Abstract
Aims and objectives
The past 2 decades witnessed the strengthening of evidence favoring the role of neoadjuvant chemoradiation (CHRT) in the treatment of locally advanced rectal cancer. The study aims to evaluate the response and acute toxicities to neoadjuvant CHRT using intensity-modulated radiotherapy (IMRT) in the treatment of rectal cancer. Predictive factors to achieve pathological complete response (pCR) were analyzed, as a secondary endpoint.
Materials and methods
All consecutive patients who underwent IMRT as part of neoadjuvant CHRT in the treatment of rectal cancer between August 2014 and December 2016 at a tertiary cancer care center were accrued for the study. The cohort underwent CHRT with IMRT technique at a dose of 50.4 Gy in 28 fractions concurrent with continuous infusion of 5 fluorouracil during the first and the last 4 days of CHRT. Surgery was performed 6 weeks later and the pathological response to CHRT was noted.
Results
Forty-three subjects were accrued for the study. Radiation dermatitis and diarrhea were the only observed grade ≥3 acute toxicities. Sphincter preservation rate (SPR) was 43.3%. pCR was observed in 32.6%. Univariate and multivariate logistic regression showed that carcinoembryonic antigen was the only independent predictive factor to achieve pCR.
Conclusion
IMRT as part of neoadjuvant CHRT in the treatment of locally advanced rectal cancer is well tolerated and gives comparable results with respect to earlier studies in terms of pathological response and SPR. Further randomized controlled studies are needed to firmly state that IMRT is superior to 3-dimensional conformal radiotherapy.
Introduction
The past decade has seen preoperative concurrent chemoradiation (CHRT) followed by surgery 6 weeks later as the dominating trend in the management of carcinoma of the rectum.Citation1 Despite the fact that there are benefits of preoperative radiotherapy, the toxicities associated with the conventional broad fields or improper radiation techniques at some places were of significant concern for the oncologists.Citation2
The past 2 decades have also seen the emergence of intensity-modulated radiotherapy (IMRT) along with gradual replacement of conventional techniques. IMRT hails with advantages of lower doses delivered to the organs at risk (OARs) as it makes the dose cloud or isodose lines conform to the shape of the volume of interest. The reduced dose to OARs like small bowel and urinary bladder translates into decreased toxicities, both acute and late, as seen in studies involving pelvic malignancies such as cervical, endometrial, and prostate cancers.Citation3–Citation5 However, this is derived largely from prospective and retrospective data rather than planned randomized studies comparing IMRT vs conventional techniques.
Materials and methods
After the ethical board approval (Institutional Review Board, Rajiv Gandhi Cancer Hospital and Research Centre, Delhi, India) of the prospective observational study, all patients undergoing CHRT for locally advanced rectal cancers were evaluated for response and toxicity profile. All consecutive patients attending the radiation department of a tertiary care cancer hospital opting for IMRT technique between August 2014 and December 2016 were accrued for this study. Written informed consent was obtained from all the patients who participated in this study. The cohort underwent whole abdomen MRI with contrast and serum carcinoembryonic antigen (CEA) levels prior to the treatment.
Patients were immobilized with the help of orfit-ray™ (Orfit Industries, Wijnegem, Belgium) thermoplastic cast and CT simulation was performed with SOMATOM sensation open™ (Global Siemens Healthcare, Erlanger, Germany) in supine position. Contrast-enhanced CT scans were performed with 3 mm slice thickness along with bladder protocol (the patient is asked to void and then drink 700 mL water and the scan is performed on having the sensation to pass urine). Contouring was done using Varian Eclipse™ Version 10 (Varian Medical Systems, Palo Alto, CA, USA) according to the Radiation Therapy Oncology Group (RTOG) guidelines.Citation6 Conventional fractionation IMRT was used (total dose of 50.4 Gy with daily fractions of 1.8 Gy, 5 days a week) along with concurrent chemotherapy with 5 fluorouracil (5FU) 1000 mg/m2 in continuous infusion during the first and last 4 days of radiation. Patients were assessed weekly for acute toxicities such as skin reactions, vomiting, cystitis, diarrhea, and hematological toxicities. RTOG scoring scale was used to grade acute toxicities.Citation7
At 6 weeks post-CHRT, contrast MRI-based response evaluation was done using RECIST 1.1 criteria.Citation8 Surgery – either low anterior resection (LAR) or abdominoperineal resection – was performed, and pathological response to neoadjuvant CHRT was graded according to the College of American Pathologist guidelines.Citation9 The primary endpoint was to evaluate down-staging and pathological response to CHRT. The secondary endpoint was to find out factors predictive for pathological complete response (pCR) to CHRT.
Statistical analysis
The descriptive statistics for quantitative variables are presented using mean (with SD), while categorical variables are presented in frequencies along with respective percentages. To compare categorical variables, Chi-square test or Fisher’s exact test was used according to the nature of data. Univariate and multivariate logistic regression were used to identify the associated independent predictive factors to achieve pCR. A p-value <0.05 was considered statistically significant. All p-values reported are two-tailed. Statistical Package for the Social Sciences version 20.0 (IBM Corporation, Armonk, NY, USA) was used to carry out all statistical computations.
Results
Forty-three patients were treated with CHRT followed by surgery after 6 weeks. The pretreatment clinical characteristics have been tabulated in . The mean dose received by 95% of planning target volume was 50.17 ± 0.39 Gy. Mean volume of small bowel that received dose >45 Gy (SBV45 Gy) was 78.79 ± 48.38 cc, while mean volume of urinary bladder receiving >50 Gy was 24.73 ± 7.93%. Diarrhea and radiation dermatitis were the only observed grade ≥3 acute toxicities (). The relationship between diarrhea and SBV45 Gy has been depicted in . It was noted that acute diarrheal toxicity of grades ≥3 was experienced when the SBV45 Gy exceeded 120 cc.
Table 1 Pretreatment clinical characteristics
Table 2 Acute toxicities
Table 3 The relationship between grades of diarrhea and SBV45 Gy
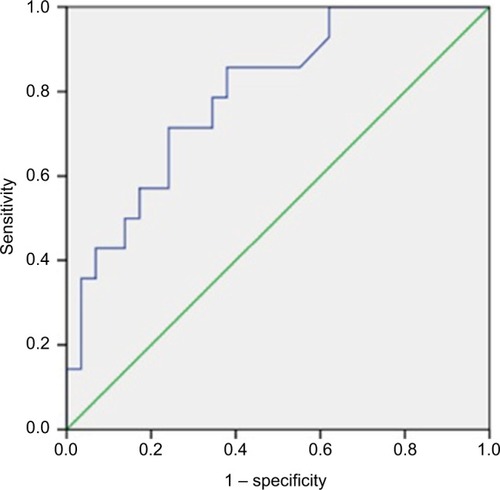
All patients underwent concurrent chemotherapy with continuous 5FU infusion. Chemotherapy dose reduction was done in 11.6% of patients during the second cycle in view of toxicities. Radiological response assessment with MRI at 6 weeks showed complete response [CR] in only one patient. Of the 30 lower rectal tumors (<5 cm from anal verge), 13 patients underwent LAR, and hence, sphincter preservation rate (SPR) was 43.3%. Pathological response assessment showed complete pathological response in 32.60%, moderate response in 30.20%, minimal response in 23.3%, and poor response in 14%. The details of down-staging (both “T” and “N” stages) have been tabulated in and . Univariate and multivariate logistic regression showed that pretreatment CEA level was the only independent predictive factor of pCR (). Additionally, a cutoff value of pretreatment CEA of ≤4.80 ng/mL (sensitivity – 71.4%; specificity – 75.9%) was derived using receiver operating characteristic (ROC) curve to predict pCR (). Moreover, it was observed that the pretreatment CEA level was significantly associated with the length of the disease and overall clinical stage (). The outcomes of different CHRT studies using IMRT are summarized in .
Table 4 Pathological down-staging – T stage
Table 5 Pathological down-staging – N stage
Table 6 Univariate and multivariate analyses with pCR as a dependent variable
Table 7 Relationship of preoperative CEA level with patient and disease-related characteristics
Table 8 Summary of studies which used IMRT for the treatment of rectal cancer
Discussion
Clinical results of IMRT for rectal cancer as a part of CHRT have not been adequately documented worldwide. The studies mentioned in the literature are difficult to compare, as the protocols of both radiotherapy (total dose, boost technique, and dose per fraction) and chemotherapy vary between studies. The results of various studies using IMRT as part of CHRT are summarized in .
Grade ≥3 skin toxicities varied from 0.03% to 21% among different IMRT studies, compared to 12% in the present study (). Dermatitis was observed predominantly over the medial thigh and perineum. This occurred more in lesions of the lower rectum where doses were delivered up to or beyond the anal verge.
One of the advantages of IMRT over 3-dimensional conformal radiotherapy (3DCRT) lies in its ability to spare the small bowel. In a study conducted by Yang et al, the cases with grade ≥2 diarrhea were higher in rectal cancer patients who were treated with 3DCRT as part of CHRT in contrast to IMRT (32% vs 11%).Citation27 Concurrently, RTOG 0822 (which was a Phase II trial using IMRT) did not result in significant difference in gastrointestinal toxicities.Citation10 Nevertheless, this was a single-arm study, with IMRT as part of CHRT and the outcomes were compared with the results of RTOG 0247.Citation30 Grade ≥3 diarrhea was experienced by 9.3% of the patients (4/43) in the present study, while the same has been reported between 1% and 18% among other IMRT studies (). It was noted that acute diarrheal toxicity grade ≥3 was experienced when the SBV45 Gy exceeded 120 cc. This is lower if compared to the QUANTEC guidelines, suggesting SBV45 Gy as 195 cc. This difference in the values could be a result of the variations in the ethnicity, contouring, planning, beam angles, and diets.
Compared to small bowel, urinary bladder has more tolerance toward radiation. Even though IMRT has more dosimetric avoidance of urinary bladder than 3DCRT, it is unlikely to reflect clinically. All patients in our study had only grade 1 cystitis. Except for one study conducted by Hernando-Requejo et al, showing grade ≥3 genitourinary (GU) toxicities of up to 5%, the remaining IMRT series failed to identify any grade ≥3 GU toxicities.Citation12,Citation16,Citation18,Citation25,Citation26 Similarly, grade ≥3 hematological toxicities are less in almost all the IMRT studies, ranging between 0%–6%.Citation14–Citation17,Citation22,Citation24,Citation26,Citation28 Nonetheless, another study which is a Phase I trial using hypofractionated IMRT in rectal cancer showed 13% grade ≥3 anemia. This study was discontinued due to toxicities. Apart from two cases (4.7%) of neutropenia, no other grade ≥2 hematological toxicities were observed in the present study.
Despite toxicity reduction, sphincter preservation is another advantage of CHRT which converts abdominoperineal resection candidates to LAR ones. The German rectal cancer study demonstrated SPR of 39% in those who underwent preoperative CHRT by the conventional technique, while the present study showed 43.3%.Citation1 Yet another study using IMRT by helical tomotherapy achieved an SPR of 85.2%.Citation16
pCR rates range from 0%–50% among various IMRT studies.Citation12,Citation22,Citation24,Citation25,Citation28 This wide range might be due to varying radiation doses, dose per fraction, chemotherapy regimen used, and certain factors of tumor biology are still unexplored and under research. Among these, Cubillo et al, who delivered equivalent 2 Gy per fraction (EQD2) of 60.4 Gy using the simultaneous integrated boost technique along with bevacizumab or cetuximab, achieved a pCR rate of 50%.Citation13 As depicted in , only three studies have delivered EQD2 dose of 49.4 Gy (1.8 Gy per fraction to the tumor) without any boost.Citation21,Citation26,Citation30 Of these, only one study has used 5FU or capecitabine and reported a pCR rate of 19.4%, compared to 32.6% in the present study.Citation30
Predictors to achieve pCR have been extensively studied. A meta-analysis demonstrated interval to surgery as the independent variable to achieve the same.Citation31 Another meta-analysis evaluated the role of pretreatment CEA level and concluded that a normal level of CEA predicted more pCR.Citation32 The present study nevertheless kept one variable as a constant, as the interval to surgery was at a mean of 45 days with an SD of 3 days. The current study suggests that the independent predictive factor to achieve pCR is the pretreatment CEA level when surgery is performed at a mean interval of 45 days. Further, an ROC curve showed pretreatment CEA of ≤4.80 ng/mL (sensitivity – 71.4%; specificity – 75.9%) as a cutoff value to predict pCR (). It was also noted that the rectal cancer patients with high pretreatment CEA levels (>5 ng/mL) demonstrated a significant association with increasing length of the disease and overall clinical stage (). Similarly, Filiz et al demonstrated a statistically significant correlation between preoperative serum CEA levels and overall clinical stage.Citation33 However, the authors did not find any relationship with the length of the disease. The data from this study should be interpreted with caution as the number of patients was small.
Conclusion
IMRT as part of neoadjuvant CHRT in the treatment of locally advanced rectal cancer is well tolerated and gives comparable results with earlier studies in terms of pathological response, acute toxicities, and SPR. Pretreatment CEA turned out to be the independent predictor to achieve pCR when surgery was performed at a mean interval of 45 days. Further randomized controlled studies are needed to categorically state that IMRT is superior to 3DCRT.
Acknowledgments
The authors thank Dr DK Shukla (ICMR, Delhi) for his statistical analysis of this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- SauerRBeckerHHohenbergerWPreoperative versus postoperative chemoradiotherapy for rectal cancerN Engl J Med2004351171731174015496622
- BossetJFMeneveauNPavyJJLes complications intestinales tardives de la radiothérapie adjuvante des cancers rectaux [Late intestinal complications of adjuvant radiotherapy of rectal cancers]Cancer Radiother199716770774 French9614893
- NuttingCMConveryDJCosgroveVPRowbottomCPadhaniARWebbSDearnaleyDPReduction of small and large bowel irradiation using an optimized intensity-modulated pelvic radiotherapy technique in patients with prostate cancerInt J Radiat Oncol Biol Phys200048364965611020560
- PortelanceLChaoKSGrigsbyPWBennetHLowDIntensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and paraaortic irradiationInt J Radiat Oncol Biol Phys200151126126611516876
- RoeskeJCLujanARotmenschJWaggonerSEYamadaDMundtAJIntensity-modulated whole pelvic radiation therapy in patients with gynecologic malignanciesInt J Radiat Oncol Biol Phys20004851613162111121668
- MyersonRJGarofaloMCEl NaqaIElective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlasInt J Radiat Oncol Biol Phys200974382483019117696
- CoxJDStetzJPajakTFToxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC)Int J Radiat Oncol Biol Phys1995315134113467713792
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer2008452228247
- WashingtonMKBerlinJBrantonPProtocol for the examination of specimens from patients with primary carcinoma of the colon and rectumArch Pathol Lab Med2009133101539155119792043
- HongTSMoughanJGarofaloMCNRG Oncology Radiation Therapy Oncology Group 0822: a Phase 2 study of preoperative chemoradiation therapy using intensity modulated radiation therapy in combination with capecitabine and oxaliplatin for patients with locally advanced rectal cancerInt J Radiat Oncol Biol Phys2015931293626163334
- EngelsBPlatteauxNVan den BeginRPreoperative intensity-modulated and image-guided radiotherapy with a simultaneous integrated boost in locally advanced rectal cancer: report on late toxicity and outcomeRadiother Oncol2013110115515924239243
- Hernando-RequejoOLopezMCubilloAComplete pathological responses in locally advanced rectal cancer after preoperative IMRT and integrated-boost chemoradiationStrahlenther Onkol2014190651552024715243
- CubilloAHernando-RequejoOGarcia-GarciaEA prospective pilot study of target-guided personalized chemotherapy with intensity-modulated radiotherapy in patients with early rectal cancerAm J Clin Oncol2012372117121
- ZhuJLiuFGuWConcomitant boost IMRT-based neoadjuvant chemoradiotherapy for clinical stage II/III rectal adenocarcinoma: results of a phase II studyRadiat Oncol201497024606870
- ZhuJLianPLiuFPhase II trial of first-line chemoradiotherapy with intensity-modulated radiation therapy followed by chemotherapy for synchronous unresectable distant metastases rectal adenocarcinomaRadiat Oncol201381023295152
- HuangMYChenCFHuangCMHelical tomotherapy combined with capecitabine in the preoperative treatment of locally advanced rectal cancerBiomed Res Int2014201435208324949438
- ArbeaLMartinez-MongeRDiaz-GonzalezJAFour-week neoadjuvant intensity-modulated radiation therapy with concurrent capecitabine and oxaliplatin in locally advanced rectal cancer patients: a validation phase II trialInt J Radiat Oncol Biol Phys201183258759322079731
- AristuJJArbeaLRodriguezJPhase I-II trial of concurrent capecitabine and oxaliplatin with preoperative intensity-modulated radiotherapy in patients with locally advanced rectal cancerInt J Radiat Oncol Biol Phys200871374875518164861
- BallonoffAKavanaghBMcCarterMPreoperative capecitabine and accelerated intensity-modulated radiotherapy in locally advanced rectal cancer: a phase II trialAm J Clin Oncol200831326427018525306
- FreedmanGMMeropolNJSigurdsonERPhase I trial of preoperative hypofractionated intensity-modulated radiotherapy with incorporated boost and oral capecitabine in locally advanced rectal cancerInt J Radiat Oncol Biol Phys20076751389139317394942
- Gasent BlesaJMGarde NogueraJLaforga CanalesJBPhase II trial of concomitant neoadjuvant chemotherapy with oxaliplatin and capecitabine and intensity-modulated radiotherapy (IMRT) in rectal cancerJ Gastrointest Cancer201243455356122371167
- LiJLJiJFCaiYPreoperative concomitant boost intensity-modulated radiotherapy with oral capecitabine in locally advanced mid-low rectal cancer: a phase II trialRadiother Oncol201110214921903285
- RichettiAFogliataAClivioANeo-adjuvant chemo-radiation of rectal cancer with volumetric modulated arc therapy: summary of technical and dosimetric features and early clinical experienceRadiat Oncol201051420170490
- ZhuJGuWLianPA phase II trial of neoadjuvant IMRT-based chemoradiotherapy followed by one cycle of capecitabine for stage II/ III rectal adenocarcinomaRadiat Oncol2013813023718210
- ParekhATruongMTPashtanIAcute gastrointestinal toxicity and tumor response with preoperative intensity modulated radiation therapy for rectal cancerGastrointest Cancer Res201365–613714324312687
- JabbourSKPatelSHermanJMIntensity-modulated radiation therapy for rectal carcinoma can reduce treatment breaks and emergency department visitsInt J Surg Oncol2012201289106722934164
- YangTJOhJHSonCHApteADeasyJOWuAGoodmanKAPredictors of acute gastrointestinal toxicity during pelvic chemoradiotherapy in patients with rectal cancerGastrointest Cancer Res201365–612913624312686
- SamuelianJMCallisterMDAshmanJBYoung-FadokTMBoradMJGundersonLLReduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancerInt J Radiat Oncol Biol Phys20128251981198721477938
- NgSYColbornKLCambridgeLHajjCYangTJWuAJGoodmanAKAcute toxicity with intensity modulated radiotherapy versus 3-dimensional conformal radiotherapy during preoperative chemoradiation for locally advanced rectal cancerRadiother Oncol2016121225225727751605
- WongSJWinterKMeropolNJRadiation Therapy Oncology Group 0247: a randomized Phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancerInt J Radiat Oncol Biol Phys20128241367137521775070
- PetrelliFSgroiGSartiEBarniSIncreasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studiesAnn Surg2016263345846424263329
- YuHChenWCaiYPretreatment serum CEA as a predictive biomarker for the response to neoadjuvant chemoradiotherapy: a meta-analysis in rectal cancerJ Carcinog Mutagen20156237
- FilizAISuculluIKurtYKarakasDOGulecBAkinMLPersistent high postoperative carcinoembryonic antigen in colorectal cancer patients – is it important?Clinics (Sao Paulo)200964428729419488584