Abstract
Purpose
It is well demonstrated that being married is associated with a better prognosis in multiple types of cancer. However, whether the protective effect of marital status varied across race/ethnicity and gender in patients with hepatocellular carcinoma remains unclear. Therefore, we aimed to evaluate the roles of race/ethnicity and gender in this relationship.
Patients and methods
We identified eligible patients from Surveillance, Epidemiology and End Results (SEER) database during 2004–2012. Overall and cancer-specific survival differences across marital status were compared by Kaplan–Meier curves. We also estimated crude hazard ratios (CHRs) and adjusted hazard ratios (AHRs) with 95% confidence intervals (CIs) for marital status associated with survival by race/ethnicity and gender in Cox proportional hazard models.
Results
A total of 12,168 eligible patients diagnosed with hepatocellular carcinoma were included. We observed that married status was an independent protective prognostic factor for overall and cancer-specific survival. In stratified analyses by race/ethnicity, the AHR of overall mortality (unmarried vs married) was highest for Hispanic (AHR =1.25, 95% CI, 1.13–1.39; P<0.001) and lowest for Asian or Pacific Islander (AHR =1.13; 95% CI, 1.00–1.28; P=0.042). Stratified by gender, the AHR was higher in males (AHR =1.27; 95% CI, 1.20–1.33; P<0.001). Conclusion: We demonstrated that married patients obtained better survival advantages. Race/ethnicity and gender could influence the magnitude of associations between marital status and risk of mortality.
Introduction
Hepatocellular carcinoma (HCC) is the fifth frequently diagnosed malignancy for males and the ninth for females worldwide.Citation1,Citation2 Although the incidence of liver cancer is less frequent than that of breast and colorectal cancers, it is the second cause of cancer-related death and estimated to account for ~745,000 deaths in 2012.Citation1 During the past few decades, several advanced therapies including systemic chemotherapy and radiofrequency ablation have shown the modest improvement in overall survival.Citation3–Citation5 Despite those achievements, the prognosis of HCC still remained dismal with an overall 1-year survival rate of <50%.Citation6 Considering high mortality and poor prognosis of HCC, it is still urgent to reduce the risk of mortality associated with HCC.
Recently, results from considerable literature have demonstrated that married patients have favorable survival outcomes compared to the unmarried in various cancer types, such as breast, colorectal, pancreatic, gastric, and prostate cancers.Citation7–Citation14 This interesting phenomenon raised great public concerns. It is postulated that the survival benefits of marriage are associated with earlier cancer detection and receipt of definitive treatment.Citation15–Citation19 Moreover, better economic status and social support contribute to lower cancer mortality in married patients. Previously published articles also indicated that marital status was considered as a prognostic factor of better survival in liver cancer.Citation19,Citation20 Less well investigated, however, is the influence of race/ethnicity and gender in the association between being married and overall prognosis of HCC. Therefore, we performed a population-based study to fill the gap on racial and gender differences in marriage-associated survival benefits,
Patients and methods
Patient selection and data extraction
We obtained data from the Surveillance, Epidemiology, and End Results (SEER) database using the SEER*Stat 8.2.1 software. The SEER collected information from 18 population-based cancer registries from 1973 to 2012 and represented ~30% of the American population.Citation11 We identified first primary hepatocellular carcinoma who were aged ≥18 years at diagnosis between 2004 and 2012. Histological types for HCC were limited to 8,170, 8,171, 8,172, 8,173, 8,174, and 8,175 according to the International Classification of Diseases for Oncology-3 (ICD-O-3). We excluded cases diagnosed by death certificates or autopsy, or with unknown information about follow-up time, marital status, stage, and grade. We classified marital status into four groups: married, divorced/separated, widowed, and single at the time of diagnosis. Due to the similar survival disadvantages of being unmarried (divorced, separated, widowed, and single), we clustered those together as the unmarried group in further analysis. We defined race/ethnicity as non-Hispanic white (NHW), Black, Hispanic, and Asian or Pacific Islander (API). Demographic and clinical information about gender, age, histology, grade, stage, and definite therapies was extracted from the SEER database. The data accessed from SEER are freely available and do not require approval from an institutional review board or ethics committee. No personal identifying information was used in the current study; therefore, we did not require any informed consent.
Statistical analysis
Chi-square test was conducted to compare clinical characteristics with different marital statuses among hepatocellular carcinoma. Kaplan–Meier curves and log-rank tests were adopted to compare survival difference in relation to marital status. Multivariable Cox proportional hazards regressions were conducted to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for overall and cancer-specific survival among different marital statuses. Furthermore, we conducted analyses to explore advantages of being married by race and gender. All analyses were two sided, and a P-value of <0.05 indicated statistically significant. All statistical analyses were performed using the IBM SPSS Statistics, Version 20.0, and figures were created using the GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
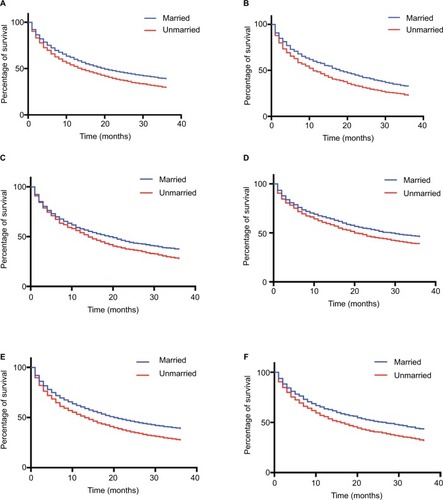
The cohort totally included 12,168 eligible cases of HCC during 2004–2012. The detailed flowchart of selection is shown in . As shown in , there were 9,355 (76.9%) males and 2,813 (23.1%) females. Among included individuals, 7,076 (58.2%) patients were married, 1,645 (13.5%) patients were divorced/separated, 1,157 (9.5%) patients were widowed, and 2,290 (18.8%) patients were single at the diagnosis (). The married rate was low in female and Black patients, and the rate decreased with the year from 2004 to 2012. Compared to unmarried groups, married patients received more surgery and radiation. In males, the percentages of unmarried patients were 39.3% for NHWs, 58.3% for Blacks, 37.7% for Hispanics, and 20.1% for APIs (). In females, the proportions were 52.6%, 74.6%, 58.1%, and 40.4%, respectively ().
Table 1 Baseline clinicopathological characteristics of patients with hepatocellular carcinoma in SEER database
Table 2 Baseline demographic characteristics of male patients stratified by race (%)
Table 3 Baseline demographic characteristics of female patients stratified by race (%)
Figure 1 Flowchart for included patients from the Surveillance, Epidemiology, and End Results database.
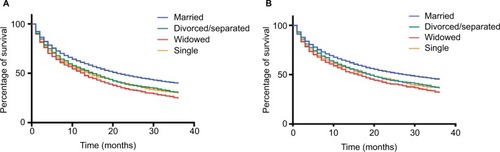
As shown in , the significant difference of overall and cancer-specific mortality was observed between married groups and unmarried groups (divorced/separated, widowed, and single) (both log-rank test P<0.0001). In multivariate Cox regression models, unmarried status was associated with higher risk of overall mortality (the married as reference, divorced/separated, 1.20, 95% CI, 1.13–1.28; widowed, 1.17, 95%CI, 1.09–1.26; single, 1.25, 95% CI, 1.18–1.32) (), and similar results were found when cancer-specific survival was analyzed (). In addition to marital status, other variables such as age, gender, year, race, grade, stage, surgery, and radiation were identified as prognostic factors.
Table 4 Univariate and multivariate analyses of OS in patients with HCC
Table 5 Univariate and multivariate analyses of cancer-specific survival in patients with HCC
Figure 2 Kaplan–Meier survival curves according to marital status (married, divorced/separated, widowed, and single) in patients with hepatocellular carcinoma.
Notes: (A) Overall survival. (B) cancer-specific survival.
Subsequently, we performed stratified analysis of overall mortality by race/ethnicity and gender. The influence of marital status on overall survival was consistent among race/ethnicity and gender, though the magnitude of the association varied (). For both race/ethnicity and gender, unmarried individuals were more likely to be inferior to married individuals in overall survival (). For different race/ethnicity, the HR of being unmarried was the largest in Hispanic (adjusted HR [AHR], 1.25, 95% CI, 1.13, 1.39), followed by Black (AHR, 1.20, 95% CI, 1.07, 1.35) and NHW (AHR, 1.19, 95% CI, 1.12, 1.27), while HR in API was the smallest (AHR 1.13; 95% CI, 1.00–1.28). As for gender, the influence of being married on prognosis was greater in males (AHR, 1.27; 95% CI, 1.20–1.33), whereas less effect was observed in females (AHR 1.12; 95% CI, 1.02–1.23).
Table 6 Crude and adjusted HRs for overall survival associated with marital status (unmarried vs married) by gender and race
Discussion
Previous studies had demonstrated that married patients were more likely to possess better prognosis of primary liver cancer.Citation19,Citation20 However, to date, survival differences of marital status stratified by race/ethnicity and gender had not been adequately investigated. Therefore, we conducted this population-based study to explore whether race and gender differences could influence the impact of marital status on the prognosis. Our results confirmed previous results that married patients experienced a lower risk of overall and cancer-specific mortality than unmarried patients. Furthermore, we observed variations in the association of being married and prognosis across race/ethnicity and gender. For different races/ethnicities, the association between being married and survival was stronger in Hispanic patients and was weaker in Asian or Pacific Islander patients, which indicated that unmarried Hispanic patients were at the highest risk of mortality in relation to other groups. Compared to males, the impact of being married on overall survival attenuated in females. Although the association between marriage and survival benefits was consistent, it should be noted that the magnitude of this association varied across race/ethnicity and gender. Thus, gender and race/ethnicity might partly explain the influence of marital status on overall survival.
Differences in the relationship between marital status and mortality by race and gender may be attributable to several reasons. First, married patients possessed more financial resources, such as greater income, better employment, and insurance, which ultimately influence the access to early diagnosis and timely medical care.Citation15 Second, social supports also contributed to a better prognosis. It was well documented that depression and stress were associated with tumor progression and metastasis.Citation21–Citation24 Compared to unmarried counterparts, married patients displayed less distress and depression after diagnosis of cancer because their spouses shared the mental burden and provided them sufficient social support.Citation25,Citation26 Goodwin et alCitation27 demonstrated that females with depression experienced a worse survival after a diagnosis of breast cancer. Conversely, breast cancer patients with emotional support enjoyed increased survival.Citation28 It has been well documented that stress and depression would impair the immune function and lead to worse prognosis.Citation22,Citation29 Moreover, dysregulation of various hormones induced by psychological factors, such as cortisol and norepinephrine,Citation22,Citation30 weakens immune systems by suppressing counts and functions of natural killer cells.Citation31,Citation32
Inevitably, there were several potential limitations in our study. First, some important information, such as chemotherapy, subsequent therapy, and comorbidities such as HBV infection, was not available in the SEER database. Meanwhile, socioeconomic status of patients also influenced the cancer prognosis. We could not adjust these factors for survival. Second, since marital status was recorded at the diagnosis, we lack data regarding changes in marital status after diagnosis, which may affect the results. Third, as a retrospective research, it was inevitable and liable to introduce some confounders into studies. Given these limitations, the results should be interpreted with caution.
Conclusion
Notwithstanding these potential limitations, our study demonstrated that being married at the time of diagnosis had a lower risk of mortality across HCC, though this association varied across race/ethnicity and gender. In the consideration of decreased rates of married status, more social support and comprehensive interventions should be given to these populations.
Acknowledgments
This study was funded by the Key Program of the National Natural Science Foundation of China (No 81330011) and the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (No 81121002). The authors acknowledge the efforts of the SEER Program tumor registries in the creation of the SEER database.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- AltekruseSFMcGlynnKAReichmanMEHepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005J Clin Oncol20092791485149119224838
- LlovetJMBruixJSystematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survivalHepatology200337242944212540794
- TiongLMaddernGJSystematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinomaBr J Surg20119891210122421766289
- DhanasekaranRKoobyDAStaleyCAKauhJSKhannaVKimHSComparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC)J Surg Oncol2010101647648020213741
- ShawJJShahSARising incidence and demographics of hepatocellular carcinoma in the USA: what does it mean?Expert Rev Gastroenterol Hepatol20115336537021651354
- BaineMSahakFLinCChakrabortySLydenEBatraSKMarital status and survival in pancreatic cancer patients: a SEER based analysisPLoS One201166e2105221698253
- AbdollahFSunMThuretRThe effect of marital status on stage and survival of prostate cancer patients treated with radical prostatectomy: a population-based studyCancer Causes Control20112281085109521643929
- OsborneCOstirGVDuXPeekMKGoodwinJSThe influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancerBreast Cancer Res Treat2005931414716184457
- QiuMYangDXuRImpact of marital status on survival of gastric adenocarcinoma patients: results from the surveillance epidemiology and end results (SEER) databaseSci Rep201662109826876653
- LiQGanLLiangLLiXCaiSThe influence of marital status on stage at diagnosis and survival of patients with colorectal cancerOncotarget2015697339734725749515
- ChenDNSongCGOuyangQWDifferences in breast cancer characteristics and outcomes between Caucasian and Chinese women in the USOncotarget2015614127741278225904050
- XiaoWJZhuYDaiBConditional survival among patients with adrenal cortical carcinoma determined using a national population-based surveillance, epidemiology, and end results registryOncotarget2015642449554496226510907
- ShiRLChenQYangZMarital status independently predicts gastric cancer survival after surgical resection – an analysis of the SEER databaseOncotarget2016711132281323526840093
- AizerAAChenMHMcCarthyEPMarital status and survival in patients with cancerJ Clin Oncol201331313869387624062405
- PinquartMDubersteinPRAssociations of social networks with cancer mortality: a meta-analysisCrit Rev Oncol Hematol201075212213719604706
- WangLWilsonSEStewartDBHollenbeakCSMarital status and colon cancer outcomes in US surveillance, epidemiology and end results registries: does marriage affect cancer survival by gender and stage?Cancer Epidemiol201135541742221466984
- WangXDQianJJBaiDSLiZNJiangGQYaoJMarital status independently predicts pancreatic cancer survival in patients treated with surgical resection: an analysis of the SEER databaseOncotarget2016717248802488727036036
- HeXKLinZHQianYXiaDJinPSunLMMarital status and survival in patients with primary liver cancerOncotarget2016839649546496329029403
- WuCChenPQianJJEffect of marital status on the survival of patients with hepatocellular carcinoma treated with surgical resection: an analysis of 13,408 patients in the surveillance, epidemiology, and end results (SEER) databaseOncotarget2016748794427945227769053
- PowellNDTarrAJSheridanJFPsychosocial stress and inflammation in cancerBrain Behav Immun201330SupplS41S4722790082
- Moreno-SmithMLutgendorfSKSoodAKImpact of stress on cancer metastasisFuture Oncol20106121863188121142861
- ReicheEMNunesSOMorimotoHKStress, depression, the immune system, and cancerLancet Oncol200451061762515465465
- TongGGengQChengJEffects of psycho-behavioral interventions on immune functioning in cancer patients: a systematic reviewJ Cancer Res Clin Oncol20141401153324037489
- CairneyJBoyleMOffordDRRacineYStress, social support and depression in single and married mothersSoc Psychiatry Psychiatr Epidemiol200338844244912910340
- GoldzweigGAndritschEHubertAPsychological distress among male patients and male spouses: what do oncologists need to know?Ann Oncol201021487788319822532
- GoodwinJSZhangDDOstirGVEffect of depression on diagnosis, treatment, and survival of older women with breast cancerJ Am Geriatr Soc200452110611114687323
- Soler-VilaHKaslSVJonesBAPrognostic significance of psychosocial factors in African-American and white breast cancer patients: a population-based studyCancer20039861299130812973855
- GarssenBGoodkinKOn the role of immunological factors as mediators between psychosocial factors and cancer progressionPsychiatry Res1999851516110195316
- SoodAKLutgendorfSKStress influences on anoikisCancer Prev Res (Phila)20114448148521464029
- SephtonSELushEDedertEADiurnal cortisol rhythm as a predictor of lung cancer survivalBrain Behav Immun201330SupplS163S17022884416
- SephtonSESapolskyRMKraemerHCSpiegelDDiurnal cortisol rhythm as a predictor of breast cancer survivalJ Natl Cancer Inst20009212994100010861311