Abstract
Esophageal cancer (EC) is an extremely aggressive, lethal malignancy that is increasing in incidence worldwide. At present, definitive chemoradiotherapy is accepted as the standard treatment for locally advanced EC. The EC guidelines recommend a radiation dose of 50.4 Gy for definitive treatment, yet the outcomes for patients who have received standard-dose radiotherapy remain unsatisfactory. However, some studies indicate that a higher radiation dose could improve local tumor control, and may also confer survival benefits. Some studies, however, suggest that high-dose radiotherapy does not bring survival benefit. The available data show that most failures occurred in the gross target volume (especially in the primary tumor) after definitive chemoradiation. Based on those studies, we hypothesize that at least for some patients, more intense local therapy may lead to better local control and survival. The aim of this review is to evaluate the radiation dose, fractionation strategies, and predictive factors of response to therapy in functional imaging for definitive chemoradiotherapy in esophageal carcinoma, with an emphasis on seeking the predictive model of response to CRT and trying to individualize the radiation dose for EC patients.
Introduction
Esophageal cancer (EC) is a common cause of cancer death around the world.Citation1,Citation2 In East Asia, squamous cell carcinoma (SCC) is the most common type of EC, whereas adenocarcinoma is predominant in Western countries.Citation3,Citation4 These two types may represent different diseases, each with a distinct pathogenesis, epidemiology, prognosis, and tumor biology, including the pattern of lymph node metastasis.Citation5
At present, definitive concurrent chemoradiotherapy (CCRT) is the basic strategy for locally advanced inoperable EC patients based on the results of the pivotal study of Intergroup Radiation Therapy Oncology Group (RTOG)-8501, which significantly improved the local control (LC) and overall survival (OS) with CCRT compared with radiotherapy (RT) alone.Citation6 Furthermore, another RTOG study (#90-12) found that escalating the dose to 64.8 Gy did not confer a benefit compared with standard doses and may have contributed to a higher incidence of treatment-related death.Citation7 In the landmark INT0123 trial, dose escalation from 50.4 to 64.8 Gy also did not increase OS; this may have been due to higher toxicities and no therapeutic gain in dose escalation for pathological complete responses (pCRs) after planned radiation.Citation8 On the basis of these results, 50.4 Gy has become the accepted standard dose in Europe and North America for patients undergoing definitive CRT (DCRT). Although radiation dose escalation has failed to improve LC or OS, a dose of 60.0 Gy or more is more popular in Asian countries, where SCC is the predominant histological type.Citation9,Citation10 However, the prognosis for EC patients treated by standard-dose CRT was still disappointing, and >50% of such patients eventually developed recurrence or distant metastases and died as a result.Citation11 Many studies have been performed to describe patterns of recurrence after DCRT for EC, revealing that most failures in both early and advanced EC occurred in the gross target volume (GTV) (especially in the primary tumor) after CRT.Citation12–Citation18 Significantly, the recurrent site in the patient undergoing surgery occurred mainly in the locoregional node as well as distant metastasis, while in patients treated with DCRT it occurred mainly in the primary lesion.Citation19–Citation21 This indicates that the standard dose (50.4 Gy in 28 fractions), at least for some patients, may be inadequate to achieve a high probability of LC. Hence, for EC patients treated with DCRT, efforts should be made to intensify the treatment to improve the LC rate, which may lead to survival benefits.
In addition, on the basis of the theory of radiation biology, a radiation dose of 45–50 Gy is adequate to control microscopic tumors, 60 Gy or more is required to control gross tumors, and nearly 100 Gy is needed to cure solid tumors at conventional fractionation.Citation22 In recent years, continuous advancements in RT technology over the past decades have allowed for EC now being able to be treated with 3-dimensional (3D) treatment planning, including intensity-modulated RT (IMRT), volumetric-modulated arc therapy (VMAT) and helical tomotherapy. These modern techniques allow the oncologist to deliver higher doses of radiation with more precision to the tumor and with less toxicity to the surrounding normal tissue, which has dramatically reduced morbidity.Citation23,Citation24 Thus, with the improvements in RT techniques and chemotherapy regimens, the question has been raised as to whether high-dose RT given concurrently with effective chemotherapy could achieve similar or better survival rates compared with the standard treatment, especially for esophageal SCC (ESCC).
The aim of this review is to evaluate the radiation dose, fractionation strategies, and predictive factors of response to therapy in functional imaging for definitive CRT in EC, with an emphasis on seeking the predictive model of response to CRT and trying to individualize the radiation dose for EC patients.
Higher dose RT for DCRT
Continuous advancements in RT technology allow the oncologist to deliver higher radiation doses to the tumor with less toxicity to the surrounding normal tissue. This has led to a dramatic decline in morbidity.Citation23,Citation24 Furthermore, the simultaneous integrated boost (SIB) technique has been applied to many tumors, including in EC. Use of an SIB technique enables delivery of a higher dose to the primary tumor (2.2 Gy/f), whereas lower doses to the subclinical disease (1.8 Gy/f).Citation25,Citation26
With the improvements in treatment techniques, some researchers have tried to address the potential benefit of a higher radiation dose for EC. In the retrospective analysis by Suh et al the results also showed that patients who received a total dose ≥50.4 Gy of RT had significantly better loco-regional control (LRC, 69% vs 32%, p<0.01) and progression-free survival (PFS, 47% vs 20%, p=0.01), than patients receiving <50 Gy when treated with concurrent chemotherapy. High-dose radiation ≥50.4 Gy showed no significant OS benefit for patients with EC (28 vs 18 months, p=0.26).Citation27 He et al used modern radiation delivery techniques to determine whether high radiation dose could confer benefits in terms of LC or OS.Citation28 The results showed that high radiation dose provided a significant lower rate of local recurrence (17.9% vs 34.3%, p=0.024) compared with patients receiving low radiation dose. Furthermore, patients receiving high radiation dose did have a marginally better 5-year local-regional recurrence-free survival (68.7% vs 55.9%, p=0.052) than in the low-dose group. The 5-year OS rate showed no significant difference between the two groups (p=0.617). Although IMRT reduced the overall incidence of treatment-related toxicity, the rates of grade 3 or greater skin reaction and esophageal strictures were higher in the higher dose group. Considering the potential for better tumor LC but more severe toxicity with the higher radiation dose, He et al recommends that individualized treatment strategies be designed for patients treated with DCRT. Indeed, these studies found that radiation doses over 50.4 Gy could improve LC but not OS for EC patients.
However, some studies have shown that high-dose RT may provide a survival benefit. The study of Zhang et al found that the median survival time was 9 months for the lower dose group (≤51 Gy) and 14.5 months for the higher dose group (>51 Gy) among 69 patients treated with CCRT (p=0.054).Citation29 In line with these findings, Kim et al evaluated the correlation between radiation dose and survival for EC patients treated with DCRT. The results showed that patients in the high-dose group (≥60 Gy) had significantly better 2-year LRC (69.1% vs 50.3%, p=0.002), median PFS (16.7 vs 11.7 months, p=0.029), and median OS (35.1 vs 22.3 months, p=0.043) than in the low-dose group (<60 Gy).Citation30 Similar to these results, a retrospective study found that higher radiation dose may bring a survival benefit for EC patients.Citation31 Wolf et al also concluded that the use of radiation doses exceeding 54 Gy was associated with better OS (p=0.002).Citation32 Semrau et al in a retrospective analysis, showed that patients receiving high radiation dose had a greater 2-year OS (26.8%, vs 7.5%; p=0.0001), and PFS (17.4% vs 5.0%; p=0.0001) than the low-dose group.Citation33 A pooled analysis by Song et al investigated whether high-dose (>60 Gy) RT in DCRT could confer survival benefits compared with the low-dose RT for patients with EC.Citation34 Those results showed advantages in response rate, 5-year OS, locoregional recurrence, and distant failure compared with the standard RT arm. However, there is no evidence from prospective randomized trials to support that a higher radiation dose could bring survival benefit for EC patients.
In a recently published Phase I dose-escalation study, Yu et al suggest that it is feasible to deliver up to 70 Gy (2.8 Gy/F) to the GTV based on positron emission tomography/ computed tomography (PET/CT) in EC patients.Citation35 Thus, the use of a novel dose-escalation technique may be more likely than high-dose (>60 Gy) RT to lead to better LC and OS. A Phase II study by Chen et al evaluated the efficacy of radiation dose escalation using SIB in 60 EC patients.Citation36 RT consisted of 66 Gy at 2.2 Gy per fraction to the gross tumor and 54 Gy at 1.8 Gy per fraction to subclinical diseases simultaneously. The result showed that the 1-year LRC, distant metastasis-free survival (DMFS), disease-free survival (DFS), and OS rates were 87.6%, 78.6%, 86.0%, 80.5%, respectively. And the 2-year LRC, DMFS, DFS, and OS rates were 75.6%, 64.4%, 86.7%, 72.7%, respectively. This indicates that SIB combined with concurrent chemotherapy is feasible, with tolerable acute toxicities in EC patients, and a trend of significant improvements in LRC and OS was shown.
Radiation dose escalation for DCRT studies are summarized in . In summary, a few studies have demonstrated that higher doses than standard RT can improve local tumor control and may lead to better survival for EC patients. Although no evidence from Phase III prospective randomized trials support the additional survival benefit of dose-escalated RT in the whole population, these results may suggest that a subgroup of patients may benefit from limited dose escalation. Considering the potential survival benefits but more severe toxicity in the higher radiation dose group, the optimal radiation dose should be managed on an individual basis. Nevertheless, Phase III trials comprising a standard-dose arm using modern RT technique are warranted in the right subgroup population.
Table 1 Studies regarding high-dose RT and/or conventional-dose RT in esophageal cancer
Dose fractionation strategies for DCRT
The prognosis for EC patients treated with conventional RT alone remains discouraging despite the advances in radiotherapeutic techniques. The low survival rate and high incidence of locoregional treatment failure in EC have initiated a modification of conventionally fractionated RT. Recent studies are consistent in revealing that the accelerated reproliferation of carcinoma stem cells after RT is an important reason for the failure of RT.Citation37,Citation38 Therefore, it is speculated that the rates of LC and survival can be improved by controlling the accelerated reproliferation of carcinoma stem cells after RT. In this respect, it has been revealed that 3–4 weeks after RT, carcinoma stem cells started to have accelerated reproliferation, which provides the theoretical principle for accelerated hyperfraction RT later in the treatment process.
Several studies have investigated whether it would be possible to achieve a better curative effect from RT by adopting a late-course accelerated hyperfraction (LCAF). A Japanese Phase II study of accelerated hyperfraction plus 5-fluorouracil/cisplatin chemotherapy showed a promising result.Citation39 Similarly, Shi et alCitation40 initiated a study of late-course accelerated hyperfractionated RT (LCAFRT) for ESCC treatment and it yielded encouraging results. They found that patients with the LCAFRT regime could achieve a better 5-year survival rate (34% vs 15% for patients with conventional fractionation) and LC rate (55% vs 21% for patients with conventional fractionation). A prospective study by Zhao et al also revealed that the LCAFRT regimen offers better LC and survival compared to standard chemotherapy plus RT, as in the RTOG 85-01 and 94-05 trials.Citation41 A meta-analysis from China strengthened the evidence supporting the therapeutic benefits of LCAFRT compared with conventional fractionation for EC.Citation42 In addition, a randomized controlled trial was recently undertaken to investigate whether LCAF 3D conformal RT could achieve better results than conventional fraction (CF).Citation43 The resulting data showed that the 1-, 2- and 3-year survival rates were 79.2%, 56.3%, and 43.8%, respectively, in the LCAF group; and, in the CF group, the 1-, 2- and 3-year survival rates were 74%, 54%, and 36%, respectively (p=0.476). The 1-, 2- and 3-year LC rates were 81.3%, 62.5%, and 50%, respectively, in the LCAF group; in the CF group, the 1-, 2- and 3-year LC rates were 78%, 58%, and 42%, respectively (p=0.454). In the CF group, the incidence of radiation-induced esophagitis was lower than that in the LCAF group (72% vs 93.8%; p=0.008). It was concluded that EC patients in the LCAF group did have a slightly improved survival compared to those who received RT using conventional fractionation; the radiation toxicities, however, were greater in the LCAF group than those in the CF group. In a Phase III randomized study of LCAFRT plus concurrent chemotherapy for patients with ESCC, Zhao reported patients who received LCAFRT with concurrent chemotherapy had a tendency to better survival. But the incidence rates of grade 3 and 4 toxicities seemed higher in the LCAHRT+ CT arm (46%) than those in the LCAHRT arm (25%), and the grade 5 toxicities for the two group were 6% and 0%, respectively.Citation44
These studies suggest that the accelerated hyperfractionated schedules were effective but with an increased incidence of acute III–IV grade toxicity, which limited the combination of concurrent chemotherapy with RT. The advent of modern RT techniques and low-toxicity chemotherapy drugs may improve the clinical efficacy. The medical effects and safety observations of this kind of combination should be verified in prospective trials.
Future thinking
Much evidence has shown that there is a positive correlation between OS rate and the scale of chemoradiation in the histopathological response of patients with EC.Citation45–Citation47 Patients with CRs had a 5-year OS rate of 61.6%, which is higher than patients with an incomplete response or no response.Citation45 We speculated that if more patients with incomplete or no responses after planned radiation could achieve CR through limited radiation dose escalation, their prognosis would be better. Besides, this scheme would avoid an increase in toxicity and a decrease in survival caused by dose escalation in patients with CRs.
But how to predict the response to therapy? A great number of studies have reported the response rates after CCRT in EC.Citation6,Citation8,Citation16–Citation18,Citation48 The CR rate by stage was 89.7%–97% for T1, 50%–60% for T2 to T3, and 17%–39% for T4. For early-stage EC, most patients achieved a CR after CRT, while for locally advanced patients, CRT generally resulted in CR rates of 20%–50%. Apart from the tumor stage, patients with EC receiving RT also showed disparate treatment responses. Unfortunately, a large number of patients were resistant to CRT, which resulted in persistent disease or immediate local failure. For radiosensitive patients, the standard radiation dose of 50.4 Gy may be sufficient to obtain a pCR. But for the resistant lesions or advanced-stage EC, it is difficult to achieve a pCR at this dose. Even a dose escalation could not produce a better response; it may merely increase treatment-related toxicities in such refractory cases. We speculated that a subgroup of patients with partial remission after the standard radiation of 50.4 Gy could achieve better responses through limited radiation dose escalations without increased treatment toxicities. Hence, the identification of the predictive and prognostic factors will help to guide the oncologist in making informed decisions regarding the optimal radiation dose for treating ESCC, and indicate who have greater possibility benefit from limited radiation dose escalation.
Predictive value of fluorodeoxyglucose (FDG) PET/CT parameters
The ability to identify some factors to predict or assess treatment response at an early stage after the start of treatment would be of great value. Extensive research shows that metabolic-related parameters, such as standardized uptake value (SUV), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of the primary tumor have the potential to become valuable predictors and prognostic biomarkers in EC patients.Citation49–Citation52
Kato et al assessed the potential value of metabolic-related parameters in predicting the response to treatment.Citation53 The SUVmean before CRT in the non-CR group and the CR group were 10.2 and 4.9, respectively. The SUVmean after treatment in the two groups was 3.7 and 1.4, respectively. The changes in SUV of the CR patients were significantly lower than those of the non-CR patients (p<0.05). The author concluded that the SUVmean before CRT of the primary tumor has the potential to become a valuable predictor for response (p<0.05). Similarly, Atsumi et al performed a study to assess the efficacy of metabolic-related parameters for the prediction of response in DCRT for EC.Citation54 The results showed that the SUVmax values for the CR arm were higher than that in the non-CR arm, and all 18 patients in the low-SUV group had a CR. The data suggest that the SUVmax was a valuable predictor for response. However, Javeri et al evaluated the initial standardized unit value of FDGPET and its association with the degree of pathological response after CRT.Citation55 Their work revealed that SUV higher than the median (10.1) was associated with a better pathological response (p=0.06). Similarly, Levine et al and Rizk et al also reported a high initial SUVmax was associated with better response.Citation56,Citation57 These conflicting results could be potentially attributed to differences in patient populations, tumor histology types, grouping criteria, as well as treatment, but they might also indicate that predictive value of SUV values are unreliable.
Recently, some studies have considered that SUVmax, which reflects only a single point in the tumor, may not always be representative of the whole tumor.Citation58 In contrast to SUVmax, volumetric parameters such as MTV and TLG represent the dual characteristics of tumor volume and the degree of tumor uptakes FDG.Citation59 Therefore, volumetric parameters based on 18F-FDG PET/CT have been proposed as a more valuable biomarker for predicting survival or response to CRT than SUVmax in patients with EC. Roedl et al evaluated the value of volumetric parameters in predicting response to CRT in patients with EC. They found that a decrease in the metabolic tumor diameter between pre- and posttreatment was the single best predictor of tumor response and survival outcome.Citation60 In another study, Jayachandran et al evaluated the value of MTV on PET scanning in predicting response to CRT in patients with EC. They found that the MTV2.5 and TLG2.5 were valuable markers for predicting the tumor response.Citation61 Another study also showed that the changes in MTV and TLG between pre- and posttreatment were more precise for predicting pathological response than ΔSUVmax.Citation62 However, they showed that none of these parameters were very accurate in predicting a pCR and that the volumetric parameters had a marginally higher accuracy than SUVmax in predicting treatment response to CRT. Overall, available data suggest that these metabolic parameters may be useful as predictors of treatment response, while the ability to predict accurately is still limited.
Predictive value of CT perfusion parameters
CT perfusion is a promising imaging tool in oncology; it can visualize changes in the tumor’s vascular physiology and introduces elements of functional diagnostics in morphological imaging.Citation63,Citation64 Respecting this fact could be potentially useful in monitoring the response of the tumor to the CRT.
Stefanovic et al evaluated the value of the CT perfusion parameters in predicting response to CRT.Citation65 In their study, 40 patients with SCC were reevaluated after CRT. The CT perfusion parameter values after the CRT were significantly correlated with tumor regression grade. These results showed that CT perfusion imaging can predict the response to CRT. Hansen et al also reported that CT perfusion parameters could be an early predictor of treatment response to CRT in EC.Citation66
To further investigate the utility of each perfusion parameter for predicting histopathologic response in EC following chemoradiation, a great deal of research was performed. In the study performed by Hayano et al, they found that higher baseline blood flow (BF), higher baseline blood volume (BV) and low mean transit time (MTT) correlated significantly with a good response.Citation67 In another EC study, Makari et al examined changes in tumor perfusion before and after chemoradiation in ESCC.Citation68 The results showed that responders had a significantly higher baseline BF and a significantly shorter baseline MTT than non-responders, while BV did not in ESCC. Similar to the findings already mentioned, Djuric-Stefanovic et al questioned whether the CT perfusion parameters could be useful to predict the pCR of EC to CRT.Citation69 The results showed that BFpost-CRT, BVpost-CRT, and permeability surfacepost-CRT were significantly lower and MTTpost-CRT was significantly higher in the pCR group. The investigators concluded that CT perfusion parameters enable accurate prediction of pCR of EC to CRT, which could be useful in improving patient selection for further treatment.
In conclusion, both FDG PET parameters and CT perfusion parameters could be a good predictor for treatment response. Such predictive factors could help to identify the subgroups that are more likely to benefit from radiation dose escalation.
Individualized radiation dose escalation based on decrease of tumor FDG uptake
The available data indicate that a decrease in tumor FDG uptake correlates with OS and pathological response for patients with EC.Citation70 Cuenca et al performed a study showing that metabolic response during CRT for locally advanced EC has a great prognostic value.Citation71 Using a 50% decrease in SUVmax as a cut-off, the 2-year OS in the good metabolic responders and poor responders was 62% and 27%, respectively (p=0.016). Similarly, Javeri et al declared that the higher the decrease of tumor FDG uptake after treatment, the better the survival of EC patients.Citation72 Metabolic response using PET/ CT is a surrogate for histopathological response in predicting sensitivity to treatments of patients with EC.Citation73 Evaluating the decrease in tumor FDG uptake could help to identify good responders to CRT. Thus, individualized radiation dose escalation based on decrease in tumor FDG uptake after standard radiation of 50.4 Gy could be feasible. According to the European Organization for Research and Treatment of Cancer criteria, metabolic response on FDGPET was divided into the following four types: complete metabolic response (CMR), partial metabolic response (PMR), stable metabolic disease, and progressive metabolic disease.Citation74,Citation75 CMR was defined as complete resolution of 18F-FDG uptake within the tumor volume so that it was indistinguishable from surrounding normal tissue. On the basis of our conjecture, patients with CMR may not need to receive dose escalation after the standard radiation of 50.4 Gy, which may reduce the treatment-related toxicities. Patients with PMR may benefit from dose escalation after a conventional radiation dose of 50.4 Gy. Of those with minor residual tumor after planned radiation, limited dose escalation would achieve CMR without increasing toxicities. These patients could benefit the most from dose escalation. For those with major residual tumor and no metabolic response after planned radiation, limited dose escalation may not change the persistence of local disease because of tumor resistance to CRT. Above all, it is necessary to determine the feasibility of individualized radiation dose escalation after planned chemoradiation based on the decrease in tumor FDG uptake.
Individualized radiation dose escalation based on flow-metabolic phenotypes
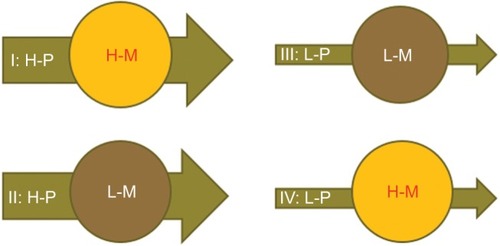
It has been reported that malignant tumors differ in terms of BF perfusion and glucose metabolism phenotype.Citation76–Citation78 For example, there are: 1) high-perfusion high-metabolism tumors; 2) low-perfusion low-metabolism tumors; 3) high-perfusion low-metabolism tumors; and 4) low-perfusion high-metabolism tumors (). A combined assessment of the flow-metabolic phenotype of EC using integrated 18F-FDG PET/perfusion CT may be of additional value in assessing the response to therapy as well as in identifying the patients who might be more likely to benefit from radiation dose escalation. Several studies have investigated the relationship between 18F-FDG PET and perfusion CT, demonstrating that the balance between tumor vascularity and glucose metabolism offers massive information regarding tumor treatment response.Citation76–Citation78 Tumors with matched high perfusion and glucose metabolism show a constitutive up-regulation of angiogenesis and metabolism; tumors with matched low perfusion and glucose metabolism likely reflect necrosis; whereas tumors that present with low perfusion and high glucose metabolism show an adaptation to hypoxia.Citation79 Thus, integrated 18F-FDG PET/perfusion CT makes it possible to distinguish different phenotypes; this may be useful in guiding individualized dose escalation based on functional imaging in patients with EC.
Conclusion
In conclusion, the available data reveal that LC after DCRT for EC remains a problem and that most local failures occur within the primary tumor. This indicates that the standard RT dose (50.4 Gy in 28 fractions) may be inadequate to achieve a high probability for LC for some subgroup patients. It is warranted to explore potential ways of improving LC, including IMRT and VMAT techniques, radiation dose escalation, and the use of more effective dose fractionation strategies. Some studies have found that the use of a higher dose and late course of accelerated hyperfraction radiation may lead to better LC and survival for EC patients undergoing CRT. Nevertheless, there is no evidence from Phase III randomized trials to support the additional benefit of dose-escalated RT. Although there is a potential for better tumor LC, there is also an associated higher incidence of toxicity. Therefore, the higher radiation dose should be used with caution on an individual basis in patients with EC.
It has been reported that EC probably has variable sensitivities to CRT. Therefore further studies will be required to: 1) identify the factors involved in EC sensitivity to radiation; 2) determine the causes of recurrence and non-control from molecular biology perspectives; and 3) individually determine the target region of RT, fractionated dose, and total dose to increase both LC and survival rates and decrease the rates of metastasis in patients with EC.
Acknowledgments
This study was funded by the Natural Science Foundation of China (NSFC 81672995) and The Key Research and Development Program of Shandong Province (2016GSF201133).
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- KumagaiKRouvelasITsaiJAMeta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancersBr J Surg2014101432133824493117
- WheelerJBReedCEEpidemiology of esophageal cancerSurg Clin North Am20129251077108723026270
- AjaniJAGastroesophageal cancers: progress and problemsJ Natl Compr Canc Netw20086981381418998257
- SongYLiLOuYIdentification of genomic alterations in oesophageal squamous cell cancerNature20145097498919524670651
- CooperJSGuoMDHerskovicAChemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01). Radiation Therapy Oncology GroupJAMA1999281171623162710235156
- MinskyBDNeubergDKelsenDPFinal report of intergroup trial 0122 (ECOG PE-289, RTOG 90-12): Phase II trial of neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagusInt J Radiat Oncol Biol Phys199943351752310078631
- MinskyBDPajakTFGinsbergRJINT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapyJ Clin Oncol20022051167117411870157
- HiguchiKKoizumiWTanabeSCurrent management of esophageal squamous-cell carcinoma in Japan and other countriesGastrointest Cancer Res20093415316119742141
- MengXWangJSunXCetuximab in combination with chemo-radiotherapy in Chinese patients with non-resectable, locally advanced esophageal squamous cell carcinoma: a prospective, multicenter phase II trailRadiother Oncol2013109227528024128808
- LiMZhangXZhaoFLuoYKongLYuJInvolved-field radiotherapy for esophageal squamous cell carcinoma: theory and practiceRadiat Oncol2016111826846932
- BedenneLMichelPBouchéOChemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102J Clin Oncol200725101160116817401004
- RawatSKumarGKakriaASharmaMKChauhanDChemoradiotherapy in the management of locally advanced squamous cell carcinoma esophagus: is surgical resection requiredJ Gastrointest Cancer201344327728423389866
- PöttgenCStuschkeMRadiotherapy versus surgery within multimodality protocols for esophageal cancer a meta-analysis of the randomized trialsCancer Treat Rev201238659960422116018
- KawaguchiYNishiyamaKMiyagiKSuzukiOItoYNakamuraSPatterns of failure associated with involved field radiotherapy in patients with clinical stage I thoracic esophageal cancerJpn J Clin Oncol20114181007101221665908
- van HagenPHulshofMCvan LanschotJJPreoperative chemoradiotherapy for esophageal or junctional cancerN Engl J Med2012366222074208422646630
- KatoKNakajimaTEItoYPhase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for Stage II–III esophageal carcinomaJpn J Clin Oncol201343660861523585687
- RohatgiPRSwisherSGCorreaAMFailure patterns correlate with the proportion of residual carcinoma after preoperative chemoradiotherapy for carcinoma of the esophagusCancer200510471349135516130133
- NakagawaSKandaTKosugiSOhashiMSuzukiTHatakeyamaKRecurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomyJ Am Coll Surg2004198220521114759776
- ReidTDDaviesILMasonJRobertsSACrosbyTDLewisWGStage for stage comparison of recurrence patterns after definitive chemoradiotherapy or surgery for oesophageal carcinomaClin Oncol (R Coll Radiol)201224961762422386923
- SugiyamaMMoritaMYoshidaRPatterns and time of recurrence after complete resection of esophageal cancerSurg Today201242875275822370963
- FletcherGHClinical dose-response curves of human malignant epithelial tumoursBr J Radiol1973465411124630323
- LiJCLiuDChenMQDifferent radiation treatment in esophageal carcinoma: a clinical comparative studyJ BUON201217351251623033291
- ChenYJLiuAHanCHelical tomotherapy for radiotherapy in esophageal cancer: a preferred plan with better conformal target coverage and more homogeneous dose distributionMed Dosim200732316617117707195
- LeclercMMaingonPHamoirMA dose escalation study with intensity modulated radiation therapy (IMRT) in T2N0, T2N1, T3N0 squamous cell carcinomas (SCC) of the oropharynx, larynx and hypopharynx using a simultaneous integrated boost (SIB) approachRadiother Oncol2013106333334023541643
- YuWWZhuZFFuXLSimultaneous integrated boost intensity-modulated radiotherapy in esophageal carcinoma: early results of a phase II studyStrahlenther Onkol20141901197998624609941
- SuhYGLeeIJKoomWSHigh-dose versus standard-dose radiotherapy with concurrent chemotherapy in stages II–III esophageal cancerJpn J Clin Oncol201444653454024771865
- HeLAllenPKPotterARe-evaluating the optimal radiation dose for definitive chemoradiotherapy for esophageal squamous cell carcinomaJ Thorac Oncol2014991398140525122435
- ZhangZLiaoZJinJDose-response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapyInt J Radiat Oncol Biol Phys200561365666415708243
- KimHJSuhYGLeeYCDose-response relationship between radiation dose and loco-regional control in patients with stage II–III esophageal cancer treated with definitive chemoradiotherapyCancer Res Treat201749366967727737537
- ChenCYLiCCChienCRDoes higher radiation dose lead to better outcome for non-operated localized esophageal squamous cell carcinoma patients who received concurrent chemoradiotherapy? A population based propensity-score matched analysisRadiother Oncol2016120113613927207358
- WolfMCZehentmayrFSchmidtMHölzelDBelkaCTreatment strategies for oesophageal cancer – time-trends and long term outcome data from a large tertiary referral centreRadiat Oncol201276022501022
- SemrauRHerzogSLVallböhmerDKocherMHölscherAHMüllerRPPrognostic factors in definitive radiochemotherapy of advanced inoperable esophageal cancerDis Esophagus201225654555422133297
- SongTLiangXFangMWuSHigh-dose versus conventional-dose irradiation in cisplatin-based definitive concurrent chemoradiotherapy for esophageal cancer: a systematic review and pooled analysisExpert Rev Anticancer Ther201515101157116926235427
- YuWCaiXWLiuQSafety of dose escalation by simultaneous integrated boosting radiation dose within the primary tumor guided by (18)FDG-PET/CT for esophageal cancerRadiother Oncol2015114219520025586952
- ChenJGuoHZhaiTRadiation dose escalation by simultaneous modulated accelerated radiotherapy combined with chemotherapy for esophageal cancer: a phase II studyOncotarget2016716227112271926992206
- WithersHRTaylorJMMaciejewskiBThe hazard of accelerated tumor clonogen repopulation during radiotherapyActa Oncol19882721311463390344
- TrottKRCell repopulation and overall treatment timeInt J Radiat Oncol Biol Phys1990194107110752211245
- JeremicBShibamotoYAcimovicLAccelerated hyperfractionated radiation therapy and concurrent 5-fluorouracil/cisplatin chemotherapy for locoregional squamous cell carcinoma of the thoracic esophagus: a phase II studyInt J Radiat Oncol Biol Phys1998405106110669539560
- ShiXHYaoWLiuTLate course accelerated fractionation in radiotherapy of esophageal carcinomaRadiother Oncol19995112126
- ZhaoKLShiXHJiangGLWangYLate-course accelerated hyper-fractionated radiotherapy for localized esophageal carcinomaInt J Radiat Oncol Biol Phys200460112312915337547
- ZhangYWChenLBaiYZhengXLong-term outcomes of late course accelerated hyper-fractionated radiotherapy for localized esophageal carcinoma in Mainland China: a meta-analysisDis Esophagus201124749550121309922
- WangJHLuXJZhouJWangFA randomized controlled trial of conventional fraction and late course accelerated hyperfraction three-dimensional conformal radiotherapy for esophageal cancerCell Biochem Biophys201262110711221858589
- ZhaoKLShiXHJiangGLLate course accelerated hyperfractionated radiotherapy plus concurrent chemotherapy for squamous cell carcinoma of the esophagus: a phase III randomized studyInt J Radiat Oncol Biol Phys20056241014102015990003
- TongDKLawSKwongDLChanKWLamAKWongKHHistological regression of squamous esophageal carcinoma assessed by percentage of residual viable cells after neoadjuvant chemoradiation is an important prognostic factorAnn Surg Oncol20101782184219220217248
- ChaoYKChanSCLiuYHPretreatment T3–4 stage is an adverse prognostic factor in patients with esophageal squamous cell carcinoma who achieve pathological complete response following preoperative chemoradiotherapyAnn Surg2009249339239619247024
- HammoudZTKeslerKAFergusonMKSurvival outcomes of resected patients who demonstrate a pathologic complete response after neoadjuvant chemoradiation therapy for locally advanced esophageal cancerDis Esophagus2006192697216643172
- NishimuraYSuzukiMNakamatsuKKanamoriSYagyuYShigeokaHProspective trial of concurrent chemoradiotherapy with protracted infusion of 5-fluorouracil and cisplatin for T4 esophageal cancer with or without fistulaInt J Radiat Oncol Biol Phys200253113413912007951
- HattMLe PogamAVisvikisDPradierOCheze Le RestCImpact of partial-volume effect correction on the predictive and prognostic value of baseline 18F-FDG PET images in esophageal cancerJ Nucl Med2012531122022213819
- MuijsCTBeukemaJCPruimJA systematic review on the role of FDG-PET/CT in tumour delineation and radiotherapy planning in patients with esophageal cancerRadiother Oncol201097216517120541273
- HuhJWMinJJLeeJHKimHRKimYJThe predictive role of sequential FDG-PET/CT in response of locally advanced rectal cancer to neoadjuvant chemoradiationAm J Clin Oncol201235434034421422901
- CapirciCRubelloDPasiniFThe role of dual-time combined 18-fluorodeoxyglucose positron emission tomography and computed tomography in the staging and restaging workup of locally advanced rectal cancer, treated with preoperative chemoradiation therapy and radical surgeryInt J Radiat Oncol Biol Phys20097451461146919419820
- KatoHFukuchiMMiyazakiTPrediction of response to definitive chemoradiotherapy in esophageal cancer using positron emission tomographyAnticancer Res2007274C2627263317695425
- AtsumiKNakamuraKAbeKPrediction of outcome with FDG-PET in definitive chemoradiotherapy for esophageal cancerJ Radiat Res201354589089823520267
- JaveriHXiaoLRohrenEInfluence of the baseline 18F-fluoro-2-deoxy-D-glucose positron emission tomography results on survival and pathologic response in patients with gastroesophageal cancer undergoing chemoradiationCancer2009115362463019130466
- LevineEAFarmerMRClarkPPredictive value of 18-fluorodeoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancerAnn Surg2006243447247816552197
- RizkNPTangLAdusumilliPSPredictive value of initial PET-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinomaJ Thorac Oncol20094787587919487968
- LarsonSMErdiYAkhurstTTumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysisClin Positron Imaging19992315917114516540
- ChungMKJeongHSParkSGMetabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancerClin Cancer Res200915185861586819737951
- RoedlJBColenRRHolalkereNSFischmanAJChoiNCBlakeMAAdenocarcinomas of the esophagus: response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET-CT. Comparison to histopathologic and clinical response evaluationRadiother Oncol200889327828618701180
- JayachandranPPaiRKQuonAPostchemoradiotherapy positron emission tomography predicts pathologic response and survival in patients with esophageal cancerInt J Radiat Oncol Biol Phys201284247147722381904
- StiekemaJVermeulenDVegtEDetecting interval metastases and response assessment using 18F-FDG PET/CT after neoadjuvant chemoradiotherapy for esophageal cancerClin Nucl Med2014391086286725140549
- MilesKALeeTYGohVExperimental Cancer Medicine Centre Imaging Network GroupCurrent status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomographyEur Radiol20122271430144122367468
- BellomiMViottiSPredaLD’AndreaGBonelloLPetraliaGPerfusion CT in solid body-tumours. Part II: clinical applications and future developmentRadiol Med20101156858874 Italian20221706
- Djuric-StefanovicAMicevMStojanovic-RundicSPeskoPSaranovicDJAbsolute CT perfusion parameter values after the neoadjuvant chemoradiotherapy of the squamous cell esophageal carcinoma correlate with the histopathologic tumor regression gradeEur J Radiol201584122477248426467704
- Lundsgaard HansenMFallentinELauridsenCComputed tomography (CT) perfusion as an early predictive marker for treatment response to neoadjuvant chemotherapy in gastroesophageal junction cancer and gastric cancer a prospective studyPLoS One201495e9760524845062
- HayanoKOkazumiSShutoKPerfusion CT can predict the response to chemoradiation therapy and survival in esophageal squamous cell carcinoma: initial clinical resultsOncol Rep200718490190817786353
- MakariYYasudaTDokiYCorrelation between tumor blood flow assessed by perfusion CT and effect of neoadjuvant therapy in advanced esophageal cancersJ Surg Oncol200796322022917450532
- Djuric-StefanovicASaranovicDMicevMDoes the computed tomography perfusion imaging improve the diagnostic accuracy in the response evaluation of esophageal carcinoma to the neoadjuvant chemoradiotherapy? Preliminary studyJ BUON201419123724424659670
- OnalCTorunNGulerOCYildirimBAPrognostic value of metabolic response measured by 18F-FDG-PET in oesophageal cancer patients treated with definitive chemoradiotherapyNucl Med Commun201637121282128927612030
- CuencaXHennequinCHindiéEEvaluation of early response to concomitant chemoradiotherapy by interim 18F-FDG PET/CT imaging in patients with locally advanced oesophageal carcinomasEur J Nucl Med Mol Imaging201340447748523371374
- JaveriHXiaoLRohrenEThe higher the decrease in the standardized uptake value of positron emission tomography after chemoradiation, the better the survival of patients with gastroesophageal adenocarcinomaCancer2009115225184519219685531
- StilesBMSalzlerGJorgensenAComplete metabolic response is not uniformly predictive of complete pathologic response after induction therapy for esophageal cancerAnn Thorac Surg20139651820182523895888
- YoungHBaumRCremeriusUMeasurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study GroupEur J Cancer199935131773178210673991
- KimMKRyuJSKimSBValue of complete metabolic response by (18)F-fluorodeoxyglucose-positron emission tomography in oesophageal cancer for prediction of pathologic response and survival after preoperative chemoradiotherapyEur J Cancer20074391385139117512192
- Veit-HaibachPSchmidDStrobelKCombined PET/CT-perfusion in patients with head and neck cancersEur Radiol201323116317322772151
- GohVRodriguez-JustoMEngledowAAssessment of the metabolic flow phenotype of primary colorectal cancer: correlations with microvessel density are influenced by the histological scoring methodEur Radiol20122281687169222527369
- GohVEngledowARodriguez-JustoMThe flow-metabolic phenotype of primary colorectal cancer: assessment by integrated 18F-FDG PET/perfusion CT with histopathologic correlationJ Nucl Med201253568769222454485
- PadhaniARMilesKAMultiparametric imaging of tumor response to therapyRadiology2010256234836420656830