Abstract
The Danish Collaborative Bacteraemia Network (DACOBAN) research database includes microbiological data obtained from positive blood cultures from a geographically and demographically well-defined population serviced by three clinical microbiology departments (1.7 million residents, 32% of the Danish population). The database also includes data on comorbidity from the Danish National Patient Registry, vital status from the Danish Civil Registration System, and clinical data on 31% of nonselected records in the database. Use of the unique civil registration number given to all Danish residents enables linkage to additional registries for specific research projects. The DACOBAN database is continuously updated, and it currently comprises 39,292 patients with 49,951 bacteremic episodes from 2000 through 2011. The database is part of an international network of population-based bacteremia registries from five developed countries on three continents. The main purpose of the DACOBAN database is to study surveillance, risk, and prognosis. Sex- and age-specific data on background populations enables the computation of incidence rates. In addition, the high number of patients facilitates studies of rare microorganisms. Thus far, studies on Staphylococcus aureus, enterococci, computer algorithms for the classification of bacteremic episodes, and prognosis and risk in relation to socioeconomic factors have been published.
Introduction
Bacteremia is a serious condition, with a 30-day mortality of 15%–30% and ranked among the top-seven causes of death in developed countries.Citation1 The term “bacteremia” also covers fungemia, and is defined as the presence of bacteria or fungi in the bloodstream associated with clinical symptoms, distinguishing it from blood contamination.Citation2,Citation3 In practical terms, bacteremia is based on the detection of bacteria or fungi in blood cultures (BCs) ordered on suspicion of a possible bloodstream infection in an ill patient. The distinction between bacteremia and blood contamination requires due consideration. However, the most effective means of capturing all data is the electronic recording of bacteria and fungi in BCs, as performed by clinical microbiological laboratories.Citation2
The Danish Collaborative Bacteraemia Network (DACOBAN) database is a research database compiled from administrative data. The main purpose of the database is to provide Danish population-based bacteremia data for studies related to surveillance, risk, and prognosis. DACOBAN covers 32% of the Danish population, and is part of a multinational bacteremia collaborative network that incorporates bacteremic data from all of Finland, as well as geographically and demographically well-defined regions in Canada, Australia, and Sweden.Citation4,Citation5
Compilation of the study database
Linkage between data sources
All Danish residents have a unique personal identification number (the civil registration number) used in all administrative registries, which enables their linkage.Citation6 Date of birth and sex are incorporated into this number.
Core data set
The core data set consists of microbiological data on all positive BCs from the clinical microbiology departments at the three hospitals in Aalborg, Herlev, and Hvidovre (), which use the same laboratory information system (ADBakt; Autonik, Sköldinge, Sweden). The data are derived from patients with a civil registration number (ie, Danish residents), which comprise 99.5% of all positive BCs. BCs are rarely ordered by physicians outside the hospital setting, but if they are, they are normally collected in the outpatient area at the nearest hospital and are thereby captured by the DACOBAN database. All departments use automated BC systems (Bactec™ [BD, Franklin Lakes, NJ, USA] or BacT/Alert® [bioMérieux, Marcy l’Etoil, France]) and conventional identification methods,Citation7 possibly supplemented with automatic methods, such as Vitek® 2 (bioMérieux) or MALDI-TOF (Bruker, Bremen, Germany).
Table 1 Hospital and population data
Linkage between the core data set and other registries
The Danish National Patient Registry (DNPR) includes all hospital diagnoses and surgical procedures from 1977 onward.Citation8 The DNPR comprises inpatients from the whole period, and outpatients and emergency visits since 1995. As all data are received electronically from the hospitals’ patient administrative systems, the DNPR is updated shortly after discharge of the patient. For patients in the DACOBAN database, we have access to their hospital history since 1977, including information on number and length of admissions, minor and major surgical procedures, and specific disease entities.
The Danish Civil Registration System, which was implemented in 1968, is updated daily.Citation6 This registry contains information on the patients’ vital status, including date of death, disappearance, or emigration if relevant. In addition to linkage to other health registries, physicians at the three clinical microbiology departments prospectively record additional clinical variables for the DACOBAN, as outlined in the following sections.
Survey frequency
The DACOBAN database receives core data when physicians at the three clinical microbiology departments validate the laboratory data. Currently, the DACOBAN database includes all of 2011, and future updates are expected for full years 6–12 months after their expiry, from the laboratories, the DNPR, and the Danish Civil Registration System. The latest date of the patients’ vital status derived from the Danish Civil Registration SystemCitation6 is September 27, 2012.
Data-resource area and population coverage
The Danish tax-funded welfare system provides free access to health care by general practitioners and at public hospitals. Only 1% of hospital beds are in the private sector, and these are used solely for elective admissions. The public health service has a regional structure, which is subject to change, with the most recent change occurring in 2007. Until 2007, Denmark was divided into 14 counties, which were reorganized into five regions. This reorganization had very little impact on the background population of the DACOBAN database, and almost none for the present Capital Region, but it led to a 17% increase in the population when North Jutland County was reorganized into the North Denmark Region.Citation9 Population data can be obtained from Statistics Denmark (http://www.statbank.dk, in English) for the calculation of incidence data.
The admission of all acutely ill patients to the nearest public hospital in their region of residence and the submission of all of the hospitals’ BCs to the three clinical microbiology departments prompts population-based coverage of the DACOBAN database.Citation10 However, the main Danish referral hospital (Rigshospitalet), which is situated in Copenhagen, has bacteremia episodes that are not captured by the DACOBAN database. We estimate that approximately 16% of bacteremic episodes among residents in the Capital Region occur at the Rigshospitalet, with a preponderance of nosocomial episodes among 0- to 40-year-old patients occurring in highly specialized wards (such as oncology, hematology, or nephrology) in this hospital (unpublished data). We plan to include microbiological data from Rigshospitalet in the DACOBAN database after taking its different laboratory information system (MADS [http://www.madsonline.dk]) into account.
Therefore, the DACOBAN database covers all positive BCs from the North Denmark Region and approximately 84% of positive BCs from the Capital Region; given the distribution of BCs between these two regions in the DACOBAN database (data not shown), coverage is estimated to be 88%. These two geographically and demographically well-defined administrative regions have a background population of approximately 1.7 million (32% of Denmark’s population, ).Citation5 Currently, the data cover the 12-year period from January 1, 2000 through December 31, 2011.
In future, DACOBAN data will include positive BCs from additional municipalities in the Capital Region (background population approximately 0.3 million), which were incorporated into the Herlev catchment area in May 2013.
Measures
Analytical units
A BC set comprises a number of BC bottles drawn simultaneously from the patient (), and is the unit used in the literature to derive bacteremic episodes.Citation11 We cannot derive BC sets from the DACOBAN database,Citation12 because each clinical microbiology department uses its own laboratory-specimen numbering. Therefore, we have to use dates instead of BC sets, which is a reasonable assumption because BC bottles drawn on the same date presumably represent the same bacteremic episode. Using dates, we apply commonly used algorithms for contamination episodes based on the likelihood of a given microbial species to originate from the skin flora versus the bloodstream,Citation11 characterizing the remaining BCs as representing true bacteremia. Therefore, we compute bacteremic episodes on the basis of consecutive dates of positive BCs.Citation3,Citation12
Patients and bacteremic episodes
Currently, the DACOBAN database includes 39,292 patients with 49,951 bacteremic episodes. The age-related incidences () and other main characteristics () do not differ materially from bacteremic patients in other population-based studies.Citation13,Citation14
Figure 1 Age-related bacteremic episodes per 10,000 person years among 49,951 bacteremic episodes, 2000–2011. Each column covers 1 year. Age 101–106 years (n=21) merged.
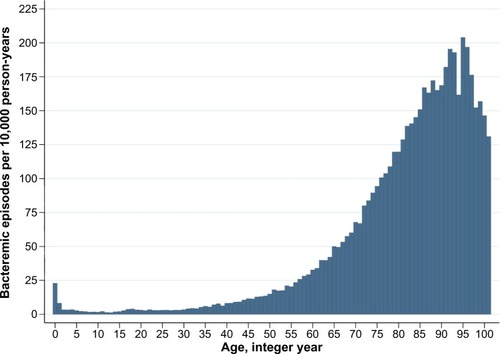
Table 2 Main characteristics of the 49,951 bacteremic episodes, 2000–2011
Main categories and variables
We divided the DACOBAN database into variables related to basic characteristics, microbiology, comorbidity, vital status, and physicians’ supplementary variables ().
Table 3 Main variables
Basic characteristics
The best-estimate date represented the baseline date of the bacteremic episode. The start of the bacteremic episode was defined as the date of drawing the BC. However, the date of the draw was missing in 4,228 bacteremic episodes (8.5%). We substituted the date of the draw with the date of BC receipt in clinical microbiology departments for these episodes. For the remaining 45,723 bacteremic episodes, 98.4% had ≤1 day between the draw date and the receipt date. From 2013, the electronic requisition of all BCs in the Capital Region precluded missing draw dates. Bacteremic episodes and the acquisition of bacteremia were computer-derivedCitation12 on the basis of generally accepted criteria.Citation11,Citation15,Citation16
Microbiological data
Detection systems (eg, different media or numbers of bottles) differ between clinical microbiology departments () and over time within the same department.Citation17 However, the main caveat relates to data on antibiotic resistance. The three departments have not coordinated on procedures, minimum inhibitory concentration values, or standard panels, yet all three departments participate in the same external quality-assessment schemes, primarily the UK National External Quality Assessment Service (http://www.ukneqasmicro.org.uk). Work is ongoing to evaluate past methods for antibiotic resistance that apply to all departments, but before this is implemented, generations of these data may differ between the departments, and caution in their interpretation is warranted.
Comorbidity
Comorbidity was assessed on the basis of the Charlson Comorbidity Index, in which 19 major disease categories (eg, malignancy, cardiovascular diseases, and diabetes mellitus) were assigned a score, with higher scores given to more severe diseases.Citation18,Citation19 We used the first-time diagnosis of a Charlson disease between 1977 (the year of implementation of the DNPR) and the best-estimate date. We had access to all DNPR data for DACOBAN patients, which enables individual linkage for studies comprising other comorbidities than those found in the Charlson Comorbidity Index, as well as surgical procedures.
Vital status
We had complete follow-up information for the vital status of all 39,292 patients except one. On the latest status date (September 27, 2012), 14,375 (36.6%) patients were alive, eleven (0.03%) had disappeared, 192 (0.49%) had emigrated, and the remaining 24,713 (62.9%) were deceased.
Physicians’ supplementary variables
For part of the data (Aalborg, 2007–2008; Herlev, 2006–2011; Hvidovre, 2006–2008), covering 8,645 patients with 9,672 bacteremic episodes (19.4%), physicians at the clinical microbiology departments prospectively recorded clinical data in an electronic database that is linked to the DACOBAN database. This enabled the retrieval of clinical data, such as possible focus of infection, empiric and final antibiotic treatment, and intravascular catheterization. These data represent routine assessments based on clinical data and information from positive microbiological specimens other than BCs that are generated by physicians at clinical microbiology departments in close cooperation with physicians at the clinical wards in which the patients were hospitalized. Variables related to antibiotic treatment were incorporated into an algorithm that determined whether the treatment was appropriate.
Research from the database
The population-based data and the high number of patients and bacteremic episodes make the DACOBAN database suitable for surveillance studies.Citation5,Citation7 DACOBAN data were recently included in a multinational surveillance study conducted by Laupland et al.Citation5 The study included an unprecedentedly high number (ie, 18,430) of incident Staphylococcus aureus bacteremic episodes. Incidence rates with detailed information on regional differences were reported separately for community-acquired and nosocomial bacteremias and methicillin-resistant and methicillin-susceptible episodes from developed countries on three continents during a 9-year period. Another cohort study of enterococci based on data from DACOBAN revealed some clinically relevant findings on infective endocarditis in 25% of adult patients with Enterococcus faecalis bacteremia.Citation7 We also found that only 17.7% of patients with E. faecalis and 7.4% with E. faecium bacteremia received appropriate antimicrobial therapy within the first day after admission.
In a study using the physicians’ supplementary variables as the gold standard, we assessed the validity of computer-derived algorithms for contamination versus bacteremic episodes, and among episodes of bacteremia, their acquisition (ie, community, health care-related, or nosocomial) and whether they were monomicrobial or polymicrobial.Citation12 The results showed high validity for contamination versus bacteremia and for monomicrobial versus polymicrobial bacteremia, but they were less reliable for acquisition.
Koch et al used the unique civil registration number to link DACOBAN data to socioeconomic variables maintained by Statistics Denmark, generating the first bacteremia study showing that low income, low level of education, and low social status were all strong predictors of higher 30-day mortality.Citation20 The same authors matched 4,117 community-acquired bacteremia patients by sex, age, and residency to 41,170 population controls to assess socioeconomic status as a risk factor for bacteremia.Citation21 Low education and low income rendered up to a 70% increased risk of bacteremia, even after adjusting for preexisting chronic diseases and drug and alcohol abuse.
Part of the DACOBAN data overlap the North Denmark Bacteremia Research Database, which is a well-established research database with prospectively derived data (eg, focus on infection and antibiotic treatment) that has been the source of roughly 60 publications.Citation9 Linkage to this database enables studies that supply clinical data and further validate the retrospective data of the DACOBAN database.
Strengths and weaknesses
The main strengths of the DACOBAN database are its catchment area of well-defined geographic regions with valid population statistics, the high number of patients, the ability to distinguish between incident and nonincident bacteremic episodes due to the patients’ longitudinal data, the possibility of complete follow-up for both short-term and long-term mortality, and the flexibility of linking to other administrative registries or research databases. Because patients are recruited from a well-defined geographic/demographic region, the DACOBAN database fulfills one prerequisite for participating in the multinational bacteremia collaborative network. The estimated coverage of 88% almost fulfills the other prerequisite stating that ≥90% of positive BCs have to be identified. In general, we think that the population-based principles are fulfilled, but unpublished data indicate that for the lower catchment in the Capital Region, caution is warranted in interpreting nosocomial bacteremic episodes among 0- to 40-year-old patients from this region. Such episodes comprised 2,989 of the 49,951 DACOBAN database episodes (6%).
The high number of patients enables precise statistical measures, and is advantageous for the study of rare microorganisms, which are often difficult to find in sufficient numbers among data from single clinical wards. Thus far, the DACOBAN database covers 12 years, an appropriate time span for studying longitudinal aspects, such as the recurrence of bacteremic episodesCitation22 or the incidence of microorganisms related to changes in laboratory procedures.Citation17
Most prognostic bacteremia studies report only in-hospital mortality, mainly because mortality after discharge is difficult to obtain in most non-Scandinavian countries. In-hospital mortality is heavily influenced by the length of admission. The DACOBAN database enables time-derived follow-up periods, such as 30-day or 365-day mortality. The Danish Civil Registration System is virtually 100% accurate, which also applies to the date of death.Citation6
The civil registration number facilitates linkage to other registries, including the retrieval of matched controls from the background population using incidence-density sampling.Citation23 It also enables the retrieval of supplementary data from clinical microbiology departments, such as information on negative BCsCitation24 or other specimen types. Further examples include socioeconomic data as described earlier, redemptions of prescribed drugs from pharmacies,Citation25 and the more than 50 nationwide clinical databases that cover specific disease entities, interventions, or activities.Citation26
The lack of clinical and paraclinical data related to the acute illness encountered in bacteremia patients constitutes the main limitation of the DACOBAN database. Clinical data, apart from discharge diagnoses found in the DNPR, are not recorded electronically and are very labor-intensive to obtain. Paraclinical data, however, are recorded electronically in all hospitals. The recent compilation of all paraclini-cal data into an accessible nationwide electronic registry by actors outside of the DACOBAN group offers new unique opportunities, in contrast to earlier studies in which linking data to paraclinical data from the individual hospital was the only option.Citation27,Citation28
The clinical data in the DACOBAN database mainly relate to chronic diseases exemplified by the Charlson Comorbidity Index, which is commonly used and validated for prognostic purposes.Citation19 The DNPR is less accurate than the Danish Civil Registration System, and has undergone changes of which the researcher should be aware.Citation8 However, the computation of previous comorbid conditions as a summary adjustment measure in prognostic models is sufficient.
The physicians’ supplementary variables described earlier yield information on important clinical aspects related to the bacteremic episode, such as the possible focus of infection, the appropriateness of the antibiotic treatment, and whether the patient was intravascularly catheterized. However, care is warranted in interpreting these data, as they represent numerous physicians’ routine assessments, with no evaluation of interobserver agreement or the use of formally specified criteria.Citation12 Despite the fact that the supplementary variables have been included for only a limited period of time, the source data are recorded continuously as an integral part of the physicians’ documentation of contacts with the patients’ clinical teams. With more effective methods for handling data on the patient’s clinical state, the possible foci of infection, and clinical decision making, such data could become a valuable part of the DACOBAN database in the future.
Another limitation relates to the possible inaccuracy in determining bacteremic episodes from an array of positive BCs.Citation22,Citation29 We partly evaluated the validity of the computer algorithms used for this purpose,Citation12 but more work is required to assess its robustness, such as in prognostic models.
Data-resource access
Interested collaborators are encouraged to mail the corresponding author, who will forward the request to the DACOBAN steering committee (HCS, MA, JDK, and CØ). Linkage to other registries has to be performed by the DACOBAN group, as the civil registration number will be encrypted prior to data delivery, in accordance with Danish legislation. The DACOBAN database was approved according to the guidelines of the Regional Committee on Health Research Ethics for use of clinical and laboratory data (Danish Data Protection Agency, record 2007-41-0627). Additional permissions from the Danish Data Protection Agency will be required for external collaborators.
Acknowledgments
Contributing members of DACOBAN: Christian Østergaard (Department of Clinical Microbiology, Hvidovre Hospital, Copenhagen University Hospital, Copenhagen, Denmark), Magnus Arpi (Department of Clinical Microbiology, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark), Kim Oren Gradel (Center for National Clinical Databases – South, Odense University Hospital, Odense, Denmark), Ulrich Stab Jensen (Department of Clinical Microbiology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark), Sara Thønnings (Department of Clinical Microbiology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark), Jenny Dahl Knudsen (Department of Clinical Microbiology, Hvidovre Hospital, Copenhagen University Hospital, Copenhagen, Denmark), Kristoffer Koch (Department of Clinical Microbiology, Aalborg University Hospital, Aalborg, Denmark), Mette Pinholt (Department of Clinical Microbiology, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark), Jesper Smit (Department of Clinical Microbiology, Aalborg University Hospital, Aalborg, Denmark), Henrik Carl Schønheyder (Department of Clinical Microbiology, Aalborg University Hospital, Aalborg, Denmark), Mette Søgaard (Department of Clinical Epidemiology, Institute of Clinical Medicine, Aarhus University Hospital, Aarhus University, Aarhus, Denmark).
Disclosure
The authors report no conflicts of interest in this work.
References
- GotoMAl-HasanMNOverall burden of bloodstream infection and nosocomial bloodstream infection in North America and EuropeClin Microbiol Infect20131950150923473333
- SchønheyderHCPaulMPlacing the burden of bacteraemia in perspectiveClin Microbiol Infect20131948949123607298
- WeinsteinMPRellerLBMurphyJRLichtensteinKAThe clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observationsRev Infect Dis1983535536828811
- LauplandKBSchønheyderHCKennedyKJRationale for and protocol of a multi-national population-based bacteremia surveillance collaborativeBMC Res Notes2009214619624839
- LauplandKBLyytikainenOSøgaardMThe changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance studyClin Microbiol Infect20131946547122616816
- PedersenCBThe Danish Civil Registration SystemScand J Public Health201139222521775345
- PinholtMØstergaardCArpiMIncidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006–2009: a population-based cohort studyClin Microbiol Infect20142014515123647880
- LyngeESandegaardJLReboljMThe Danish National Patient RegisterScand J Public Health201139303321775347
- SchønheyderHCSøgaardMExisting data sources for clinical epidemiology: the North Denmark Bacteremia Research DatabaseClin Epidemiol2010217117820865114
- OlsenJBassoOSørensenHTWhat is a population-based registry?Scand J Public Health1999277810847676
- TrickWEZagorskiBMTokarsJIComputer algorithms to detect bloodstream infectionsEmerg Infect Dis2004101612162015498164
- GradelKOKnudsenJDArpiMØstergaardCSchønheyderHCSøgaardMClassification of positive blood cultures: computer algorithms versus physicians’ assessment – development of tools for surveillance of bloodstream infection prognosis using population-based laboratory databasesBMC Med Res Methodol20121213922970812
- UslanDZCraneSJSteckelbergJMAge- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, MinnesotaArch Int Med200716783483917452548
- SkogbergKLyytikäinenOOllgrenJNuortiJPRuutuPPopulation-based burden of bloodstream infections in FinlandClin Microbiol Infect201218E170E17622512663
- GarnerJSJarvisWREmoriTGHoranTCHughesJMCDC definitions for nosocomial infections, 1988Am J Infect Control1988161281402841893
- FriedmanNDKayeKSStoutJEHealth care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infectionsAnn Int Med200213779179712435215
- SøgaardMEngebjergMCLundbye-ChristensenSSchønheyderHCChanges in blood culture methodology have an impact on time trends of bacteraemia: a 26-year regional studyEpidemiol Infect201113977277620619078
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis1987403733833558716
- de GrootVBeckermanHLankhorstGJBouterLMHow to measure comorbidity. a critical review of available methodsJ Clin Epidemiol20035622122912725876
- KochKNørgaardMSchønheyderHCThomsenRWSøgaardMEffects of socioeconomic status on mortality after bacteremia in working-age patients. A Danish population-based cohort studyPLoS One20138e7008223936145
- KochKSøgaardMNørgaardMThomsenRWSchønheyderHCSocioeconomic inequalities in risk of hospitalization with community-acquired bacteremia: a Danish population-based case-control studyAm J Epidemiol201417991096110624682527
- JensenUSKnudsenJDØstergaardCGradelKOFrimodt-MøllerNSchønheyderHCRecurrent bacteraemia: a 10-year regional population-based study of clinical and microbiological risk factorsJ Infect20106019119920026352
- WacholderSSilvermanDTMcLaughlinJKMandelJSSelection of controls in case-control studies. III. Design optionsAm J Epidemiol1992135104210501595690
- SøgaardMNørgaardMPedersenLSørensenHTSchønheyderHCBlood culture status and mortality among patients with suspected community-acquired bacteremia: a population-based cohort studyBMC Infect Dis20111113921599971
- KildemoesHWSørensenHTHallasJThe Danish National Prescription RegistryScand J Public Health201139384121775349
- GreenADanish clinical databases: an overviewScand J Public Health201139687121775356
- GradelKOThomsenRWLundbye-ChristensenSNielsenHSchønheyderHCBaseline C-reactive protein level as a predictor of mortality in bacteraemia patients: a population-based cohort studyClin Microbiol Infect20101762763220545964
- GrannAFErichsenRNielsenAGFroslevTThomsenRWExisting data sources for clinical epidemiology: the clinical laboratory information system (LABKA) research database at Aarhus University, DenmarkClin Epidemiol2011313313821487452
- RobertsFJDefinition of polymicrobial bacteremiaRev Infect Dis198911102910302602769
