Abstract
Introduction
Despite a clinically relevant, statistically significant survival benefit with nab-paclitaxel plus gemcitabine and FOLFIRINOX vs single-agent gemcitabine for metastatic pancreatic cancer (mPC), little is known regarding their real-world effectiveness. We analyzed patients with mPC using a nationally representative electronic medical records database to address this unmet need.
Methods
This retrospective analysis of the Navigating Cancer database compared outcomes among patients who received first-line nab-paclitaxel plus gemcitabine, FOLFIRINOX, or gemcitabine for mPC. Effectiveness, safety, and supportive care use were examined. nab-Paclitaxel plus gemcitabine was the reference for statistical comparisons.
Results
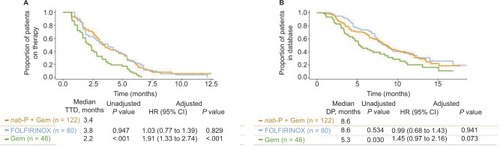
Baseline characteristics were similar except age (oldest patients were in the gemcitabine cohort followed by nab-paclitaxel plus gemcitabine, then FOLFIRINOX). Patients receiving nab-paclitaxel plus gemcitabine (n=122) demonstrated similar time to treatment discontinuation (TTD; median, 3.4 vs 3.8 months; P=0.947) and database persistence (DP; median, 8.6 vs 8.6 months; P=0.534) vs FOLFIRINOX (n=80); however, TTD (median, 3.4 vs 2.2 months; P<0.001) and DP (median, 8.6 vs 5.3 months; P=0.030) were significantly longer with nab-paclitaxel plus gemcitabine vs gemcitabine (n=46). There were more any-grade adverse events with FOLFIRINOX or gemcitabine vs nab-paclitaxel plus gemcitabine (95% or 89% vs 84%, respectively).
Conclusion
This real-world analysis confirms the phase III MPACT trial findings and demonstrates that nab-paclitaxel plus gemcitabine has effectiveness similar to that of FOLFIRINOX but greater tolerability for treating mPC despite younger patients being in the FOLFIRINOX cohort. These findings support nab-paclitaxel plus gemcitabine as an appropriate first-line treatment option for patients with mPC.
Introduction
In the USA, pancreatic cancer (PC) is the fourth leading cause of cancer-related mortality, with incidence and mortality rates predicted to increase.Citation1,Citation2 Estimates suggest that by 2030, PC will become the second leading cause of cancer-related deaths in the USA.Citation3 The 5-year relative survival rate for patients with all stages of PC is 7–8%; for patients with metastatic disease at diagnosis (52–53% of new cases), the rate decreases to 2–3%.Citation2,Citation4 The 5-year survival rate for patients with localized disease at diagnosis (9% of new cases) is only 27–29%,Citation2,Citation4 suggesting that most patients with an early-stage diagnosis will progress to advanced disease.
In 1997, gemcitabine demonstrated significant clinical benefit vs 5-fluorouracil in patients with advanced PC and was subsequently approved for treating metastatic PC (mPC).Citation5,Citation6 After two decades of phase III trials in advanced PC that failed to demonstrate clinical benefit with various gemcitabine-based combinations over gemcitabine monotherapy,Citation7–Citation14 the ACCORD (NCT00112658) and MPACT (NCT00844649) clinical trials reported a significant survival benefit with novel regimens as first-line treatment for mPC.Citation15–Citation17 In 2011, the ACCORD trial demonstrated significantly longer overall survival (OS) with FOLFIRINOX (folinic acid + 5-fluorouracil + irinotecan + oxaliplatin) vs gemcitabine (median, 11.1 vs 6.8 months; hazard ratio [HR] 0.57; P<0.001) in patients with mPC.Citation15 In 2013, the MPACT trial demonstrated a significantly longer OS with nab-paclitaxel plus gemcitabine vs gemcitabine in patients with mPC, which was confirmed in an updated report based on longer follow-up (median, 8.7 vs 6.6 months; HR 0.72; P<0.001).Citation16,Citation17 Subsequently, nab-paclitaxel plus gemcitabine and FOLFIRINOX became the preferred first-line treatment options for patients with mPC and good performance status (PS).Citation6
Cross-trial comparisons are problematic due to trial design differences. The ACCORD trial was conducted at academic centers in France and enrolled patients aged ≤75 years with an Eastern Cooperative Oncology Group (ECOG) PS ≤1.Citation15 However, the MPACT trial was an international study conducted at academic and community centers and enrolled patients with Karnofsky PS (KPS) 70–100 with no upper age restriction.Citation17 A real-world retrospective analysis highlighted the impact of eligibility criteria on clinical outcomes: gemcitabine-treated patients who would have met ACCORD (25% of the cohort) or MPACT (45% of the cohort) trial enrollment criteria experienced better outcomes than the overall cohort (median OS, 8.6 months [ACCORD eligible], 6.7 months [MPACT eligible], and 5.8 months [entire cohort]).Citation18
Patients in randomized, controlled trials do not always reflect the real-world population. For example, although the median age of patients diagnosed with PC is 70 years, elderly patients are typically underrepresented in clinical trials.Citation4,Citation19 Real-world studies analyzing clinical effectiveness and resource utilization of first-line treatment options for patients with mPC are limited. Therefore, this analysis aimed to examine the real-world comparative effectiveness of nab-paclitaxel plus gemcitabine vs FOLFIRINOX or gemcitabine as well as to compare adverse events (AEs) and supportive care utilization among these patients.
Methods
Data source
This analysis was performed using completely deidentified data from the Navigating Cancer (NC) database, which provides services to a variety of community-based oncologists who use various electronic medical record (EMR) systems. The NC database contains records mainly of medical oncology and/or hematology practices throughout the USA. At the time of the analysis, the database contained ≈1300 providers and 2,500,000 oncology patients. Institutional review board or ethics committee approval was not required because all data were deidentified.
Study design and patients
This was a retrospective analysis of patients with a primary diagnosis of mPC who received first-line nab-paclitaxel plus gemcitabine, FOLFIRINOX, or gemcitabine monotherapy between September 6, 2013, and October 6, 2014 (patients were followed up for ≥6 months or until April 6, 2015, whichever occurred later). Patients included in the analysis were those with mPC who had ≥1 visit during the study period and received ≥1 cycle of nab-paclitaxel plus gemcitabine (three doses of nab-paclitaxel), gemcitabine (three doses), or FOLFIRINOX (two doses of 5-fluorouracil). Patients who participated in any interventional clinical trial during the study period (preindex period through follow-up), received active treatment for a secondary malignancy, or continued to receive study regimens from the preindex period (60 days prior to mPC diagnosis) were excluded.
Endpoints
Primary endpoints were time to treatment discontinuation (TTD; time between first and last dose + 7 days for nab-paclitaxel plus gemcitabine and gemcitabine or + 14 days for FOLFIRINOX) and database persistence (DP; a proxy for OS). Patients were censored if no subsequent treatment was given and the end of first-line treatment was within 30 days of the data cut-off for TTD. Duration of treatment was defined as day 1 of first-line therapy until either the start of a new therapy or a gap of ≥60 days between two doses of the same regimen. Discontinuation and duration of therapy were based on nab-paclitaxel only for nab-paclitaxel plus gemcitabine, 5-fluorouracil only for FOLFIRINOX, and gemcitabine for gemcitabine monotherapy. DP was defined as the number of days between the initial dose of first-line therapy and the last available date of patient data (in the EMR database). Patients without EMR database activity within the last 30 days of the data cut-off were considered deceased; patients were censored if the last date of patient record was within 30 days of the data cut-off.
Secondary endpoints were safety (overall incidence of AEs and time to discontinuation due to an AE) and use of supportive care or premedication. Documented AEs (hereinafter referred to as AEs) were identified using International Classification of Diseases (ICD)-9 codes and laboratory values. Time to discontinuation due to an AE was determined by the occurrence of an ICD-9 code or laboratory value indicating a grade ≥3 hematologic AE within 30 days of postdiscontinuation. Supportive care or premedication included use on the day of or before receiving chemotherapy (antiemetics or steroids), as well as treatments received during chemotherapy (granulocyte colony-stimulating factor [G-CSF] or erythropoiesis-stimulating agent [ESA]). The proportion of patients receiving prophylactic G-CSF (day of or before first administration of nab-paclitaxel plus gemcitabine or FOLFIRINOX) was also determined.
An exploratory objective was to understand sequencing treatment patterns in patients switching from first- to second-line regimens. Outcomes such as the proportion of patients receiving second-line therapy, total duration of therapy (time between first dose of first-line and last dose of second-line therapies), and DP were determined. A subgroup analysis evaluating the primary endpoints was also performed in patients aged >70 years.
Statistical analyses
nab-Paclitaxel plus gemcitabine was the reference cohort, and comparisons were tested for significance between nab-paclitaxel plus gemcitabine and FOLFIRINOX or gemcitabine. Demographics and baseline characteristics between treatments were evaluated by analysis of variance for continuous variables and c2 or Fisher’s exact test for categorical variables. Median TTD and DP were calculated using the Kaplan–Meier method, and differences were evaluated by Cox proportional hazards model. Comparisons of supportive care use were made using generalized linear regression analysis with Poisson distribution and log-link function. For all multivariate analyses, age, sex, and Charlson Comorbidity Index score were included as covariates. PS could not be adjusted for as a covariate because these data were missing for 47% of patients.
Results
Baseline characteristics
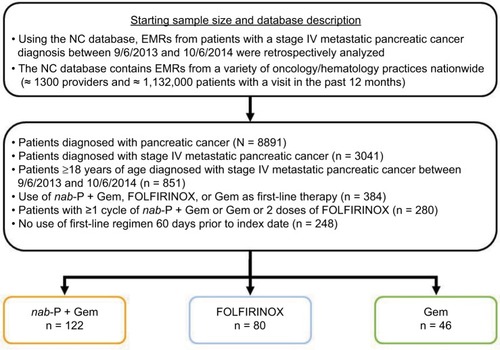
Out of 8891 patients in the database diagnosed with PC (3041 with mPC), 248 patients met the inclusion criteria for this analysis: 122 in the nab-paclitaxel plus gemcitabine cohort, 80 in the FOLFIRINOX cohort, and 46 in the gemcitabine cohort (). Baseline characteristics were generally similar among the three cohorts, with few exceptions (). Patients in the FOLFIRINOX cohort were significantly younger than those in the nab-paclitaxel plus gemcitabine cohort, and those in the nab-paclitaxel plus gemcitabine cohort were significantly younger than those in the gemcitabine cohort. The nab-paclitaxel plus gemcitabine cohort had significantly more men than did the gemcitabine cohort. Among patients with available baseline PS (53% of the population), the distribution of those with ECOG PS 0–1 and 2+ was similar between the nab-paclitaxel plus gemcitabine and FOLFIRINOX cohorts; however, there was a higher proportion of patients with ECOG PS 2+ in the gemcitabine cohort compared with the other two cohorts.
Table 1 Characteristics of patients (total study population) at baseline
Time to treatment discontinuation
The difference in TTD between patients in the nab-paclitaxel plus gemcitabine vs FOLFIRINOX cohorts was not statistically significant; however, TTD was significantly longer for patients who received nab-paclitaxel plus gemcitabine vs gemcitabine (). The findings did not change when these data were adjusted using the aforementioned covariates.
Figure 2 (A) Time to treatment discontinuation and (B) database persistence among patients who received nab-paclitaxel + gemcitabine, FOLFIRINOX, or gemcitabine.
Database persistence
The difference in DP between patients in the nab-paclitaxel plus gemcitabine vs FOLFIRINOX cohorts was not statistically significant, and findings did not change when these data were adjusted using the aforementioned covariates (). DP was significantly longer for patients who received nab-paclitaxel plus gemcitabine vs gemcitabine; however, after adjusting for covariates, the difference was no longer significant.
Safety and use of supportive care
The incidence of documented, any-grade AEs was greater among patients who received gemcitabine or FOLFIRINOX vs nab-paclitaxel plus gemcitabine, and the difference was statistically significant for FOLFIRINOX vs nab-paclitaxel plus gemcitabine (89% or 95% vs 84%, respectively; P=0.437 and P=0.021, respectively). Between the three cohorts, the most frequent all-grade and grade 3/4 hematologic AEs occurring in >10% of patients were anemia and neutropenia, respectively (). Febrile neutropenia was rare, with no statistically significant differences in its occurrence between patients in the nab-paclitaxel plus gemcitabine vs the FOLFIRINOX or gemcitabine cohorts. Nausea and vomiting as well as dehydration were the most frequent all-grade nonhematologic AEs occurring in >10% of patients between the three cohorts. The AEs that most frequently led to treatment discontinuation among those who received gemcitabine, FOLFIRINOX, or nab-paclitaxel plus gemcitabine were anemia (15%, 8%, or 2%, respectively), dehydration (7%, 5%, or 3%, respectively), nausea and vomiting (7%, 0%, or 1%, respectively), and neutropenia (2%, 6%, or 6%, respectively).
Table 2 Safety
Patients in the FOLFIRINOX cohort received more doses of G-CSF than did those in the nab-paclitaxel plus gemcitabine cohort (). A significantly greater percentage of patients treated with FOLFIRINOX vs nab-paclitaxel plus gemcitabine received prophylactic G-CSF (39% vs 8%; P<0.001). Patients in the nab-paclitaxel plus gemcitabine cohort received more doses of ESA than did those in the FOLFIRINOX cohort. Compared with the gemcitabine group, patients in the nab-paclitaxel plus gemcitabine cohort received more doses of antiemetics, ESAs, G-CSF, and steroids as supportive care.
Table 3 Supportive care use
Sequencing outcomes
Among patients who received first-line nab-paclitaxel plus gemcitabine, 20% received a 5-fluorouracil-based second-line therapy, and of those who received first-line FOLFIRINOX, 51% received a gemcitabine-based second-line therapy. A small portion of patients received second-line chemotherapy following first-line gemcitabine (n=9); therefore, these patients were not characterized in detail. The median time to next therapy for patients who received nab-paclitaxel plus gemcitabine followed by a 5-fluorouracil-based second-line therapy or FOLFIRINOX followed by a gemcitabine-based second-line therapy was 4.5 months (P=0.833). Characteristics of patients who received second-line therapy were generally similar; however, patients who received FOLFIRINOX followed by a second-line gemcitabine-based therapy were generally younger than those who received first-line nab-paclitaxel plus gemcitabine followed by a 5-fluorouracil-based second-line therapy ().
Table 4 Characteristics of patients at initiation of first-line therapy among those who received second-line therapy
The median treatment duration was similar among patients who received nab-paclitaxel plus gemcitabine followed by a 5-fluorouracil-based therapy and those who received FOLFIRINOX followed by a gemcitabine-based therapy (median, 8.7 vs 8.4 months; P=0.516). The DP was numerically longer for patients who received nab-paclitaxel plus gemcitabine followed by a 5-fluorouracil-based therapy vs those who received FOLFIRINOX followed by a gemcitabine-based therapy (median, 12.7 vs 9.1 months; P=0.477); however, this difference was not statistically significant.
Age subgroup analysis
The majority (52%) of patients who received gemcitabine were aged >70 years, whereas 44% and 16% of patients who received nab-paclitaxel plus gemcitabine or FOLFIRINOX, respectively, were aged >70 years (). There was no statistically significant difference in TTD between patients in the nab-paclitaxel plus gemcitabine vs FOLFIRINOX cohorts (median, 3.7 vs 2.1 months; P=0.734). The TTD was significantly longer for patients who received nab-paclitaxel plus gemcitabine vs gemcitabine (median, 3.7 vs 2.1 months; P=0.024). Additionally, there was no statistically significant difference in DP for those who received nab-paclitaxel plus gemcitabine vs FOLFIRINOX (median, 8.2 vs 9.4 months; P=0.453), and a significantly longer DP was observed for those who received nab-paclitaxel plus gemcitabine than gemcitabine (median, 8.2 vs 5.2 months; P=0.024).
Discussion
This analysis provides real-world evidence of similar effectiveness with nab-paclitaxel plus gemcitabine and FOLFIRINOX in treating patients with mPC, as determined by TTD and DP, despite patients who received FOLFIRINOX being significantly younger than those who received nab-paclitaxel plus gemcitabine. Additionally, with the exception of anemia, there was a lower incidence of all-grade AEs among patients who received nab-paclitaxel plus gemcitabine vs FOLFIRINOX. Importantly, this analysis confirmed the phase III MPACT trial findings: nab-paclitaxel plus gemcitabine was more effective than gemcitabine in the real-world setting.
DP, used in this study as a proxy for OS, for patients who received nab-paclitaxel plus gemcitabine in this analysis was similar to the OS reported for the regimen in the MPACT trial.Citation16 However, the DP among patients who received FOLFIRINOX in this analysis was shorter than the OS reported for the ACCORD trial.Citation15 Comparing real-world and clinical trial data is a flawed process, but there might be potential explanations for the suboptimal outcomes observed for patients treated with FOLFIRINOX. In everyday practice, physicians frequently modify the FOLFIRINOX regimen when treating patients with PC,Citation20 which may influence outcomes for patients receiving FOLFIRINOX. Further, a retrospective analysis reported that only 25% of real-world patients with mPC would meet ACCORD trial eligibility criteria.Citation18 The relationship between these real-world findings and the respective clinical trial results may be a reflection of the enrolled cohorts. The ACCORD trial was conducted at academic centers in France and enrolled patients aged ≤75 years with an ECOG PS ≤1, whereas the MPACT trial was an international trial conducted at academic and community centers and enrolled patients with a KPS ≥70 with no upper age restriction.Citation15,Citation17 Although DP was a proxy for survival and direct comparisons cannot be made between studies, these data suggest that MPACT trial results may be reflective of real-world outcomes.
Treatment sequencing plans (pairing of the appropriate first-line therapy with subsequent treatments) for patients with mPC are being actively investigated. In this analysis, there was a greater effectiveness with nab-paclitaxel plus gemcitabine followed by a 5-fluorouracil-based therapy vs FOLFIRINOX followed by gemcitabine-based therapy; however, differences were not statistically significant, possibly due to small sample sizes (n=25 and 41, respectively). Nevertheless, these findings support nab-paclitaxel plus gemcitabine as a first-line therapy for patients with mPC. Understanding how a treatment plan can be built is important, given that nab-paclitaxel plus gemcitabine is now the most frequently used first-line therapy for treating mPC in the USA.Citation21
Observations in elderly patients in this analysis were consistent with what has been reported in the literature. Among patients who received nab-paclitaxel plus gemcitabine, a slightly shorter DP was observed for those aged >70 years vs the entire cohort. A similar survival trend was observed for patients aged ≥65 years who received nab-paclitaxel plus gemcitabine during the MPACT trial.Citation16,Citation22 Among patients who received FOLFIRINOX, DP was slightly longer among those aged >70 years vs the entire cohort. However, it should be noted that in this analysis, only 13 out of 80 patients in the FOLFIRINOX cohort were aged >70 years, and PS data were lacking for many patients, thus making it difficult to draw conclusions.
The costs associated with managing AEs during PC treatment are often considerable.Citation23 Therefore, identification of the AE profile for real-world patients is quite relevant from an economic perspective. Generally, the incidence of AEs herein was similar to what has been reported in the ACCORD and MPACT trials; however, rates of neutropenia and fatigue among patients in the FOLFIRINOX cohort were lower than what was reported in the ACCORD trial.Citation15 Possible explanations for these differences may lie in prophylactic G-CSF use or the likely use of modified FOLFIRINOX regimens. The distinction between FOLFIRINOX and modified FOLFIRINOX was not available for the current analysis. In both the ACCORD trialCitation15 and the present analysis, an appreciable proportion of patients receiving FOLFIRINOX used G-CSF to avoid or ameliorate neutropenia.Citation24 A real-world retrospective study of US hospital data reported the monthly G-CSF support cost associated with FOLFIRINOX to be $4793,Citation25 which may pose a hardship for some patients. With the exception of anemia, the incidence of all-grade AEs was higher for patients in the FOLFIRINOX vs nab-paclitaxel plus gemcitabine cohort. Interestingly, the rate of grade 3/4 hematologic AEs was higher among patients who received gemcitabine vs nab-paclitaxel plus gemcitabine, which was not the case in the MPACT trial.Citation17 This may be explained by the difference in age between patients in the gemcitabine cohort in the present analysis vs those who received gemcitabine during the MPACT trial (mean, 72 years; median, 63 years, respectively) or by the fact that, in the present analysis, patients in the gemcitabine cohort were ≈5 years older than those who received nab-paclitaxel plus gemcitabine.
There are numerous limitations to the current methodology and analysis. Treatment effectiveness was assessed by DP, a proxy for, but not true measure of, survival. Actual death dates were not recorded in the EMRs; therefore, DP was used to estimate survival. This proxy measure is imperfect because switching to a new oncologist, entering hospice care, or moving to a new care facility could have resulted in disappearance from the EMR, thus underestimating survival. Additionally, this was not a randomized study, and many baseline characteristics fundamentally differed among the cohorts. However, these imbalances were adjusted for in the TTD and DP analyses. PS was available for only 53% of the population and could not be adjusted for as a covariate, which may have confounding effects. AEs were assessed using ICD-9 codes and laboratory values only, meaning that subjective AEs, such as neuropathy, may be underreported.
The present analysis validates the findings established by the MPACT trial of superiority of nab-paclitaxel plus gemcitabine vs gemcitabine outside of randomization, based on physicians’ decisions, in a real-world population. Otherwise, the effectiveness of nab-paclitaxel plus gemcitabine and that of FOLFIRINOX were comparable, which may not have been predicted based on improper cross-trial comparison. Patients who received nab-paclitaxel plus gemcitabine generally had a lower incidence of AEs and used less G-CSF (but more ESAs, antiemetics, and steroids) than those who received FOLFIRINOX. In conclusion, in the absence of a head-to-head trial with FOLFIRINOX, this real-world analysis supports nab-paclitaxel plus gemcitabine as a preferred first-line treatment option for many patients with mPC.
Acknowledgments
Writing assistance was provided by Aaron Runkle, PhD, MediTech Media, Ltd, through funding by Celgene Corporation. The authors were fully responsible for all content and editorial decisions for this manuscript. Funding for this study was provided by Celgene Corporation.
Previous or duplicate publication: Presented as posters at the 2016 Gastrointestinal Cancers Symposium, January 21–23, 2016, San Francisco, CA. Abstracts presented at the 2016 American Society of Clinical Oncology annual meeting, June 3–7, 2016, Chicago, IL.
Disclosure
Fadi Braiteh has served as a speaker and consultant for and received honoraria from Celgene Corporation. Claudio Faria, Monika Parisi, Manish B Patel, and Quanhong Ni are employees of Celgene Corporation. Siyeon Park reports no conflict of interest.
References
- Pancreatic Cancer Action Network: The alarming rise of pancreatic cancer deaths in the U.S.2012 Available from: https://www.pancan.org/reports/report-the-alarming-rise-of-pancreatic-cancer-deaths-in-the-u-s-2/Accessed October 3, 2016
- American Cancer SocietyCancer Facts and Figures2016 Available from: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdfAccessed October 3, 2016
- RahibLSmithBDAizenbergRRosenzweigABFleshmanJMMatrisianLMProjecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United StatesCancer Res201474112913292124840647
- Surveillance, Epidemiology, and End Results Program: SEER stat facts sheets: pancreas cancer Updated 2016. Available from: http://seer.cancer.gov/statfacts/html/pancreas.htmlAccessed October 3, 2016
- BurrisHAIIIMooreMJAndersenJImprovements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trialJ Clin Oncol1997156240324139196156
- NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma V22016 https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdfAccessed October 3, 2016
- ColucciGLabiancaRDi CostanzoFRandomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 studyJ Clin Oncol201028101645165120194854
- KindlerHLNiedzwieckiDHollisDGemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303)J Clin Oncol201028223617362220606091
- GoncalvesAGilabertMFrancoisEBAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancerAnn Oncol201223112799280522771827
- CunninghamDChauIStockenDDPhase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancerJ Clin Oncol200927335513551819858379
- PoplinEFengYBerlinJPhase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology GroupJ Clin Oncol200927233778378519581537
- Abou-AlfaGKLetourneauRHarkerGRandomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancerJ Clin Oncol200624274441444716983112
- StathopoulosGPSyrigosKAravantinosGA multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancerBr J Cancer200695558759216909140
- MooreMJGoldsteinDHammJErlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials GroupJ Clin Oncol200725151960196617452677
- ConroyTDesseigneFYchouMFOLFIRINOX versus gemcitabine for metastatic pancreatic cancerN Engl J Med2011364191817182521561347
- GoldsteinDEl-MaraghiRHHammelPNab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trialJ Natl Cancer Inst20151072dju41325638248
- Von HoffDDErvinTArenaFPIncreased survival in pancreatic cancer with nab-paclitaxel plus gemcitabineN Engl J Med2013369181691170324131140
- PeixotoRDHoMRenoufDJEligibility of metastatic pancreatic cancer patients for first-line palliative intent nab-paclitaxel plus gemcitabine versus FOLFIRINOXAm J Clin Oncol Epub201541
- HutchinsLFUngerJMCrowleyJJColtmanCAJrAlbainKSUnder-representation of patients 65 years of age or older in cancer-treatment trialsN Engl J Med1999341272061206710615079
- PeddiPFLubnerSMcWilliamsRMulti-institutional experience with FOLFIRINOX in pancreatic adenocarcinomaJOP201213549750122964956
- AbramsTAMeyerGMoloneyJPatterns of chemotherapy (CT) use in a population-based US-wide cohort of patients (pts) with metastatic pancreatic cancer (MPC)J Clin Oncol201432suppl 5 abstr 4131
- TaberneroJChioreanEGInfanteJRPrognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancerOncologist201520214315025582141
- AttardCLBrownSAlloulKMooreMJCost-effectiveness of FOLFIRINOX for first-line treatment of metastatic pancreatic cancerJ Clin Oncol201230suppl 4 abstr 199
- GhalautPSSenRDixitGRole of granulocyte colony stimulating factor (G-CSF) in chemotherapy induced neutropeniaJ Assoc Physicians India20085694294419322972
- KimGPParisiMPatelMReal world hospital costs associated with nab-paclitaxel plus gemcitabine (nab-P+G) and FOLFIRINOX (FFX) as first line (1L) treatment (tx) for metastatic pancreatic adenocarcinoma (MPAC)J Clin Oncol201634suppl abstr e15741