjuvant chemotherapy for resected colon cancer: comparison of the prognostic value of tumour and patient related factorsInt J Colorectal Dis201126448349221212966
- SarfatiDHillSBlakelyTThe effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: a retrospective cohort studyBMC Cancer2009911619379520
- GrønbergBHSundstrømSKaasaSInfluence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapyEur J Cancer201046122225223420471248
- LüchtenborgMJakobsenEKrasnikMLinklaterKMMellemgaardAMøllerHThe effect of comorbidity on stage-specific survival in resected non-small cell lung cancer patientsEur J Cancer201248183386339522795582
- LastJMA Dictionary of Epidemiology4th edNew York, NYOxford University Press2001
- FeinsteinAThe pre-therapeutic classification of co-morbidity in chronic diseasesJ Chronic Dis197023455468
- BarnettKMercerSWNorburyMWattGWykeSGuthrieBEpidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional studyLancet20123809836374322579043
- De RijkeJMSchoutenLJten VeldeGPMWInfluence of age, comorbidity and performance status on the choice of treatment for patients with non-small cell lung cancer; results of a population-based studyLung cancer (Amsterdam, Netherlands)2004462233245
- LangerCJNeglected and Underrepresented Subpopulations: Elderly and Performance Status 2 Patients with Advanced-Stage Non-Small-Cell Lung CancerClinical Lung Cancer2006S126S13716764753
- QuoixEOptimal pharmacotherapeutic strategies for elderly patients with advanced non-small cell lung cancerDrugs & aging2011281188589422054229
- GroseDDevereuxGBrownLScottish Lung Cancer ForumVariation in comorbidity and clinical management in patients newly diagnosed with lung cancer in four Scottish centersJ Thorac Oncol20116350050921258251
- PattersonREFlattSWSaquibNMedical comorbidities predict mortality in women with a history of early stage breast cancerBreast Cancer Res Treat2010122385986520077000
- AhernTPLashTLThwinSSSillimanRAImpact of acquired comorbidities on all-cause mortality rates among older breast cancer survivorsMed Care2009471737919106734
- LashTLMorVWielandDFerrucciLSatarianoWSillimanRAMethodology, design, and analytic techniques to address measurement of comorbid diseaseJ Gerontol A Biol Sci Med Sci200762328128517389725
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- LashTLCollapsing high-end categories of comorbidity may yield misleading resultsClin Epidemiol20091111520865081
- JørgensenTLHallasJFriisSHerrstedtJComorbidity in elderly cancer patients in relation to overall and cancer-specific mortalityBr J Cancer201210671353136022353805
- BushDSmithBYoungerJMichaelsonJSThe non-breast-cancer death rate among breast cancer patientsBreast Cancer Res Treat2011127124324920927583
- SchonbergMAMarcantonioERLiDSillimanRANgoLMcCarthyEPBreast cancer among the oldest old: tumor characteristics, treatment choices, and survivalJ Clin Oncol201028122038204520308658
- CoeberghJWJanssen-HeijnenMLPostPNRazenbergPPSerious co-morbidity among unselected cancer patients newly diagnosed in the southeastern part of The Netherlands in 1993–1996J Clin Epidemiol199952121131113610580775
- PatnaikJLByersTDiguiseppiCDenbergTDDabeleaDThe influence of comorbidities on overall survival among older women diagnosed with breast cancerJ Natl Cancer Inst2011103141101101121719777
- TammemagiCMNeslund-DudasCSimoffMKvalePImpact of comorbidity on lung cancer survivalInt J Cancer2003103679280212516101
- IrisaKMasagoKTogashiYSignificance of pretreatment comorbidities in elderly patients with advanced non-small-cell lung cancer treated with chemotherapy or epidermal growth factor receptor-tyrosine kinase inhibitorMed Oncol201229118519221136210
- Janssen-HeijnenMLHoutermanSLemmensVELouwmanMWMaasHACoeberghJWPrognostic impact of increasing age and comorbidity in cancer patients: a population-based approachCrit Rev Oncol Hematol200555323124015979890
- HoutermanSJanssen-HeijnenMLVerheijCDComorbidity has negligible impact on treatment and complications but influences survival in breast cancer patientsBr J Cancer200490122332233715162155
- LemmensVEvan HalterenAHJanssen-HeijnenMLVreugdenhilGRepelaer van DrielOJCoeberghJWAdjuvant treatment for elderly patients with stage III colon cancer in the southern Netherlands is affected by socioeconomic status, gender, and comorbidityAnn Oncol200516576777215817594
- Janssen-HeijnenMLLemmensVEvan den BorneBEBiesmaBOeiSBCoeberghJWNegligible influence of comorbidity on prognosis of patients with small cell lung cancer: a population-based study in The NetherlandsCrit Rev Oncol Hematol200762217217817197191
- BattafaranoRJPiccirilloJFMeyersBFImpact of comorbidity on survival after surgical resection in patients with stage I non-small cell lung cancerJ Thorac Cardiovasc Surg2002123228028711828287
- ReadWLTierneyRMPageNCDifferential prognostic impact of comorbidityJ Clin Oncol200422153099310315284260
- PiccirilloJFTierneyRMCostasIGroveLSpitznagelELJrPrognostic importance of comorbidity in a hospital-based cancer registryJAMA2004291202441244715161894
- BerglundAWigertzAAdolfssonJImpact of comorbidity on management and mortality in women diagnosed with breast cancerBreast Cancer Res Treat2012135128128922829398
- RiihimäkiMThomsenHBrandtASundquistJHemminkiKDeath causes in breast cancer patientsAnn Oncol201223360461021586686
- KendalWSDying with cancer: the influence of age, comorbidity, and cancer siteCancer200811261354136218286532
- van de Poll-FranseLVHaakHRCoeberghJWJanssen-HeijnenMLLemmensVEDisease-specific mortality among stage I-III colorectal cancer patients with diabetes: a large population-based analysisDiabetologia20125582163217222526616
- Helweg-LarsenKThe Danish Register of Causes of DeathScand J Public Health201139Suppl 7262921775346
- GjersøePAndersenSEMølbakAGWulffHRThomsenOOReliability of death certificates. The reproducibility of the recorded causes of death in patients admitted to departments of internal medicineUgeskr Laeger19981603550305034 Danish9739603
- WexelmanBAEdenERoseKMSurvey of New York City resident physicians on cause-of-death reporting, 2010Prev Chronic Dis201310E7623660118
- GeraciJMEscalanteCPFreemanJLGoodwinJSComorbid disease and cancer: the need for more relevant conceptual models in health services researchJ Clin Oncol200523307399740416234509
- FloresMBRochaGZDamas-SouzaDMObesity-induced increase in tumor necrosis factor-α leads to development of colon cancer in miceGastroenterology2012143374175322677195
- ForteVPandeyAAbdelmessihRObesity, diabetes, the cardiorenal syndrome, and risk for cancerCardiorenal Med20122214316222851963
- TsuganeSInoueMInsulin resistance and cancer: epidemiological evidenceCancer Sci201010151073107920345478
- SainzJRudolphAHoffmeisterMEffect of type 2 diabetes predisposing genetic variants on colorectal cancer riskJ Clin Endocrinol Metab2012975E845E85122419714
- NascaPCImmunity and cancer riskNascaPCPastidesHFundamentals of Cancer EpidemiologySudbury, MAJones and Bartlett Publishers, Inc2008334358
- HemminkiKLiuXJiJSundquistJSundquistKEffect of autoimmune diseases on risk and survival in histology-specific lung cancerEur Respir J20124061489149522323571
- TakkoucheBRegueira-MéndezCEtminanMBreast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysisJ Natl Cancer Inst2008100201439144718840819
- KhuderSAHerialNAMutgiABFedermanDJNonsteroidal antiinflammatory drug use and lung cancer: a metaanalysisChest2005127374875415764753
- TerryMBGammonMDZhangFFAssociation of frequency and duration of aspirin use and hormone receptor status with breast cancer riskJAMA2004291202433244015161893
- BardiaAOlsonJEVachonCMEffect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort studyBreast Cancer Res Treat2011126114915520669045
- SassanoAPlataniasLCStatins in tumor suppressionCancer Lett20082601–2111918180097
- ThomsenRWFarkasDKFriisSEndocarditis and risk of cancer: a Danish nationwide cohort studyAm J Med20131261586723260503
- KwanMLHabelLASlatteryMLCaanBNSAIDs and breast cancer recurrence in a prospective cohort studyCancer Causes Control200718661362017404892
- BlairCKSweeneyCAndersonKEFolsomARNSAID use and survival after breast cancer diagnosis in post-menopausal womenBreast Cancer Res Treat2007101219119716823508
- HolmesMDChenWYLiLHertzmarkESpiegelmanDHankinsonSEAspirin intake and survival after breast cancerJ Clin Oncol20102891467147220159825
- ChanATOginoSFuchsCSAspirin use and survival after diagnosis of colorectal cancerJAMA2009302664965819671906
- ChlebowskiRTMcTiernanAWactawski-WendeJDiabetes, metformin, and breast cancer in postmenopausal womenJ Clin Oncol201230232844285222689798
- KaplanMAPekkolayZKucukonerMType 2 diabetes mellitus and prognosis in early stage breast cancer womenMed Oncol20122931576158022083554
- GonzalezECFerranteJMVan DurmeDJPalNRoetzheimRGComorbid illness and the early detection of cancerSouth Med J200194991392011592754
- ExtermannMInteraction between comorbidity and cancerCancer Control200714132217242667
- Wheatley-PricePBlackhallFThatcherNThe influence of sex in non-small cell lung cancerOnkologie2009321054754819816068
- ShugarmanLRMackKSorberoMERace and sex differences in the receipt of timely and appropriate lung cancer treatmentMed Care200947777478119536007
- Di MaioMSignorielloSMorabitoAPrognostic impact of education level of patients with advanced non-small cell lung cancer enrolled in clinical trialsLung Cancer201276345746422297086
- BerglundALambeMLüchtenborgMSocial differences in lung cancer management and survival in South East England: a cohort studyBMJ Open201223pii:e001048
- DaltonSOFrederiksenBLJacobsenESocioeconomic position, stage of lung cancer and time between referral and diagnosis in Denmark, 2001–2008Br J Cancer201110571042104821897390
- EgebergRHalkjaerJRottmannNHansenLHoltenISocial inequality and incidence of and survival from cancers of the colon and rectum in a population-based study in Denmark, 1994–2003Eur J Cancer200844141978198818667301
- AartsMJVoogdACDuijmLECoeberghJWLouwmanWJSocioeconomic inequalities in attending the mass screening for breast cancer in the south of The Netherlands – associations with stage at diagnosis and survivalBreast Cancer Res Treat2011128251752521290176
- SheppardAJChiarelliAMMarrettLDNishriEDTrudeauMEStage at diagnosis and comorbidity influence breast cancer survival in First Nations women in Ontario, CanadaCancer Epidemiol Biomarkers Prev201120102160216721803843
- HillSSarfatiDBlakelyTSurvival disparities in indigenous and non-indigenous New Zealanders with colon cancer: the role of patient comorbidity, treatment and health service factorsJ Epidemiol Community Health201064211712320056966
- LandLHDaltonSOJørgensenTLEwertzMComorbidity and survival after early breast cancer. A reviewCrit Rev Oncol Hematol201281219620521536452
- YasmeenSXingGMorrisCChlebowskiRTRomanoPSComorbidities and mammography use interact to explain racial/ethnic disparities in breast cancer stage at diagnosisCancer2011117143252326121246529
- FrederiksenBLOslerMHarlingHLadelundSJørgensenTDo patient characteristics, disease, or treatment explain social inequality in survival from colorectal cancer?Soc Sci Med20096971107111519695753
- TammemagiCMNerenzDNeslund-DudasCFeldkampCNathansonDComorbidity and survival disparities among black and white patients with breast cancerJAMA2005294141765177216219879
- DaltonSORossLDüringMInfluence of socioeconomic factors on survival after breast cancer – a nationwide cohort study of women diagnosed with breast cancer in Denmark, 1983–1999Int J Cancer2007121112524253117680561
- GuptaSKLamontEBPatterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementiaJ Am Geriatr Soc200452101681168715450045
- RajiMAKuoYFFreemanJLGoodwinJSEffect of a dementia diagnosis on survival of older patients after a diagnosis of breast, colon, or prostate cancer: implications for cancer careJAMA Intern Med20081681820332040
- VaethPASatarianoWAAlcohol consumption and breast cancer stage at diagnosisAlcohol Clin Exp Res19982249289349660324
- AllemaniCBerrinoFKroghVDo pre-diagnostic drinking habits influence breast cancer survival?Tumori201197214214821617706
- DesaiMMBruceMLKaslSVThe effects of major depression and phobia on stage at diagnosis of breast cancerInt J Psychiatry Med1999291294510376231
- FlemingSTPursleyHGNewmanBPavlovDChenKComorbidity as a predictor of stage of illness for patients with breast cancerMed Care200543213214015655426
- KiefeCIFunkhouserEFouadMNMayDSChronic disease as a barrier to breast and cervical cancer screeningJ Gen Intern Med19981363573659669564
- RichALTataLJFreeCMHow do patient and hospital features influence outcomes in small-cell lung cancer in England?Br J Cancer2011105674675221829191
- CykertSDilworth-AndersonPMonroeMHFactors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancerJAMA2010303232368237620551407
- KennedyGDRajamanickamVO’connorESOptimizing surgical care of colon cancer in the older adult populationAnn Surg2011253350851421169811
- RuethNMParsonsHMHabermannEBSurgical treatment of lung cancer: predicting postoperative morbidity in the elderly populationJ Thorac Cardiovasc Surg201214361314132322341420
- MorrisEJTaylorEFThomasJDThirty-day postoperative mortality after colorectal cancer surgery in EnglandGut201160680681321486939
- Van LeeuwenBLRosenkranzKMFengLLDepartment of Surgical Oncology, MD Anderson Cancer CenterThe effect of under-treatment of breast cancer in women 80 years of age and olderCrit Rev Oncol Hematol201179331532020655242
- van SteenbergenLNRuttenHJCreemersGJPruijtJFCoeberghJWLemmensVELarge age and hospital-dependent variation in administration of adjuvant chemotherapy for stage III colon cancer in southern NetherlandsAnn Oncol20102161273127819880434
- BradleyCJGivenCWDahmanBFitzgeraldTLAdjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancerJAMA Intern Med20081685521529
- LuoRGiordanoSHFreemanJLZhangDGoodwinJSReferral to medical oncology: a crucial step in the treatment of older patients with stage III colon cancerOncologist20061191025103317030645
- GrossCPGuoZMcAvayGJAlloreHGYoungMTinettiMEMultimorbidity and survival in older persons with colorectal cancerJ Am Geriatr Soc200654121898190417198496
- GraySWLandrumMBLamontEBMcNeilBJJaklitschMTKeatingNLImproved outcomes associated with higher surgery rates for older patients with early stage nonsmall cell lung cancerCancer201211851404141121800285
- PasettoLMFalciCBassoUAdjuvant treatment for elderly patients with colon cancer. An observational studyAnticancer Res2008284C2513251818751443
- DySMSharkeyPHerbertRHaddadKWuAWComorbid illnesses and health care utilization among Medicare beneficiaries with lung cancerCrit Rev Oncol Hematol200659321822516829124
- O’ConnorTLEdgeSBKossoffEBFactors affecting the delivery of adjuvant/neoadjuvant chemotherapy in older women with breast cancerJ Geriatr Oncol201234320328
- BoothCMShepherdFAPengYAdjuvant chemotherapy for non-small cell lung cancer: practice patterns and outcomes in the general population of Ontario, CanadaJ Thorac Oncol20127355956622307012
- HuCYDelclosGLChanWDuXLAssessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancerMed Oncol20112841062107420714945
- HershmanDLWangXMcBrideRJacobsonJSGrannVRNeugutAIDelay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survivalInt J Radiat Oncol Biol Phys20066551353136016765531
- GoldHTDoHTDickAWCorrelates and effect of suboptimal radiotherapy in women with ductal carcinoma in situ or early invasive breast cancerCancer2008113113108311518932243
- WingetMHossainSYasuiYScarfeACharacteristics of patients with stage III colon adenocarcinoma who fail to receive guideline-recommended treatmentCancer2010116204849485620578180
- PungliaRSSaitoAMNevilleBAEarleCCWeeksJCImpact of interval from breast conserving surgery to radiotherapy on local recurrence in older women with breast cancer: retrospective cohort analysisBMJ2010340c84520197326
- DavidoffAJTangMSealBEdelmanMJChemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancerJ Clin Oncol201028132191219720351329
- GrossCPMcAvayGJGuoZTinettiMEThe impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancerCancer2007109122410241917510973
- EarleCCNeumannPJGelberRDWeinsteinMCWeeksJCImpact of referral patterns on the use of chemotherapy for lung cancerJ Clin Oncol20022071786179211919235
- BirkmeyerNJGoodneyPPStukelTAHillnerBEBirkmeyerJDDo cancer centers designated by the National Cancer Institute have better surgical outcomes?Cancer2005103343544115622523
- OnegaTDuellEJShiXDemidenkoEGottliebDGoodmanDCInfluence of NCI cancer center attendance on mortality in lung, breast, colorectal, and prostate cancer patientsMed Care Res Rev200966554256019454624
- CraftPSBuckinghamJMDahlstromJEVariation in the management of early breast cancer in rural and metropolitan centres: implications for the organisation of rural cancer servicesBreast201019539640120452216
- SchonbergMAMarcantonioERNgoLSillimanRAMcCarthyEPDoes life expectancy affect treatment of women aged 80 and older with early stage breast cancers?J Geriatr Oncol20123181622368726
- GentilJDabakuyoTSOuedraogoSPoillotMLDejardinOArveuxPFor patients with breast cancer, geographic and social disparities are independent determinants of access to specialized surgeons. A eleven-year population-based multilevel analysisBMC Cancer201212135122889420
- BlaisSDejardinOBoutreuxSLaunoyGSocial determinants of access to reference care centres for patients with colorectal cancer – a multilevel analysisEur J Cancer200642173041304817029939
- KrzyzanowskaMKReganMMPowellMEarleCCWeeksJCImpact of patient age and comorbidity on surgeon versus oncologist preferences for adjuvant chemotherapy for stage III colon cancerJ Am Coll Surg2009208220220919228531
- O’GradyMASlaterESigurdsonERAssessing compliance with national comprehensive cancer network guidelines for elderly patients with stage III colon cancer: the Fox Chase Cancer Center Partners’ initiativeClin Colorectal Cancer201110211311621859563
- VinodSKSidhomMAGabrielGSLeeMTDelaneyGPWhy do some lung cancer patients receive no anticancer treatment?J Thorac Oncol2010571025103220453689
- KeatingNLLandrumMBKlabundeCNAdjuvant chemotherapy for stage III colon cancer: do physicians agree about the importance of patient age and comorbidity?J Clin Oncol200826152532253718487570
- HamakerMESchreursWHUppelschotenJMSmorenburgCHBreast cancer in the elderly: retrospective study on diagnosis and treatment according to national guidelinesBreast J2009151263319141131
- FieldTSDoubeniCFoxMPUnder utilization of surveillance mammography among older breast cancer survivorsJ Gen Intern Med200823215816318060463
- van GilsCWKoopmanMMolLRedekopWKUyl-de GrootCAPuntCJAdjuvant chemotherapy in stage III colon cancer: guideline implementation, patterns of use and outcomes in daily practice in The NetherlandsActa Oncol2012511576422122695
- GironésRTorregrosaDGómez-CodinaJMaestuITeniasJMRosellRLung cancer chemotherapy decisions in older patients: the role of patient preference and interactions with physiciansClin Transl Oncol201214318318922374421
- Van RynMBurkeJThe effect of patient race and socio-economic status on physicians’ perceptions of patientsSoc Sci Med200050681382810695979
- Cavalli-BjörkmanNGlimeliusBStrangPEqual cancer treatment regardless of education level and family support? A qualitative study of oncologists’ decision-makingBMJ Open201224pii:e001248
- SiminoffLAGrahamGCGordonNHCancer communication patterns and the influence of patient characteristics: disparities in information-giving and affective behaviorsPatient Educ Couns200662335536016860520
- SchonbergMASillimanRAMcCarthyEPMarcantonioERFactors noted to affect breast cancer treatment decisions of women aged 80 and olderJ Am Geriatr Soc201260353854422283600
- MandelblattJSSheppardVBHurriaACancer Leukemia Group BBreast cancer adjuvant chemotherapy decisions in older women: the role of patient preference and interactions with physiciansJ Clin Oncol201028193146315320516438
- NeugutAIMatasarMWangXDuration of adjuvant chemotherapy for colon cancer and survival among the elderlyJ Clin Oncol200624152368237516618946
- BarronTIConnollyRBennettKFeelyJKennedyMJEarly discontinuation of tamoxifen: a lesson for oncologistsCancer2007109583283917243168
- McCowanCShearerJDonnanPTCohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancerBr J Cancer200899111763176818985046
- van Herk-SukelMPvan de Poll-FranseLVVoogdACNieuwenhuijzenGACoeberghJWHeringsRMHalf of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysisBreast Cancer Res Treat2010122384385120058066
- HershmanDLKushiLHShaoTEarly discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patientsJ Clin Oncol201028274120412820585090
- BickellNALeParFWangJJLeventhalHLost opportunities: physicians’ reasons and disparities in breast cancer treatmentJ Clin Oncol200725182516252117577028
- HadjiPZillerVKyvernitakisJPersistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysisBreast Cancer Res Treat2013138118519123334803
- KoroukianSMXuFBakakiPMDiaz-InsuaMToweTPOwusuCComorbidities, functional limitations, and geriatric syndromes in relation to treatment and survival patterns among elders with colorectal cancerJ Gerontol A Biol Sci Med Sci201065332232920018824
- NewschafferCJBushTLPenberthyLEBellantoniMHelzlsourKDiener-WestMDoes comorbid disease interact with cancer? An epidemiologic analysis of mortality in a cohort of elderly breast cancer patientsJ Gerontol A Biol Sci Med Sci1998535M372M3789754143
- LemmensVEJanssen-HeijnenMLHoutermanSWhich comorbid conditions predict complications after surgery for colorectal cancer?World J Surg200731119219917180570
- YoodMUOwusuCBuistDSMortality impact of less-than-standard therapy in older breast cancer patientsJ Am Coll Surg20082061667518155570
- GiordanoSHDuanZKuoYFHortobagyiGNGoodwinJSUse and outcomes of adjuvant chemotherapy in older women with breast cancerJ Clin Oncol200624182750275616782915
- BuistDSIchikawaLProutMNReceipt of appropriate primary breast cancer therapy and adjuvant therapy are not associated with obesity in older women with access to health careJ Clin Oncol200725233428343617687148
- McCarthyEPNgoLHRoetzheimRGDisparities in breast cancer treatment and survival for women with disabilitiesAnn Intern Med2006145963764517088576
- WangSWongMLHamiltonNDavorenJBJahanTMWalterLCImpact of age and comorbidity on non-small-cell lung cancer treatment in older veteransJ Clin Oncol201230131447145522454424
- RichALTataLJFreeCMInequalities in outcomes for non-small cell lung cancer: the influence of clinical characteristics and features of the local lung cancer serviceThorax201166121078108421785158
- LinnBSLinnMWGurelLCumulative illness rating scaleJ Am Geriatr Soc19681656226265646906
- HuangCWSunLCShihYLThe impact on clinical outcome of high prevalence of diabetes mellitus in Taiwanese patients with colorectal cancerWorld J Surg Oncol2012107622553992
- NagelJMBückerSWoodMLess advanced stages of colon cancer in patients with type 2 diabetes mellitus: an unexpected finding?Exp Clin Endocrinol Diabetes2012120422422822231920
- PaganoEFilippiniCDi CuonzoDFactors affecting pattern of care and survival in a population-based cohort of non-small-cell lung cancer incident casesCancer Epidemiol201034448348920444663
- KlabundeCNLeglerJMWarrenJLBaldwinLMSchragDA refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patientsAnnals of epidemiology20071785849017531502