Abstract
Introduction
DANBIO is a research register and a data source for rheumatologic diseases (rheumatoid arthritis [RA], axial spondyloarthritis, and psoriatic arthritis) for monitoring clinical quality at the national, regional, and hospital levels.
Study population
The register includes patients with rheumatologic diseases who are treated at a hospital or a private rheumatologic clinic. Registration is mandatory for all patients with RA regardless of treatment and also for patients with other diagnoses if treated with biological disease-modifying antirheumatic drugs. Since 2006, the registration has been done electronically, including patient-reported outcome measures registered electronically by the patients with the use of touch screens.
Main variables
Core variables such as diagnosis, year of diagnosis, age, and sex are registered at the beginning. Data entered at later visits included the following: patient-reported outcomes for disease activity, pain, fatigue, functional status, and physician-reported objective measures of disease activity, treatment, C-reactive protein, and, when indicated, imaging. For subgroups of patients, the variables such as quality of life, sociodemographic factors, lifestyle, and comorbidity are also registered.
Descriptive data
The DANBIO cohort comprised ∼26,000 patients with RA, 3,200 patients with axial spondyloarthritis, and 6,200 patients with psoriatic arthritis in 2015. DANBIO has high nationwide coverage and completeness on key data variables. More than 60 original papers as well as annual reports of clinical quality (since 2005) have been published.
Conclusion
DANBIO is a powerful register for research in rheumatologic diseases and furthermore serves as a Clinical Quality Register with the aim of monitoring treatment quality in patients with RA in Denmark.
Introduction
The database, DANBIO, is a nationwide, Danish register for research use in rheumatologic diseases such as rheumatoid arthritis (RA), axial spondyloarthritis (Ax SpA), and psoriatic arthritis (PsA). It also serves as a clinical database that monitors clinical quality of treatment by the use of selected quality indicators for patients with RA in Denmark.
Study population and data collection
Up to the year 2000, no routine-based nationwide reporting of patients with RA existed in Denmark, and patient files rarely comprised quantitative patient-reported outcome data. The introduction of new biological disease-modifying antirheumatic drugs (bDMARDs) triggered the formation of a nationwide voluntary register, which aimed to survey indications for treatment, efficacy, and adverse events in rheumatologic patients who received biological therapies in routine care.
In the beginning, data were collected on paper forms, which were subsequently scanned into the register at the DANBIO office.Citation1 In 2006, an online version of DANBIO was introduced, and the aims were extended to include patients regardless of treatment and also to collect patient-reported outcomes regarding, eg disability, pain, lifestyle, and quality of life by the use of dedicated touch screens in the waiting room (). This is routinely done before consultation with the doctor, and the information from the touch screen is available during the consultation. DANBIO has been approved by the National Board of Health as a Clinical Quality Register, and since 2006, reporting to the register has been mandatory. For clinical quality registers, the usual requirement for obtaining an informed patient consent before registration is not needed. Only rarely do patients object to be included in the register, and in these cases, it is up to the physician to decide whether to register the patient or not.
RA, Ax SpA, and PsA are chronic diseases that require lifelong monitoring and treatment. A patient is therefore reported to the database at the time of diagnosis, referral to specialized treatment, including biological therapy at the hospital, or in a private rheumatologic clinic. At the first registration, the diagnosis, date of diagnosis, age, sex, and previous medical treatment are registered. At later visits, and at least once yearly, information regarding patient’s disease activity (including pain and functional status) is collected via touch screens, and objective measures (eg, swollen and tender joint counts, C-reactive protein) are entered by the physician.
The patient groups with Ax SpA and PsA are not included at the moment in the National Clinical Quality Program, and therefore reporting is only mandatory for patients with RA and for the other patient groups only when treated with bDMARDs. In the beginning of 2015, the DANBIO cohort comprised ∼26,000 patients with RA, 3,200 patients with Ax SpA, and 6,200 patients with PsA treated with conventional synthetic DMARDs and/or bDMARDs.
Main variables
gives the key variables of the register. Some variables are collected via touch screens (patient-reported outcome measures [PROMs]), and others are entered by the physician.
Table 1 Key variables in the DANBIO register
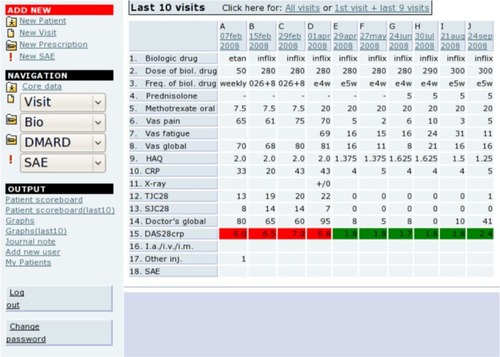
The use of touch screens for data collection has been validated to perform equally well as paper forms ().Citation2 The variables are collected in a standardized format, often using tick boxes, which have been shown to have a high validity.Citation1 shows the scoreboard for entering and reviewing individual patient data. In connection with ie, queries from DANBIO and research projects, the departments fill in missing data by checking against the hospital patient records.
The completeness of data is generally high (ie, 96%, 93%, 83%, 90%, and 93% for information on the name of the treating doctor, diagnosis, year of diagnosis, DAS28, and HAQ, respectively). National coverage of DANBIO is assessed annually in two ways: for patients in biological treatment by comparing to those registered in the patient record systems of each rheumatologic department (94% in 2013) and by calculation of all incident patients with RA in DANBIO by comparison to the Danish National Patient Registry (NPR; 85% in 2013). Since NPR is an administrative registerCitation3 in which RA diagnosis may be registered for patient contacts before the final diagnosis of the patient has been established, it cannot serve as a golden standard; however, it is the most valid nationwide register available for such comparison. Further, local medical record audits have shown that the diagnosis in DANBIO is valid. Therefore, it is assumed that the true coverage of DANBIO is higher than this estimate.Citation4
Follow-up
At least once-a-year follow-up information is collected in DANBIO, including PROMs such as the level of function and level of pain. Disease activity, type of treatment, treatment effectiveness, and side effects of treatment (if any) are registered by the physician.Citation5
Systematic monitoring of patients with RA with real-time feedback to the physician is feasible. It is documented that regular registration of disease activity for patients with RA results in improvement in disease activity, although the goal of treat to target is not achieved in a substantial proportion of patients in routine care.Citation6
Quality indicators
The current quality indicators for RA are shown in . The indicators include goals for whether patients are “treated to target” (ie, remission or low disease activity) by follow-up of patients with measurement and registration of disease activity level, functional status, pain, quality of life, and X-ray status. A standard for each indicator is set by the DANBIO steering committee and by consensus among the members of the Danish Society of Rheumatology setting goals for how large a fraction of patients who should meet the indicator criteria. The indicator results are published at country, regional, and hospital department levels in the Annual Clinical Quality Report.Citation4 An example of supplementary results from the quality report is presented in that gives the number of adult rheumatologic patients initiating bDMARDs in Denmark over time and the differences in prescription pattern between geographical regions.Citation4
Figure 3 Number of adult rheumatologic patients initiating bDMARDs in Denmark over time across the geographical regions.
Abbreviation: bDMARDs, biological disease-modifying antirheumatic drugs.
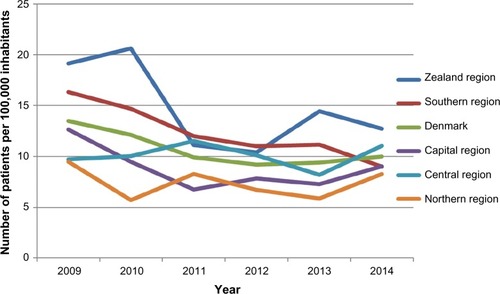
Table 2 Overview of the DANBIO quality indicators monitored in the Annual Clinical Quality Report
Examples of research
DANBIO is used in routine care for systematic monitoring of the patients, but it is also an extensive research database.
DANBIO data have been used for research with focus on both short- and long-term evaluation of treatment responses, remission rates, and drug adherence.Citation7–Citation10 Furthermore, research has been performed on cancer incidence and precancerous development following treatments with biological treatment regimes,Citation11,Citation12 as well as on the influence of tobacco smoking on treatment response.Citation13
More than 60 peer-reviewed articles have been published to date with the use of DANBIO data and >170 abstracts have been presented at international conferences and congresses.
DANBIO is easily linked to other data sources by the use of the unique personal identification number, assigned to all Danish citizens. With regulatory approval for specific research purposes, DANBIO data can be merged with ie, the NPR, the Danish Cancer Registry, or administrative registers holding socioeconomic information in Statistic Denmark.
A nationwide biobank (ie, blood and synovial fluid) associated with DANBIO has been established since 2015 through public funding.
Administrative issues and funding
DANBIO has an independent steering committee with representatives from the Danish Society of Rheumatology, DRFO (Danske Reumatologer og Fysiurgers Organisation), Junior Rheumatologists (Yngre Reumatologer), and the hospital owners. The daily administration is handled in the DANBIO general office at Rigshospitalet, Glostrup, and is staffed by the Head of Secretariat as well as a secretary.
DANBIO has been approved as a clinical quality register by the Danish authorities – the National Board of Health and the Danish Data Protection Agency – and is sponsored by the public hospital owners (Danish Regions) under the organization of the Danish Clinical Registries – a national improvement program. The pharmaceutical companies that provide biological treatments in rheumatology contribute to the development of the information technology platform through unrestricted grants, which have been approved by the public hospital owners. The sponsors have no influence on the register setup, data collection, data analysis, or publication of results. These issues are all administered by the steering committee.
Conclusion
DANBIO serves as a powerful register for research in rheumatologic diseases and additionally as a clinical quality register with the aim of monitoring quality by the use of selected clinical indicators for patients with RA in Denmark.
The register has existed since 2000, and patients diagnosed at the hospital or at private rheumatologic clinic are registered. Since 2006, the registration, including PROMs, has been electronically registered directly into the database with the use of touch screens.
DANBIO has high nationwide coverage of patients with rheumatologic diseases and high completeness on key data variables. Data have been used for a large number of research projects and each year a National Clinical Quality Report is published.
Acknowledgments
This paper was funded by the Program for Clinical Research Infrastructure established by the Lundbeck Foundation and the Novo Nordisk Foundation and administered by the Danish Regions. DANBIO thanks the Danish Regions for supporting the register. All the Departments of Rheumatology in Demark and the private rheumatologic clinics are acknowledged for using the register. Niels Steen Krogh, Zitelab ApS, is acknowledged for the continuous technical development and support of the register.
Disclosure
The authors report no conflicts of interest in this work.
References
- HetlandMLUnkerskovJRavnTRoutine database registration of biological therapy increases the reporting of adverse events twentyfold in clinical practice. First results from the Danish Database (DANBIO)Scand J Rheumatol200534404415903024
- SchefteDBHetlandMLAn open-source, self-explanatory touch screen in routine care. Validity of filling in the bath measures on ankylosing spondylitis disease activity index, function index, the health assessment questionnaire and visual analogue scales in comparison with paper versionsRheumatology2010499910419920097
- LyngeESandegaardJLReboljMThe Danish national patient registerScand J Public Health Suppl2011397 suppl3033
- DANBIO [homepage on the Internet]Danbio’s National Clinical Quality Report 2014 (Report in Danish) Available from: www.danbio-online.dkAccessed July 01, 2015
- HetlandMLDANBIO – powerful research database and electronic patient recordRheumatology201150697721148154
- HetlandMHJensenDVKroghNSMonitoring patients with rheumatoid arthritis in routine care: experiences from a treat-to-target strategy using the DANBIO registryClin Exp Rheumatol201432suppl 85141146
- HetlandMLChristensenIJTarpUAll Departments of Rheumatology in DenmarkDirect comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registryArthritis Rheum2010621223220039405
- ØrnbjergLMØstergaardMBøyesenPImpact of tumour necrosis factor inhibitor treatment on radiographic progression in rheumatoid arthritis patients in clinical practice: results from the nationwide Danish DANBIO registryAnn Rheum Dis2013721576322532636
- GlintborgBØstergaardMDreyerLTreatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registryArthritis Rheum201163238239021279995
- GlintborgBOstergaardMKroghNSDreyerLKristensenHLHetlandMLPredictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumour necrosis factor: results from 8 years’ surveillance in the Danish nationwide DANBIO registryAnn Rheum Dis201069112002200820511613
- DreyerLMellemkjærLAndersenARIncidences of overall and site specific cancers in TNFα inhibitor treated patients with rheumatoid arthritis and other arthritides – a follow-up study from the DANBIO registryAnn Rheum Dis2013721798222945500
- CordtzRMellemkjærLGlintborgBHetlandMLDreyerLMalignant progression of precancerous lesions of the uterine cervix following biological DMARD therapy in patients with arthritisAnn Rheum Dis20157471479148025744102
- HøjgaardPGlintborgBHetlandMLAssociation between tobacco smoking and response to tumour necrosis factor α inhibitor treatment in psoriatic arthritis: results from the DANBIO registryAnn Rheum Dis201474122130213625063827