Abstract
Objective
The aim of this retrospective study was to evaluate the efficacy and prognostic factors of bortezomib and dexamethasone (BD) chemotherapy regimen in the treatment of newly diagnosed multiple myeloma (MM) patients in our hospital.
Methods
A total of 47 newly diagnosed MM patients treated in our hospital from May 2010 to September 2016 were included in this study. All the enrolled patients received at least two cycles of BD chemotherapy regimen.
Results
The overall response rate after treatment was 68.5% with a complete response of 23.4%, very good partial response of 17.0%, partial response of 21.3% and minor response of 6.8%. The median time of overall survival (OS), progression-free survival (PFS) and time to progression (TTP) of the treated patients were 36.0, 19.0 and 18.0 months, respectively; the mean OS, PFS and TTP were 36.0, 19.3 and 18.8 months, respectively. Though some adverse events had occurred, none of the patients was discontinued from treatment. Level of albumin, β2-microglobulin and cytogenetic abnormalities were prognostic factors for OS, and plasma cell percentage in bone marrow, β2-microglobulin and cytogenetic abnormalities were prognostic factors for PFS as revealed by log-rank test of univariate analysis; no prognostic factors for OS and PFS were detected by COX regression of multivariate analysis.
Conclusion
Our study demonstrated that BD regimen was effective and well tolerated in newly diagnosed MM patients, and prognostic factors for patients’ survival include level of albumin, plasma cell percentage in bone marrow, β2-microglobulin and cytogenetic abnormalities.
Introduction
Multiple myeloma (MM) is the second most common hematological malignancy and has a very high incidence especially in the elderly people.Citation1 Disease symptoms of MM include skeletal destruction, bone marrow failure, normal immunoglobulin production suppression and insufficiency of renal function.Citation2 Treatment regimens of MM are developing very rapidly in decades.Citation3–Citation6 From the year 2000, a revolution for MM therapy has begun as a result of the emergence of new agents such as lenalidomide and bortezomib.Citation7–Citation9
For significantly improved clinical response in MM patients in clinical trials, bortezomib was approved by the US Food and Drug Administration (FDA) in 2003, and it has become the first-line therapy for MM since then.Citation10,Citation11 The ubiquitin-proteasome pathway can be blocked by selectively and reversibly inhibiting the activity of proteolysis of the proteasome complex, thus exerting an effect on anti-myeloma.Citation2 Although overwhelming number of studies all over the world have investigated the role of bortezomib in MM in vivo and in vitro, very few studies from People’s Republic of China have summarized the effect, tolerability and prognostic factors of bortezomib for newly diagnosed MM up to date.Citation12–Citation14 Although in recent years more new agents such as next generation of proteasome inhibitors (eg, carfilzomib) and immunomodulatory drugs (eg, pomalidomide) have been used in clinical trials and approved by the FDA,Citation6,Citation15,Citation16 they are currently not available in the Chinese market. As a result, chemotherapy regimens including bortezomib are still the standardized treatment for MM in People’s Republic of China. In order to better understand the role and effect of bortezomib in myeloma, summarizing and analyzing the prognostic factors in newly diagnosed MM patients treated by bortezomib is warranted.
Methods
Forty-seven newly diagnosed MM patients treated in Zhongda Hospital of Southeast University, People’s Republic of China from May 2010 to September 2016 were enrolled in this study. All the patients had received at least two cycles of bortezomib and dexamethasone (BD) regimen. The diagnosis was evaluated and confirmed by the International Myeloma Working Group (IMWG) criteria.Citation17,Citation18
This study was waived from the requirement of obtaining patient informed consent because the patients remained anonymous in the study. All aspects of the study conformed to the Declaration of Helsinki. This study was approved by the Institutional Review Board of Zhongda Hospital of Southeast University (2016ZDSYLL018.1).
The included patients were treated with the following regimen: bortezomib (1.3 mg/m2 subcutaneously or intravenously on days 1, 4, 8 and 11 of the 21-day cycle or 1.3 mg/m2 subcutaneously or intravenously on days 1, 8, 15 and 22 of every 35-day cycle) combined with dexamethasone 8 or 16 mg was given to patients on the day of or 1 day after bortezomib therapy. If grade 3 or 4 hematological toxicities occurred in patients, the next chemotherapy would be postponed until the recovery of blood cells and dosage of bortezomib also be reduced in subsequent cycles. Granulocyte colony-stimulating factor would be given to the needed patients to boost the counts of white blood cells (WBC) less than 0.5×109/L. For the included patients who were eligible for hematopoietic stem cell transplantation (SCT), 5 received autologous SCT and 1 was received allogeneic SCT.
Demographics of patients including age when diagnosed, sex, performance status, International Staging System (ISS) stage, Durie-Salmon (DS) stage, type of M proteins, classification of bone destruction, plasma cell percentage in bone marrow, level of hemoglobin and platelet, serum calcium, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, blood urea nitrogen, creatinine, C-reaction protein, β2-microglobulin, extramedullary disease, urine protein occurrence, number of patients who underwent SCT and cytogenetic abnormalities were collected. Overall survival (OS) was defined as the time from the first administration of BD regimen to patient death, progression-free survival (PFS) was calculated as the time from the initial administration of BD regimen to the identification date of progressive disease (PD) or death and time to progression (TTP) was calculated as the time from the first administration of BD regimen to progression of disease or to the initiation of other therapy. Responses of patients were evaluated according to the IMWG uniform response criteria: complete response (CR) was defined by the absence of M protein in serum and urine and confirmed by the disappearance of soft tissue plasmacytomas and presence of <5% bone marrow plasma cells; very good partial response (VGPR) met all of the criteria for partial response (PR) and also M proteins were detected by immunofixation but not on electrophoresis or serum M protein reduction ≥90% and urine M protein <100 mg/24 h; PR was defined by at least a 50% reduction of serum M proteins and urine M protein <200 mg/24 h; minor response (MR) was defined by ≥25% but <49% reduction of serum M protein and reduction of 24-h urine M protein by 50%–89% but still exceeded 200 mg/24 h and PD was called when M protein was >25% or increased in serum or urine, or increased bone marrow plasma cells, bone lesions or plasmacytomas, or new hypercalcemia occurred. Adverse events were evaluated according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.Citation19,Citation20 OS, PFS and TTP were analyzed by Kaplan–Meier method. Log-rank test was used to identify the univariate prognostic factors independently associated with OS and PFS, and Cox regression was applied to identify the multivariate prognostic factors independently associated with OS and PFS. All statistical analyses were performed by SPSS version 22.0 statistical software (Chicago, IL, USA). The value of p less than 0.05 was considered statistically significant.
Results
Patient characteristics
The clinical characteristics of the included 47 patients before the BD regimen therapy are shown in . Disease stages were confirmed by DS classification and ISS. The median age of MM patients was 65 (41–86) years. MM subtype of most patients (70.2%) were IgG and IgA. Six patients received SCT. Among the 19 patients who underwent chromosome testing, 3 were found to have cytogenetic abnormalities.
Table 1 Clinical characteristic of the included MM patients
Response to therapy
Responses to BD regimen are demonstrated in . Overall response was seen in 68.5% patients: CR was achieved in 11 patients (23.4%), VGPR was achieved in 8 patients (17.0%), PR was achieved in 10 patients (21.3%) and MR was seen in 3 patients (6.8%).
Table 2 Response in the MM patients after treatment
Safety
Adverse events of the treatment are listed in . The most common toxic effects occurring during treatment were neutropenia and peripheral neuropathy. Other commonly reported toxic effects were anemia, thrombocytopenia, infection, diarrhea, vomiting, deep venous thrombosis and fatigue. None of the patients were discontinued from treatment because of adverse events.
Table 3 Major adverse events of MM patients
Survival and prognostic factors
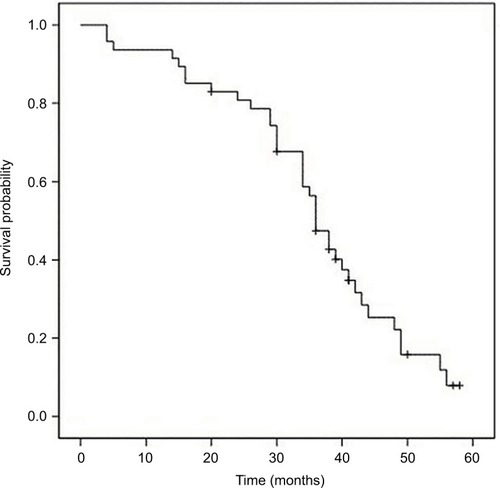
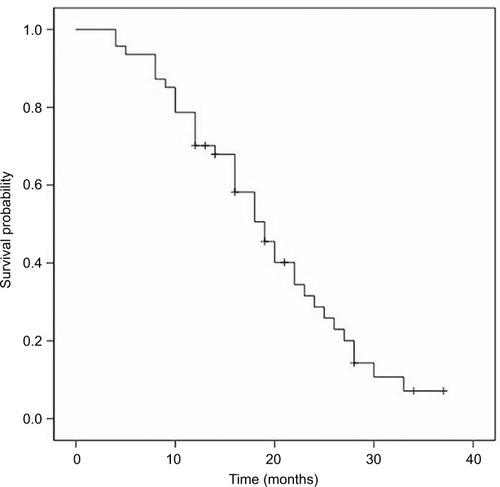
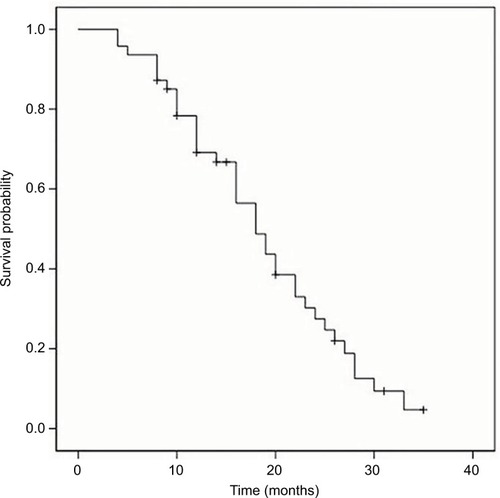
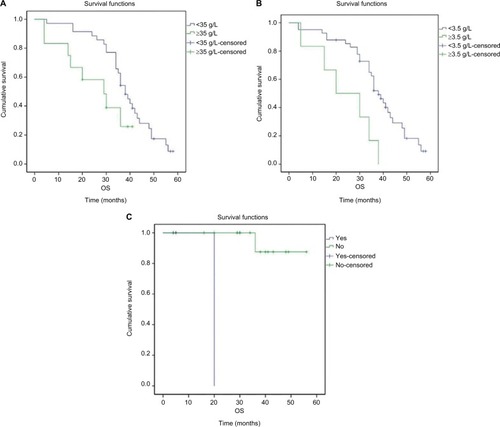
The median OS, PFS and TTP of these MM patients were 36.0 months (95% confidence interval [CI]: 32.8–39.2 months), 19.0 months (95% CI: 15.7–22.3 months) and 18.0 months (95% CI: 14.4–21.6 months), respectively; the mean OS, PFS and TTP were 36.0 months (95% CI: 31.8–40.3), 19.3 months (95% CI: 16.6–22.0) and 18.8 months (95% CI: 16.2–21.3), respectively (–).
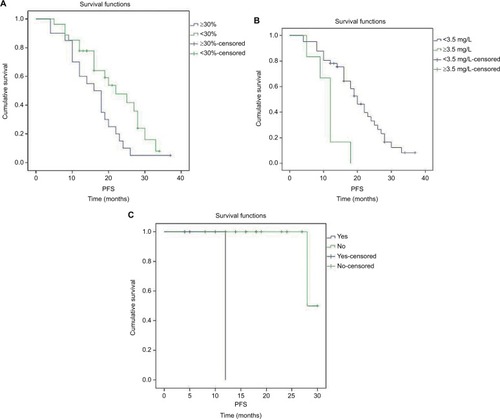
Associations between patient characteristics and OS and PFS were also evaluated. In the present study, log-rank test indicated that level of albumin, β2-microglobulin and cytogenetic abnormalities were prognostic factors for OS; high level of albumin as well as β2-microglobulin and cytogenetic abnormalities was associated with poor OS (, ). Plasma cell percentage in bone marrow, β2-microglobulin and cytogenetic abnormalities were prognostic factors for PFS; high percentage of plasma cells in bone marrow, high β2-microglobulin and cytogenetic abnormalities were associated with poor PFS (, ). No prognostic factors for OS and PFS were revealed by COX regression of multivariate analysis.
Table 4 Prognostic factors for OS
Table 5 Prognostic factors for PFS
Discussion
MM is a plasma disorder that accounts for approximately 10% of all hematologic malignancies.Citation21 It is considered as an incurable disease with a median survival of about 3 to 4 years if treated by conventional regimen.Citation21,Citation22 Multiple factors play roles in determining the best course of therapy and so the treatment of MM is quite individualized.Citation23 BD regimen is currently the standard chemotherapy regimen for MM patients in People’s Republic of China. For the included 47 newly diagnosed MM patients in our study, median time of OS, PFS and TTP were 36.0, 19.0 and 18.0 months, respectively, which were similar to the studies reported previously.Citation12,Citation17 For safety and tolerability, our results indicated that neutropenia and peripheral neuropathy were the two most common side effects caused by BD regimen. No patient was stopped from treatment because of those side effects, and this also proved that BD regimen was relatively safe and well tolerated.Citation24–Citation27
In this study, level of albumin, β2-microglobulin and cytogenetic abnormalities were prognostic factors for OS; plasma cell percentages in bone marrow, β2-microglobulin and cytogenetic abnormalities were prognostic factors for PFS. It was indicated in our study that prognosis of patients with higher levels of albumin, β2-microglobulin and plasma cell percentage in bone marrow may be worse than those patients with lower levels of these parameters. For the published studies analyzing prognostic factors of survival of Chinese MM patients, our results were not so consistent with them.Citation12–Citation14,Citation17 In the study by Lu et al,Citation12 sex, ISS stage, number of FISH abnormalities and extramedullary disease were the prognostic factors for survival, and no prognostic factors were observed by Guo et al.Citation14 More interestingly, theoretically high levels of albumin should be correlated with better outcome, but opposite results were found in this study. The potential explanation for the discrepancies might be because of the different groups of patients and centers in People’s Republic of China. Inadequate cytogenetic data, small sample size and heterogeneity of MM patients are also the reasons. We believe that with more standard treatment strategies as well as higher qualified and multicenter studies including more patient samples, the power of the study will certainly be increased. Approval of novel therapeutic agents in the Chinese market for MM and development of precise and effective treatment will also hopefully increase response in MM patients after chemotherapy.
Acknowledgments
This study was supported by National Natural and Science Foundation of People’s Republic of China (81370673), Key Medical Project of Jiangsu Province (BL2014078) and Key Medical Discipline of Jiangsu Province (2016–2020).
Disclosure
The authors report no conflicts of interest in this work.
References
- San MiguelJFIntroduction to a series of reviews on multiple myelomaBlood2015125203039304025838347
- SonneveldPGoldschmidtHRosiñolLBortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trialsJ Clin Oncol201331263279328723897961
- PalumboAFaconTSonneveldPThalidomide for treatment of multiple myeloma: 10 years laterBlood200811183968397718245666
- BarlogieBEpsteinJSelvanayagamPAlexanianRPlasma cell myeloma—new biological insights and advances in therapyBlood19897348658792465790
- BarlogieBShaughnessyJTricotGTreatment of multiple myelomaBlood20041031203212969978
- ChenRChenBZhangXGaoCEfficacy of carfilzomib in the treatment of relapsed and (or) refractory multiple myeloma: a meta-analysis of data from clinical trialsDiscov Med20162212118919927875670
- ShaughnessyJDJrQuPUsmaniSPharmacogenomics of bortezomib test-dosing identifies hyperexpression of proteasome genes, especially PSMD4, as novel high-risk feature in myeloma treated with Total Therapy 3Blood2011118133512352421628408
- DriscollJExpression of E3 ubiquitin ligases in multiple myeloma patients after treatment with the proteasome inhibitor bortezomibCancer Transl Med201515153157
- DaiCChenDJiangYHistone H2A and H2B deubiquitinase in developmental disease and cancerCancer Transl Med201515170175
- MohtyMMalardFMohtyBSavaniBMoreauPTerposEThe effects of bortezomib on bone disease in patients with multiple myelomaCancer2014120561862324249482
- AguiarPMde Mendonca LimaTColleoniGWBStorpirtisSEfficacy and safety of bortezomib, thalidomide, and lenalidomide in multiple myeloma: an overview of systematic reviews with meta-analysesCrit Rev Oncol Hematol201711319521228427509
- LuJLuJChenWHuoYHuangXHouJClinical features and treatment outcome in newly diagnosed Chinese patients with multiple myeloma: results of a multicenter analysisBlood Cancer J201448e23925127393
- LiFXuYDengPHeterogeneous chromosome 12p deletion is an independent adverse prognostic factor and resistant to bortezomib-based therapy in multiple myelomaOncotarget20156119434944425831238
- GuoHZhouXJiangYBortezomib plus intermediate-dose dexamethasone and thalidomide in elderly untreated patients with multiple myeloma: a Chinese experienceAm J Hematol201085749950120575036
- ChenRChenBGeZEfficacy of carfilzomib in the treatment of relapsed and (or) refractory multiple myeloma: a meta analysis of individual patient data from clinical trialsBlood2016128225675
- ChenRWangYLuanCGaoCZhangXChenBEffect of pomalidomide on relapsed/refractory multiple myeloma: a systematic review and meta-analysisJ Cancer20178101801180828819377
- KibaTItoTNakashimaTBortezomib and dexamethasone for multiple myeloma: higher AST and LDH levels associated with a worse prognosis on overall survivalBMC Cancer20141446224952705
- ChenRATuYCaoYLiuLLiangYBortezomib-dexamethasone or vincristine-doxorubicin-dexamethasone as induction therapy followed by thalidomide as maintenance therapy in untreated multiple myeloma patientsJ Int Med Res20113951975198422118002
- ZengWMengFLiuZBortezomib-based chemotherapy regimens can improve response in newly diagnosed multiple myeloma patients with bcl-2 and survivin overexpressionInt J Clin Exp Pathol2014774239424625120804
- ZwicklHZwickl-TraxlerEPecherstorferMA single-center retrospective analysis of first-line therapy of multiple myeloma with bendamustine-bortezomib-dexamethasoneLeuk Lymphoma20165792065207026901249
- BianchiGAndersonKCUnderstanding biology to tackle the disease: multiple myeloma from bench to bedside, and backCA Cancer J Clin201464642244425266555
- BianchiGMunshiNCPathogenesis beyond the cancer clone(s) in multiple myelomaBlood2015125203049305825838343
- CejalvoMJde la RubiaJClinical treatment of newly diagnosed multiple myelomaExpert Rev Hematol20158559561126327587
- RichardsonPGSonneveldPSchusterMWAssessment of Proteasome Inhibition for Extending Remissions (APEX) InvestigatorsBortezomib or high-dose dexamethasone for relapsed multiple myelomaN Engl J Med2005352242487249815958804
- HarousseauJLAttalMLeleuXBortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II studyHaematologica200691111498150517043025
- SonneveldPSchmidt-WolfIGvan der HoltBBortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trialJ Clin Oncol201230242946295522802322
- LiuXHeCKMengXBortezomib-based vs non-bortezomib-based post-transplantation treatment in multiple myeloma patients: a systematic review and meta-analysis of Phase III randomized controlled trialsOnco Targets Ther201581459146926109870