 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Although asthma has recently been established as a risk factor for pneumococcal disease (PD), few studies have specifically evaluated this association in children.
Methods
We conducted a nation-wide population-based cohort study of the effect of asthma on childhood PD among all singleton live births in Denmark from 1994 to 2007, before the introduction of the 7-valent pneumococcal conjugate vaccine. All data were abstracted from Danish medical registries. Because underlying comorbidity substantially increases the PD risk in children, standard methods were used to assess the evidence of biologic interaction between comorbidity and asthma on the risk of PD.
Results
There were 2,253 cases of childhood PD among 888,655 children born in Denmark from 1994 to 2007. The adjusted incidence rate ratio of the effect of asthma on childhood PD was 2.2 (95% confidence interval [CI]: 2.0, 2.5). Age-stratified incidence rate ratios were 2.1 (95% CI: 1.8, 2.9) in children 6 months to <24 months, 4.1 (95% CI: 3.3, 5.1) in children 24 months to <60 months, and 2.3 (95% CI: 1.6, 3.2) in children ≥60 months. Evaluation of the biologic interaction between asthma and comorbidity in older children revealed that 55% (24 months to <60 months) to 73% (≥60 months) of cases among asthma-exposed children can be accounted for by the interaction between asthma and comorbidity.
Conclusion
These results confirm that asthma is an important risk factor for PD in children and suggest that children with underlying comorbidities are more sensitive to the effect of asthma on PD than children without comorbidities.
Introduction
Streptococcus pneumoniae is a leading cause of serious illness worldwide. Pneumococcal infections are especially dangerous for children and adults with immunodeficiencies, such as HIV, or with illnesses such as chronic cardiac disease and diabetes.Citation1 Pneumococcal vaccination has therefore been recommended for persons at high risk of severe illness or complications.
Asthma was first identified as an independent risk factor for pneumococcal disease (PD) in 2005 when Talbot et alCitation2 reported that asthma was associated with a 2.4-fold increased odds of PD among persons 2–49 years old enrolled in Tennessee’s Medicaid Program between 1994 and 2002. Several studies conducted since then have provided additional evidence that asthma is associated with an increased risk of PD in adults,Citation3–Citation5 but no study has provided convincing evidence that asthma is associated with an increased risk of PD in children.Citation6
Currently, pneumococcal vaccination is recommended for asthmatic adults, but it is not specifically recommended for asthmatic children. Asthma is the most common chronic disease in children,Citation7 and the prevalence of asthma is increasing worldwide.Citation8–Citation12 As the prevalence of asthma increases, so does the importance of understanding whether children with asthma are at increased risk of PD. The aim of the current study was to evaluate the association between asthma and the development of PD among Danish children born between 1994 and 2007.
Methods
Study population
The study population included all singleton live births in Denmark from January 1, 1994 through December 31, 2007. Routine childhood pneumococcal vaccination with 7-valent pneumococcal conjugate vaccine (PCV7) did not begin in Denmark until October 2007,Citation13 so few children in the study population were expected to be vaccinated. We used the unique Civil Personal Registry number assigned to all Danish citizens at birth and to residents upon immigration, which has been used in public records since 1968, to identify all live births from the Danish Civil Registration System. This continually updated national registration system includes information about date and place of birth, immigration, sex, marital status, citizenship, emigration, and vital status.Citation14 All study children were followed from birth until the diagnosis of PD, removal from the Danish Civil Registration System due to any cause, or December 31, 2007, whichever came first. The Danish Data Protection Agency provided permission to use these data (record number: 1-16-02-1-08). Because this study was based on data extracted from registries, it was exempt from human subjects review, and members of the study population did not have to provide informed consent.
Data collection
To ascertain PD, we used each subject’s unique Civil Personal Registry number to link their Danish Civil Registration System data to the Danish National Registry of Patients. The Danish National Registry of Patients began in 1977 and includes inpatient diagnoses made at nonpsychiatric hospitals and, beginning in 1995, diagnoses made at outpatient specialist clinics and emergency room visits.Citation15 We used the Danish version of International Classification of Diseases (ICD)-10 codes G00.1, A40.3, and J13.9 to ascertain pneumococcal meningitis, pneumococcal septicemia, and pneumococcal pneumonia, respectively. Diagnosis of pneumococcal pneumonia using these ICD codes was validated in a prior study in which 64% of ICD-10-identified cases had microbiologic, radiologic, and clinical evidence consistent with PD, and the remaining 36% were classified as having probable PD.Citation16
We used ICD-10 codes (J45 and J46) recorded in the Danish National Registry of Patients to ascertain asthma. Registry-based asthma diagnoses have been previously validated in Denmark and determined to be of high quality.Citation17,Citation18 We classified study subjects as having asthma if they had at least one diagnosis code indicating asthma hospitalization, emergency, or outpatient visits at any time before the diagnosis of PD, or before the end of follow-up for children who did not develop PD.
Information obtained from the Danish Medical Birth Registry included place of birth, gestational age, fetal presentation, mode of delivery, birth weight, 5-minute Apgar score, maternal place of birth, maternal age at the time of delivery, maternal cohabitation status, and maternal parity.Citation19 Maternal smoking status during pregnancy was based on self-report during the first antenatal visit and was classified as yes or no, and presence or absence of congenital malformations or selected underlying comorbidities was ascertained using ICD-10 codes recorded in the Danish National Registry of Patients ().
Statistical analyses
Children accumulated unexposed person-time from birth until an asthma diagnosis, if any, and exposed person-time thereafter. We calculated the frequency and proportion of children with and without asthma within categories of demographic variables and birth outcomes, as well as age-specific incidence rates of PD.
We used Poisson regression to estimate crude and adjusted incidence rate ratios (IRRs) and 95% confidence intervals (CIs) associating childhood asthma and PD. We included covariates that changed the crude association between asthma and PD by more than 10% in adjusted models; child’s sex was retained in the adjusted models regardless of its impact on the unadjusted measures.
Because underlying comorbidity is known to substantially increase the risk of PD in children,Citation20 and because it was strongly associated with childhood asthma exposure in our study population, we evaluated the impact of comorbidity on the association between asthma and PD in stratified analyses. The impact of congenital malformations was also assessed. For these analyses, we used Poisson regression to calculate crude and adjusted IRRs and 95% CIs stratified by the presence or absence of congenital malformations or selected underlying comorbidities. We further evaluated any observed effect measure modification for evidence of biologic interaction using standard measures (Supplementary materials).Citation21,Citation22
We conducted all statistical analyses using SAS/STAT® software, version 9.2 (SAS Institute Inc., Cary, NC, USA).Citation23
Results
Characteristics of the study population
There were 890,681 singleton live births during the study period. After excluding 2,026 records, 888,655 births were included in the analysis. Records were excluded if a child had 0 days of follow-up (number [n] =1,228), birth weight <500 g (n=90), gestational age <25 completed weeks or >45 completed weeks (n=510 and n=5, respectively), implausible gestational age and birth weight combinations (n=70), or if they met <1 exclusion criteria (n=123). The mean and median follow-up periods were both 8.0 years (interquartile range: 4.4–11.6 years).
Among the 888,655 children in the study population, 6.0% (n=53,024) received an asthma diagnosis before the end of follow-up. The mean age of asthma diagnosis was 31 months (standard deviation: ±31.0 months), and the median was 18.7 months (interquartile range: 10.7–39.6 months). A total of 6,641 children had a recorded comorbidity; cardiac disease was the most common comorbidity (32.8%), followed by renal disease (21.5%) and type 1 diabetes (20.3%).
Compared with children without asthma, children with an asthma diagnosis were more likely to be male, to have been born preterm (<37 weeks), to have a low birth weight (<2,500g), and to be born to a mother who reported smoking at the first prenatal visit (). In addition, asthmatic children had more congenital malformations (6.7% versus 4.4%, respectively) and selected underlying comorbidities (2.4% versus 0.6%, respectively) compared with nonasthmatic children.
Table 1 Selected characteristics of live births, Denmark, 1994–2007
A total of 2,253 children were diagnosed with PD during the follow-up period, and most of them were admitted as inpatients (96.3%). Pneumonia accounted for the majority of cases (72.9%), followed by septicemia (14.6%) and meningitis (12.5%). Most PD diagnoses occurred between 6 months and 24 months after birth (n=1,180; 52.7%) and the fewest occurred at more than 60 months after birth (n=255; 11.3%). There was no trend toward increasing or decreasing incidence throughout the study’s calendar period.
Age-specific PD incidence
Age-specific incidence rates for PD are presented in . The rate of PD was highest among children 6 to <24 months old (91.2 cases per 100,000 child-years) followed by children 0 to <6 months old (78.8 cases per 100,000 child-years) and children 24 to <60 months old (21.5 cases per 100,000 child-years). Children in the oldest age group had the lowest PD rates (8.0 cases per 100,000 child-years). PD incidence rates in asthmatic children were consistently higher than in nonasthmatic children, with the exception of children aged 0 to <6 months.
Table 2 Pneumococcal disease incidence rates per 100,000 child-years and IRRs of the association between childhood asthma and incident pneumococcal disease, Denmark, 1994–2007
Association between asthma and PD
The unadjusted IRR associating asthma with incident PD among all children was 2.4 (95% CI: 2.1, 2.6). Age-specific measures of association were confounded by year of birth, birth weight, congenital malformations, and underlying comorbidities. These confounders and child’s sex were therefore included in adjusted analyses. The adjusted IRR associating asthma with incident PD among all children was 2.2 (95% CI: 2.0, 2.5); in order of increasing age strata, the adjusted IRRs were 0.4 (95% CI: 0.2, 0.8) in children 0 to <6 months old, 2.1 (95% CI: 1.8, 2.5) in children 6 to <24 months old, 4.1 (95% CI: 3.3, 5.1) in children 24 to <60 months old, and 2.3 (95% CI: 1.6, 3.2) in children ≥60 months old ().
Restricting the study population to children without selected comorbidities or malformations did not substantially change the IRRs associating asthma with incident PD in any age strata. Restricting the study population to children who did have selected comorbidities or malformations, however, revealed that comorbidity was an effect modifier of the association between asthma and PD in children aged 24 to <60 months and ≥60 months ().
Table 3 IRRs for the association between asthma and incident pneumococcal disease, stratified by comorbidity and congenital malformation among the two oldest age groups only, Denmark, 1994–2007
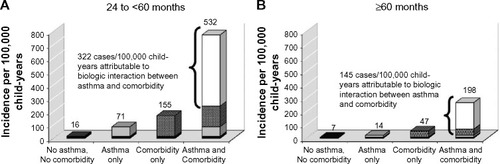
Evaluation of biologic interaction between asthma and comorbidity on the incidence of PD in these age groups showed that the rate of PD was greater among those with a recorded diagnosis of both asthma and comorbidity compared with the rate that would be expected based on the independent effects of asthma or comorbidity alone. shows that among children aged 24 to <60 months old, the unadjusted incidence rate of PD in children with both asthma and comorbidity was 7.5 times the rate in children with asthma alone, and that after adjusting for confounding variables, 55% (95% CI: 31, 79) of PD cases among asthmatic children could be attributed to the presence of both asthma and comorbidity at the same time. Among children aged ≥60 months old, the unadjusted IRR of PD in children with both asthma and comorbidity was 14 times the rate in children with asthma alone, and the adjusted percentage of PD cases attributable to biologic interaction increased to 73% (95% CI: 52, 93).
Figure 1 Biologic interaction between asthma and comorbidity on the risk of pneumococcal disease (PD), Denmark, 1994–2007.
Abbreviation: CI, confidence interval.
Discussion
This study provides evidence that asthma is an important risk factor for the development of PD in children, and it provides new insight about a potentially meaningful interaction between asthma and comorbidity on the risk of PD.
Consistent with data presented by Talbot et al,Citation2 we observed a twofold increased rate of PD among all children <18 years old following a childhood asthma diagnosis compared with person-time before an asthma diagnosis or in children who never had an asthma diagnosis after adjusting for confounding factors. The highest rate ratio occurred among children 24 to <60 months old, which was twice as large as the rate ratios observed among children 6 to <24 months old and ≥60 months old. We also observed a rate ratio less than 1 among children 0 to <6 months old, but emphasize that we do not believe that this indicates that asthma is protective against PD in this age group. Instead, we suspect that this observation is due to the difficulty of reliably diagnosing asthma in very young children.Citation24
The evaluation of a biological interaction between childhood asthma and comorbidity on the incidence of PD revealed that the combined effect of these two exposures was synergistic in older children, and that a high proportion (55% in children aged 24 to <60 months, and 73% in children aged ≥60 months) of the PD incidence among asthmatic children with comorbidities was attributable to this interaction. No synergy between childhood asthma and comorbidity was observed in children less than 2 years old. These results suggest that children more than 2 years old who have underlying comorbidities are more sensitive to the effect of asthma on PD than children more than 2 years old without comorbidities.
Juhn et alCitation3 also identified comorbidity as an effect modifier of the association between asthma and PD among Minnesota adults. They reported that the odds ratio (OR) of the effect of asthma on PD among adults with high-risk conditions was lower (OR: 1.2; P=0.86) than the OR among those without high-risk conditions (OR: 2.9; P=0.04). Similar results were observed in our study in children between 2 years and 5 years old: the association between asthma and incident PD was lower among children with underlying comorbid conditions compared with children without underlying illnesses (adjusted IRR: 2.9 versus 4.2, respectively). The lower rate ratios among children with comorbid conditions probably results from their higher risk of PD, which is sometimes called modification by the baseline risk. Juhn et alCitation3 did not assess for biological interaction on the additive scale in their study, and instead reported that there was no statistical interaction between asthma and illnesses based on the addition of an interaction term in a log-linear multivariate model. Departure from additivity is a better measure of biologic interaction, and a lack of statistical interaction – when measured as an interaction term in a log-linear multiplicative multivariate model – can easily be mistaken for a lack of biologic interaction.Citation22
Several investigators have identified potential biologic mechanisms that may explain how asthma increases the risk of PD. Two studies have identified associations between asthma and increased carriage of Streptococcus pneumoniae in the nasopharynx,Citation25,Citation26 suggesting that children with asthma may be at increased risk of PD because they are more likely to be colonized with pneumococci. Other proposed mechanisms include asthma-induced pathologic alterations that can impair clearance of pathogenic bacteria from the airwayCitation27,Citation28 and chronic airway inflammation leading to impaired respiratory immunity.Citation29,Citation30
Several limitations should be considered when interpreting the results from this study. First, the use of registry-based ICD-10 codes to identify children with asthma is likely to result in some misclassification. Underascertainment of asthma is possible if less severe cases of asthma were missed, most likely due to diagnosis or treatment only by a general practitioner. Such underascertainment would bias the IRRs describing the association between asthma and PD toward the null because some exposed children would be misclassified as unexposed. Overascertainment is also possible if some children with wheezing due to other causes, for example respiratory syncytial virus, were classified as having asthma. Although one way to increase sensitivity and specificity of asthma exposure would have been to incorporate the use of prescription asthma medications into a classification scheme for asthma exposure, we did not have access to these data for this study. We were also unable to include a mechanism by which to reclassify asthma-exposed children as unexposed if they “grew out” of an asthma diagnosis.
Although imperfect sensitivity and specificity of exposure classification is possible, several studies that have recently evaluated the quality of ICD-10-based asthma diagnoses in the Danish National Registry of Patients have found the diagnosis codes to be accurate.Citation17,Citation18 One study that used 3,550 medical records as the gold standard to validate ICD-10 inpatient asthma diagnoses recorded in the Danish National Registry of Patients reported 90% sensitivity and 99% specificity of asthma diagnoses among children aged 6–14 years old.Citation17 Another study reported 44% sensitivity and 98% specificity of asthma diagnoses recorded among 18-year-old men reporting for mandatory medical evaluation at the Danish Military Draft Board. The authors of this study subsequently demonstrated that the level of nondifferential asthma misclas-sification present in the Danish National Registry of Patients was not sufficient to nullify the association between asthma and various skin cancers that they examined.Citation18
We observed a 6% prevalence of asthma in our study population, which is less than an estimated asthma prevalence of 10%–12% based on questionnaire data collected from parents of Danish children aged 5–17 years.Citation31,Citation32 To determine the potential impact of imperfect sensitivity and specificity of exposure ascertainment on our results, we performed a bias analysis by calculating the IRR that would have been observed if asthma ascertainment only has a sensitivity equal to 50% and a specificity equal to 97%. This sensitivity is consistent with the differences between the asthma prevalence recorded in this study and that recorded in published reports, and this specificity was the minimum specificity that resulted in no negative cell frequency in the corrected table. We assumed that exposure misclassification was nondifferential and independent for cases and noncases, as the data in our study were prospectively collected. The results from this analysis indicated a minimal impact on the age-specific IRRs: the unadjusted IRR in children aged 6 to <24 months old would increase from 2.3 to 2.4 and the IRR among children 24 to <60 months old would increase from 4.8 to 5.0; the unadjusted IRRs among children 0 to <6 months old and ≥60 months old would not change.
Second, the exclusive use of ICD-10 codes to identify PD cases creates the potential for misclassification. Some PD cases could have been missed if the resulting illness was mild, if cultures were falsely negative, or due to recordkeeping errors. However, because PD is a serious disease typically requiring medical treatment, ICD-10 codes are likely to capture the most important and costly infections. If underascertainment of PD did occur, it was likely to be nondifferential due to the prospective nature of the data which is, in turn, expected to produce unbiased ratio effect estimatesCitation33 in the absence of false positives. It is, however, possible that some cases could have been falsely attributed to PD when in fact they were caused by other bacterial infections. Although we did not independently verify case status in this study, discharge diagnoses of PD have been found to have high specificity in validation studies conducted by other investigators.Citation16,Citation34
Third, although we were able to collect extensive information about pregnancy- and birth-related characteristics, we were not able to capture complete information about some social factors associated with PD. Misclassification of exposure to secondary tobacco smokeCitation35 may have occurred in this study because information about smoking was only available from mothers at the beginning of pregnancy, and no information was available from fathers or other childcare providers. In addition, we were not able to identify which children attended daycare.Citation20
Conclusion
Despite these limitations, the current study is an important contribution to the current knowledge of the association between asthma and PD. The evidence presented here indicates that asthmatic children are more likely to develop PD compared with nonasthmatic children, thereby providing support for the addition of asthma to the list of pneumococcal vaccine-eligible conditions for older children. These results also indicate that children who have asthma and another underlying comorbidity may be at especially high risk of PD, and should be carefully assessed in the clinic when presenting with bacterial illnesses.
Author contributions
Kimberly M Shea: Dr Shea oversaw all aspects of the study including conceiving of the study idea, preparing the dataset, conducting the analyses, and drafting the initial manuscript. Timothy L Lash, Susan S Jick, and Henrik T Sørensen: Drs Lash, Jick, and Sørensen assisted with the design of the study, participated in the interpretation of the results, reviewed and revised the manuscript, and approved the final manuscript for submission. Sussie Antonsen: Ms Antonsen prepared the original dataset, assisted with the statistical analyses, reviewed and revised the manuscript, and approved the final manuscript for submission.
Supplementary materials
Evaluation of observed effect measure modification for evidence of biologic interaction
Effect measure modification observed in stratified analyses was further evaluated for evidence of biologic interaction by using three standard measuresCitation1,Citation2 to determine whether the independent effects of asthma and comorbidity summed to the total effect of both factors together.
First, the interaction contrast (IC) between asthma and comorbidity was calculated by applying the following formula to crude pneumococcal disease (PD) incidence rates per 100,000 person years:
(1) where R represents the rate of disease, E represents exposure to asthma, and C is the modifying covariate which, in this case, was comorbidity. The IC represents the number of cases of disease (per 100,000 child-years) that cannot be accounted for by baseline factors among children without asthma or comorbidity, asthma only, or comorbidity only, and is therefore presumed to be attributable to a biological interaction between asthma and comorbidity.
Next, the interaction contrast ratio (ICR) and 95% confidence interval around the ICR was calculated to quantify the excess rate when both asthma and comorbidity were present at the same time relative to the baseline rate of disease that occurred when neither were present, while adjusting for important confounders of the association between asthma and PD:Citation3
(2)
The ICRs in our study were adjusted for sex, birth weight, child year of birth, and congenital malformation. We then used the ICR (to account for confounders) to calculate the attributable proportion due to interaction (AP), which quantifies the proportion of disease among exposed persons attributable to the interaction between an exposure and a modifying covariate. The AP was calculated by dividing the ICR by the IRR, comparing children with asthma and comorbidity to children without either of these:Citation3
(3)
Table S1 ICD-10 codes used to identify asthma, pneumococcal disease, and comorbidities from Danish registries
References
- RothmanKJThe estimation of synergy or antagonismAm J Epidemiol197610355065111274952
- GreenlandSLashTLRothmanKJConcepts of interactionRothmanKJGreenlandSLashTLModern Epidemiology3rd edPhiladelphia, PALippincott, Williams and Wilkins2008
- AnderssonTAlfredssonLKallbergHZdravkovicSAhlbomACalculating measures of biological interactionEur J Epidemiol20052057557916119429
Disclosure
The authors report no conflicts of interest in this work.
References
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Recomm Rep199746RR-8124
- TalbotTRHartertTVMitchelEAsthma as a risk factor for invasive pneumococcal diseaseN Engl J Med2005352202082209015901861
- JuhnYJKitaHYawnBPIncreased risk of serious pneumococcal disease in patients with asthmaJ Allergy Clin Immunol2008122471972318790525
- WattJPO’BrienKLBeninALRisk factors for invasive pneumococcal disease among Navajo adultsAm J Epidemiol200716691080108717693393
- KlemetsPLyytikäinenORuutuPRisk of invasive pneumococcal infections among working age adults with asthmaThorax201065869870220685743
- HjulerTWohlfahrtJStaum KaltoftMKochABiggarRJMelbyeMRisks of invasive pneumococcal disease in children with underlying chronic diseasesPediatrics20081221e26e3218595971
- World Health Organization [webpage on the Internet]WHO factsheet 307: asthmaGeneva, SwitzerlandWorld Health Organization Available from: http://www.who.int/mediacentre/factsheets/fs307/en/index.htmlAccessed March 30, 2014
- SunyerJAntóJMTobiasABurneyPGenerational increase of self-reported first attack of asthma in fifteen industrialized countries. European Community Respiratory Health Study (ECRHS)Eur Respir J199914488589110573237
- HartertTVPeeblesRSEpidemiology of asthma: the year in reviewCurr Opin Pulm Med2000614910608418
- ManninoDMHomaDMAkinbamiLJMoormanJEGwynnCReddSCSurveillance for asthma – United States, 1980–1999MMWR Surveill Summ2002511113
- Centers for Disease Control and Prevention (CDC)Asthma prevalence and control characteristics by race/ethnicity – United States, 2002MMWR Morb Mortal Wkly Rep200453714514814985651
- AsherMIMontefortSBjörksténBISAAC Phase Three Study GroupWorldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveysLancet2006368953773374316935684
- HarboeZBValentiner-BranthPBenfieldTLEarly effectiveness of heptavalent conjugate pneumococcal vaccination on invasive pneumococcal disease after the introduction in the Danish Childhood Immunization ProgrammeVaccine201028142642264720096392
- PedersenCBGøtzscheHMøllerJOMortensenPBThe Danish Civil Registration System. A cohort of eight million personsDan Med Bull200653444144917150149
- Department of Clinical Epidemiology, Aarhus University HospitalNational Registries, hosted and run by the National Board of HealthSørensenHTChristensenTSchlosserHKPedersenLUse of Medical Databases in Clinical Epidemiology2nd edAarhus, DenmarkSun-Tryk, Aarhus University20092329
- MahonBEEhrensteinVNørgaardMPedersenLRothmanKJSørensenHTPerinatal risk factors for hospitalization for pneumococcal disease in childhood: a population-based cohort studyPediatrics20071194e804e81217403823
- MothGVedstedPSchiøtzPONational registry diagnoses agree with medical records on hospitalized asthmatic childrenActa Paediatr200796101470147317727688
- JensenAØNielsenGLEhrensteinVValidity of asthma diagnoses in the Danish National Registry of Patients, including an assessment of impact of misclassification on risk estimates in an actual datasetClin Epidemiol20102677220865105
- KnudsenLBOlsenJThe Danish Medical Birth RegistryDan Med Bull19984533203239675544
- ButlerJEpidemiology of pneumococcal diseaseTuomanenEMitchellTMorrisonDSprattBThe PneumococcusWashington, DCASM Press2004148168
- RothmanKJThe estimation of synergy or antagonismAm J Epidemiol197610355065111274952
- GreenlandSLashTLRothmanKJConcepts of interactionRothmanKJGreenlandSLashTLModern Epidemiology3rd edPhiladelphia, PALippincott, Williams and Wilkins20087483
- SAS 92SAS Institute IncCary, NC, USA
- OlsonLMRadeckiLFrintnerMPWeissKBKorfmacherJSiegelRMAt what age can children report dependably on their asthma health status?Pediatrics20071191e93e10217200264
- BisgaardHHermansenMNBuchvaldFChildhood asthma after bacterial colonization of the airway in neonatesN Engl J Med2007357151487149517928596
- CardozoDMNascimento-CarvalhoCMAndradeALPrevalence and risk factors for nasopharyngeal carriage of Streptococcus pneumo-niae among adolescentsJ Med Microbiol200857Pt 218518918201984
- FahyJVCorryDBBousheyHAAirway inflammation and remodeling in asthmaCurr Opin Pulm Med200061152010608420
- BusseWWPathogenesis and sequelae of respiratory infectionsRev Infect Dis199113Suppl 6S477S4851862278
- BardinPGFraenkelDJSandersonGAmplified rhinovirus colds in atopic subjectsClin Exp Allergy19942454574648087657
- MessageSDJohnstonSLHost defense function of the airway epithelium in health and disease: clinical backgroundJ Leukoc Biol200475151712972516
- ThomsenSFUlrikCSLarsenKBackerVChange in prevalence of asthma in Danish children and adolescentsAnn Allergy Asthma Immunol200492550651115191018
- HermannCDe Fine OlivariusNHøstABegtrupKHollnagelHPrevalence, severity and determinants of asthma in Danish five-year-oldsActa Paediatr200695101182119016982487
- GreenlandSLashTLValidity in epidemiologic studiesRothmanKJGreenlandSLashTLModern Epidemiology3rd edPhiladelphia, PALippincott, Williams & Wilkins2008142143
- KristensenJLanghoff-RoosJSkovgaardLTKristensenFBValidation of the Danish Birth RegistrationJ Clin Epidemiol19964988938978699210
- NuortiJPButlerJCFarleyMMCigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance TeamN Engl J Med20003421068168910706897
