Abstract
Background
Eukaryotic initiation factor 3 subunit d (eIF3d) is the largest subunit of eIF3, which is shown to promote protein synthesis in cancer cells. Increased expression of eIF3d has been shown in some types of cancers, but has not been previously studied in gastric cancer (GC). Thus, the aim of this study was to analyze eIF3d expression in GC.
Patients and methods
Expression of eIF3d was detected by immunohistochemistry in GC tissues and adjacent noncancerous (ANC) tissues. Samples were obtained from 210 patients with GC who had received curative gastrectomy. Clinicopathological features and survival rate were also analyzed.
Results
Expression rates of eIF3d in GC and ANC were 45.2% and 21.0%, respectively. High expression of eIF3d protein was significantly related to tumor stage, as determined by lymph node metastasis and depth of invasion (p<0.05). The Kaplan–Meier survival curves showed that patients with high eIF3d expression had a significantly poor overall survival (p=0.005). Multivariate Cox regression analyses showed that the level of eIF3d was an independent predictive factor of poor prognosis for GC (p=0.017).
Conclusion
Expression of eIF3d was upregulated in GC. High expression of eIF3d was determined as an independent poor prognostic factor in GC. It is suggested that eIF3d could be a good biomarker in GC.
Introduction
Gastric cancer (GC) remains one of the leading causes of cancer-related mortality worldwide.Citation1 Although great improvements have been made in the treatment of GC, five-year survival rates have remained very low, approximating 20%, due to the tendency for early invasion and metastasis.Citation2 Clinically, TNM stage is predominantly used to predict the outcome of GC patients. However, growing evidence has suggested that patients with the same stage might have vastly different prognoses due to the heterogeneity of tumors.Citation3,Citation4 Therefore, it is urgent to find useful biomarkers to refine risk stratification and survival prognosis.
Eukaryotic initiation factor 3 (eIF3) is a protein complex involved in the initiation pathway. Functional eIF3 binds to the 40S ribosomal subunit and promotes the formation of the 40S initiation complex.Citation6,Citation7 It has been shown that eIF3 is the largest initiation factor composed of 13 non-identical subunits denoted as eIF3a-m.Citation5–Citation7 Several eIF3 subunits, such as eIF3a, eIF3b, eIF3c, eIF3h, eIF3i, and eIF3e, have been demonstrated to promote cell proliferation by initiating protein translation in cancers.Citation8 In addition, eIF3f subunit has been shown to be downregulated in cancers.Citation9 Recent studies have reported that deregulation of eIF3 subunits is implicated in tumorigenesis.Citation10–Citation12
The eIF3 subunit d (eIF3d), categorized as the largest subunit of eIF3, is crucial for the functional activity of eIF3.Citation13,Citation14 Recent studies have reported eIF3d over expression in several malignant tumors, including prostate cancer,Citation15 colon cancer,Citation16 and melanoma.Citation17 The eIF3d has also been identified as a potential therapeutic target in several cancer types.Citation17–Citation19 Similarly, Kim et al identified eIF3d as a predictive gene in GC. This information might be useful in establishing resistance against a combined treatment of cisplatin and fluorouracil.Citation20
The expression of eIF3d in human GC tissue and its clinical significance have not been reported in the literature. In this study, we analyzed eIF3d expression in primary GC from 210 Chinese patients by immunohistochemical analysis and investigated the relationship of eIF3d and various clinicopathological factors.
Patients and methods
Specimen source and patient information
A total of 210 patients with GC who had received curative gastrectomy in the Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, China, between January 2006 and October 2010 were enrolled in this study. Patients, age ranged from 22 to 87 years (mean age, 59.6 years). The mean follow-up period was 40.96 months with a range from 2 to 89 months. The TNM stage of tumors was evaluated according to the 7th edition of the TNM Classification of GC.Citation22 No patient underwent radiotherapy or chemotherapy before surgery. The 210 GC tissues and 195 adjacent noncancerous (ANC) tissues were obtained from these specimens for immunohistochemical staining. All these specimens were fixed with 10% formaldehyde and embedded with paraffin, and were examined by a pathologist to confirm malignancy. The above cases were approved by the ethics committee of Huadong Hospital and informed consent forms were signed.
Immunostaining analysis
A polyclonal antibody against eIF3d (ab155419; Abcam, Cambridge, MA, USA) was used in this study. Specimens were sectioned into 3- to 4-μm slices. After routine xylene dewaxing and gradient ethanol hydration, the slides were blocked with 3% hydrogen peroxide for 10 min. After using the microwave antigen repair method, the slides were incubated with the anti-eIF3d Ab (diluted 1:40 in phosphate buffered saline [PBS]) at 4°C for 24 h. Sections were washed thrice with PBS, followed by the addition of diaminobenzidine for 6 min. Slides were independently evaluated by two pathologists who were blinded to clinical data. The level of eIF3d was scored not only by staining intensity but also by the percentage of cells that exhibit eIF3d. The staining intensity was scored as 0 (no staining), 1 (weak), 2 (moderate), or 3 (intense staining). The percentage of positive cells was scored as follows: 0 (≤5%), 1 (6% to 25%), 2 (26% to 50%), and 3 (>50%). The total of the above two scores was graded as follows: 0 (score 0), 1 (score 1–2), 2 (score 3–4), and 3 (score 5–6), where the total scores of 0 or 1 were designated as low expression and 2 or 3 were designated as high expression.Citation21
Statistical analysis
The SPSS 19 software package (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Student’s t-test was used for continuous variables, whereas c2 test or Fisher’s exact test was used for proportions. The Kaplan–Meier method was used for survival analyses and the Cox regression model was used for prognostic factors, such as eIF3d and other clinicopathological features.
Results
Expression of eIF3d in GC tissues
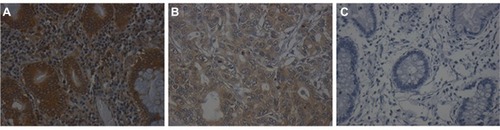
The eIF3d staining results predominantly showed cytoplasmic staining in both the GC tissues and the ANC tissues (). However, a sharp difference was found when eIF3d expression in GC was compared with that of ANC. The high eIF3d expression rate in GC tissues was 45.2% (95/210) while that in ANC tissues was 21.0% (41/195; p<0.001).
Figure 1 The expression of eIF3d in gastric cancer tissues and adjacent noncancerous tissues.
Abbreviation: eIF3d, eukaryotic initiation factor 3 subunit d.
eIF3d expression and clinicopathological features
Expression of eIF3d (high expression in 95 patients; low expression in 115 patients) and clinicopathological features were analyzed. summarizes the positive correlates of upregulated eIF3d expression with lymph node metastasis (p=0.041), tumor depth (p=0.007), and late TNM stage (III/IV) (p=0.027). The data indicate that eIF3d did not correlate significantly with gender (p=0.217), age (p=0.724), primary tumor location (p=0.403), or lymphovascular invasion (p=0.312).
Table 1 Relationship of eIF3d expression with clinicopathological parameters of tumor
eIF3d as a predictor of overall survival in GC patients
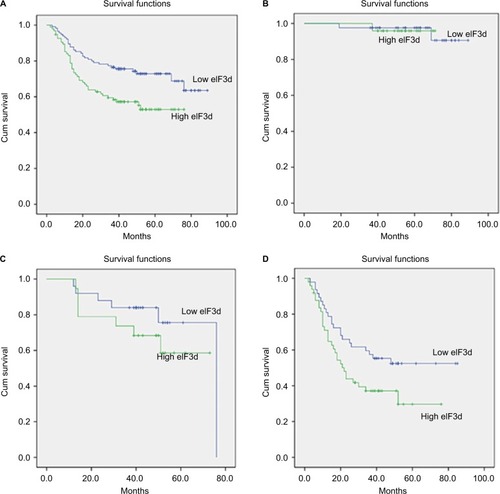
Kaplan–Meier survival curves showed that the cumulative survival rates were 75.6% (3 years) and 72.9% (5 years) in patients with lower eIF3d expression, and 59.5% (3 years) and 52.9% (5 years) in those with higher eIF3d expression. The overexpression of eIF3d was significantly correlated with poor overall survival of GC patients (p=0.005). The data were divided into subgroups according to the TNM stage. The 5-year survival rates were 97.6% and 95.8% in low and high eIF3d patients, respectively (p=0.925) in stage I and 75.6% and 58.6%, respectively (p=0.274) in stage II. However, in stage III, the 5-year survival rate in low eIF3d patients was significantly higher than that of high eIF3d patients (52.4% vs. 29.7%, p=0.046; ). Furthermore, multivariate survival analysis was performed using the Cox multivariate regression model. The results revealed that eIF3d levels (HR=1.804; 95% CI: 1.112–2.926; p=0.017), lymph nodes metastasis (HR=3.082; 95% CI: 1.101–8.629; p=0.032), distant metastasis (HR=3.776; 95% CI: 1.362–10.464; p=0.007), lymphovascular invasion (HR=1.817; 95% CI: 1.086–3.041; p=0.023), and late TNM stage (HR = 2.886; 95% CI: 1.374–6.509; p=0.005) were statistically independent forecasting factors of adverse outcome for GC ( and ).
Table 2 Univariate analysis of the correlation between clinicopathological parameters and survival time in patients with gastric cancer
Table 3 Multivariate analysis of the correlation between clinicopathological parameters and survival time in patients with gastric cancer
Figure 2 Kaplan–Meier analysis for overall survival of patients with gastric cancer according to eIF3d expression.
Abbreviations: Cum, cumulative; eIF3d, eukaryotic initiation factor 3 subunit d.
Discussion
GC is a highly heterogeneous disease with poor clinical outcomes. The traditional stage classification systems have limited capacity to predict the results of GC patients. More relevant studies should be undertaken to refine prognosis, comprehend progression, and improve treatment. Investigations have showed that protein synthesis is central to the occurrence and development of tumors. Translational control occurs in any phase of protein synthesis, and it is believed that the initiation phase of translation plays an important role during the process.Citation23,Citation24 eIF3 is the largest and most intricate eIF, consisting of thirteen subunits. This polypeptide interacts with the 40S subunit of free-floating ribosomes and stabilizes the eIF2–GTP–Met–tRNAi complex.Citation6,Citation7 eIF3d is the largest subunit of eIF3 and its function may be crucial. Some studies showed that eIF3d was required for the stable association of eIF3 subunits.Citation14 Based on these observations, it may be inferred that eIF3d is essential in promoting protein synthesis in the occurrence of cancer through certain molecular mechanisms.
Previous studies have demonstrated that eIF3d was overexpressed in several human cancers, such as prostate cancer,Citation15 colon cancer,Citation16 and melanoma,Citation17 while also resistant to chemotherapy.Citation20 There have been recent studies on expression of other subunits of eIF3 in GCCitation9,Citation25; however, the role of eIF3d in GC remains unclear.
In this study, immunohistochemical staining was used to evaluate the level of eIF3d in GC and to analyze with the resultant clinicopathology and prognosis. The data showed that eIF3d was remarkably higher in GC tissues than companion ANC tissues. In addition, eIF3d was positively correlated with the tumor stage, as determined by lymph node metastasis and depth of invasion. The Kaplan–Meier survival analysis showed that patients with high eIF3d expression had significantly poorer survival compared with patients with low eIF3d expression, particularly those in stage III. This was consistent with the result that eIF3d overexpression was more frequent in late-stage patients. Furthermore, multivariate Cox regression analyses showed that the level of eIF3d was an independent predictive factor of poor prognosis for GC as well as lymph node metastasis, serosa invasion, and lymphovascular invasion. Thus, eIF3d might serve as an efficient prognostic biomarker in GC.
In consideration of its indispensable role in protein synthesis and cell proliferation, eIF3d could be a potential target for gene therapy in GC. It was reported that knockdown of eIF3d could inhibit the growth of colon and breast cancer cells by inducing cell-cycle arrest at the G2/M phase or suppressing the Wnt/β-catenin signaling pathway.Citation16,Citation19 This study showed the relationship between eIF3d and the malignant behavior of GC at the immunohistochemical level implying that eIF3d may promote tumor growth. However, further studies should be performed at the cellular and molecular levels to reveal the underlying mechanism and should look for the possibility of making use of eIF3d in GC treatment.
Conclusion
To our knowledge, this study represents a first report identifying that eIF3d was upregulated in GC. Elevated expression of eIF3d was an independent poor prognostic factor in GC. We suggest that eIF3d could be a good biomarker in GC.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- YoonHKimNDiagnosis and management of high risk group for gastric cancerGut Liver20159151725547086
- LimLMichaelMMannGBLeongTAdjuvant therapy in gastric cancerJ Clin Oncol200523256220623216135489
- StockMOttoFGene deregulation in gastric cancerGene2005360111916154715
- DamocEFraserCSZhouMStructural characterization of the human eukaryotic initiation factor 3 protein complex by mass spectrometryMol Cell Proteomics2007671135114617322308
- JacksonRJHellenCUPestovaTVThe mechanism of eukaryotic translation initiation and principles of its regulationNat Rev Mol Cell Biol201011211312720094052
- HersheyJWThe role of eIF3 and its individual subunits in cancerBiochim Biophys Acta20151849779280025450521
- DongZZhangJTInitiation factor eIF3 and regulation of mRNA translation, cell growth, and cancerCrit Rev Oncol Hematol200659316918016829125
- LiGWangNSunCLiBDecreased expression of eukaryotic initiation factor 3f is an adverse prognostic factor for stage I-III gastric cancerWorld J Surg Oncol2014127224678890
- ZhangLPanXHersheyJWIndividual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cellsJ Biol Chem200728285790580017170115
- WangZChenJSunJCuiZWuHRNA interference-mediated silencing of eukaryotic translation initiation factor 3, subunit B (EIF3B) gene expression inhibits proliferation of colon cancer cellsWorld J Surg Oncol20121011922734884
- DongZLiuLHHanBPincheiraRZhangJTRole of eIF3 p170 in controlling synthesis of ribonucleotide reductase M2 and cell growthOncogene200423213790380115094776
- VillaNDoAHersheyJWFraserCSHuman eukaryotic initiation factor 4G (eIF4G) protein binds to eIF3c, -d, and -e to promote mRNA recruitment to the ribosomeJ Biol Chem201328846329323294024092755
- BandyopadhyayALakshmananVMatsumotoTChangECMaitraUMoe1 and spInt6, the fission yeast homologues of mammalian translation initiation factor 3 subunits p66 (eIF3d) and p48 (eIF3e), respectively, are required for stable association of eIF3 subunitsJ Biol Chem200227732360236711705997
- GaoYTengJHongYThe oncogenic role of EIF3D is associated with increased cell cycle progression and motility in prostate cancerMed Oncol201532751826036682
- YuXZhengBChaiRLentivirus-mediated knockdown of eukaryotic translation initiation factor 3 subunit D inhibits proliferation of HCT116 colon cancer cellsBiosci Rep2014346e0016125370813
- SudoHTsujiABSugyoAKnockdown of COPA, identified by loss-of-function screen, induces apoptosis and suppresses tumor growth in mesothelioma mouse modelGenomics201095421021620153416
- LiHZhouFWangHKnockdown of EIF3D suppresses proliferation of human melanoma cells through G2/M phase arrestBiotechnol Appl Biochem201562561562025322666
- FanYGuoYKnockdown of eIF3D inhibits breast cancer cell proliferation and invasion through suppressing the Wnt/beta-catenin signaling pathwayInt J Clin Exp Pathol201589104201042726617750
- KimHKChoiIJKimCGA gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patientsPLoS One201162e1669421364753
- ErolOSürenDTutuşBImmunohistochemical analysis of E-cadherin, p53 and inhibin-α expression in hydatidiform mole and hydropic abortionPathol Oncol Res201622351552126683836
- KwonSJEvaluation of the 7th UICC TNM Staging System of Gastric CancerJ Gastric Cancer2011112788522076207
- SpilkaRErnstCMehtaAKHaybaeckJEukaryotic translation initiation factors in cancer development and progressionCancer Lett2013340192123830805
- CuestaRGuptaMSchneiderRJThe regulation of protein synthesis in cancerProg Mol Biol Transl Sci20099025529220374744
- ChengYZhouJLiHClinicopathologic implications of eukaryotic initiation factor 3f and Her-2/neu expression in gastric cancerClin Transl Sci20158432032525684180
