Abstract
Objectives
The systemic status and local immune status, as determined by the neutrophil–lymphocyte ratio (NLR) or the lymphocyte ratio (LYMR) and tumor-infiltrating lymphocyte (TIL) count, respectively, have been suggested as predictors of the tumor response to neoadjuvant chemoradiotherapy (nCRT) in rectal cancer, although the utility of these measures remains controversial. We aimed to investigate the values of the LYMR, NLR and TIL count and their combinations (TIL–LYMR/TIL–NLR) in predicting pathologic complete response (pCR) after nCRT.
Patients and methods
Pretreatment biopsy samples and data from the blood tests of 92 patients with rectal cancer who underwent curative resection after nCRT were retrospectively obtained. CD8+ TILs were immunostained using an antibody against CD8. The density of CD8+ TILs was recorded as the number of CD8+ T cells per square millimeter, and the results were classified as either “high” or “low”. The LYMR and NLR were calculated using pretreatment blood test data and categorized into either “high” or “low” groups. TIL–LYMR was graded as “low,” “mid” or “high” when neither, one or both the CD8+ TIL count and LYMR were “high,” respectively. TIL–NLR was graded similarly. The associations between TILs and LYMR, NLR and their combinations (TIL–LYMR/TIL–NLR) were evaluated.
Results
pCR was significantly associated with a high LYMR, a low NLR and increased chemotherapy cycles (P=0.039, P=0.043 and P=0.015, respectively), but not with the CD8+ TIL count or carcinoembryonic antigen (CEA) level (P=0.100 and P=0.590, respectively). Additionally, 40% of patients with high LYMR and 40.7% with low NLR achieved pCR, whereas only 19.7% with low LYMR and 20.3% with high NLR did so. When the combinations were assessed, TIL–LYMR showed a positive correlation with pCR (P=0.038), while no association between TIL–NLR and pCR was found (P=0.916). In multivariate analysis, TIL–LYMR remained an independent predictor of pCR (odds ratio [OR]=1.833, 95% confidence interval [CI]=1.069–3.142, P=0.028).
Conclusion
High LYMR, low NLR and high TIL–LYMR at baseline are predictive of pCR to nCRT for patients with rectal cancer. These parameters may help identify pCR patients and provide additional information for therapeutic decision-making.
Introduction
Neoadjuvant chemoradiotherapy (nCRT) followed by surgery is the standard treatment for locally advanced rectal cancer, with the advantages of tumor downstaging and local control improvement.Citation1 For patients achieving pathologic complete response (pCR) after nCRT, a strategy called “watch and wait” can be considered as a substitute for surgery.Citation2 Such a strategy offers pCR patients a noninvasive treatment without compromising the long-term clinical outcome.Citation3,Citation4 Therefore, it is important to identify this group of patients before the initiation of treatment. Indeed, many clinicopathological and molecular factors have been suggested to be associated with pCR, although their utility in clinical practice remains controversial.Citation5–Citation8
Recently, it has been noted that the immune response plays a significant role in the antitumor effect of radiation. Tumor cell death is induced not only by the direct damage caused by radiation but also by the intratumoral immune reaction that follows.Citation9–Citation11 A high density of tumor-infiltrating lymphocytes (TILs; CD4+, CD8+) in the primary tumor has been suggested as a predictor of better tumor responses to nCRT.Citation12 In addition to the localized immune reaction, the systemic immune status, as measured with the circulating lymphocyte ratio (LYMR) and neutrophil–lymphocyte ratio (NLR), has also shown the predictive value for tumor down-staging and survival.Citation13,Citation14 However, whether the combination of these two immune reactions shows better predictive value for the tumor response remains unknown.
Based on the above evidence, we hypothesized that the local immune status, as measured by the CD8+ TIL count, and host immune status, as measured with the NLR and LYMR, could be used as predictors of the tumor response to nCRT. Therefore, the aim of this study was to explore the correlation of pretreatment local and systemic immune status values with pCR and evaluate the predictive value of their combination.
Patients and methods
Patients
From May 2006 to June 2014, a total of 92 patients with locally advanced rectal cancer who received nCRT followed by curative tumor excision were retrospectively included in this study. All patients provided pretreatment biopsy samples. None of the patients had overt metastatic disease at the beginning of the treatment. Pelvic magnetic resonance imaging or transanal ultrasound was applied to assess the primary tumor stage. All patients had stage T3/T4 disease and/or involved lymph node(s) before treatment. The total dose of radiation was 50 Gy given in 25 fractions, and four cycles of concomitant chemotherapy with capecitabine (1,000 mg/mm2 of body surface area, days 1–14) and oxaliplatin (130 mg/mm2 of body surface area, day 1) were scheduled, with one, two and one cycle given before, during and after nCRT, respectively. The number of cycles received varied from 1 to 4, depending on patient tolerance. The study was performed with approval from the institutional research ethics committee of Sun Yat-sen University Cancer Center. Written consent forms notifying the use of specimens and publication of the results were obtained on admission. The raw data in this paper has been successfully uploaded and locked onto Research Data Deposit with a RDD number of RDDA2017000321.
Immunological assessment of biopsy samples
Pretreatment biopsy samples were obtained before the initiation of nCRT. TILs were assessed by immunohistochemical staining of slides from formalin-fixed, paraffin-embedded tumor blocks using mouse monoclonal antibodies against CD8 (1:100, ZA0508; ZSGB-BIO, Beijing, China). For each slide, three random fields were chosen for the enumeration of CD8+ TIL within the area where tumor cells were considered to be most strongly stained. Then, an average was reached and considered as the number of CD8+ TIL per field. The density of TILs was defined as the number of positive CD8 lymphocytes per square millimeter and was then graded as either “high” or “low” (cutoff=80/mm2). This cutoff value yielded a minimal “P-value” in the analysis of correlation with pCR and was thus applied.
Evaluation of hematological factors
Blood samples were taken within 2 weeks before nCRT at our center. None of the patient had evidence of infectious complications such as fever, chills and headache at the time of blood withdrawal. The data from regular blood tests were assessed, including counts of white blood cells (WBCs), lymphocytes and neutrophils. The NLR was calculated as the count of neutrophils divided by the count of lymphocytes, and the LYMR was calculated as the count of lymphocytes divided by the count of WBCs. An NLR ≥2.0 and an LYMR ≥0.3 were considered elevated (high) using the “minimal P value” method. The LYMR combined with the CD8+ TIL count (TIL–LYMR) was then classified into three levels: low (both LYMR and density of CD8+ TILs were low), mid (either LYMR or density of CD8+ TILs was low) and high (both LYMR and density of CD8+ TILs were high). The combination of NLR with CD8+ TILs (CD8–NLR) was similarly grouped as low, mid or high.
Grading of tumor regression after nCRT
The tumor response to nCRT was reevaluated by two independent pathologists blinded to the initial reports according to the Mandard tumor regression grade (TRG) systemCitation15 as follows: Grade 1, complete response with absence of residual cancer and fibrosis extending through the wall; Grade 2, presence of residual tumor cells scattered through the area of fibrosis; Grade 3, increase in the number of residual cancer cells, with predominant fibrosis; Grade 4, residual cancer outgrowing the area of fibrosis and Grade 5, absence of regressive changes. Grade 1 was referred to as pCR, while Grades 2–5 were considered to represent non-pCR.
Statistical analysis
The correlation of pCR with clinicopathological parameters was analyzed using univariate analysis. Comparison of blood cell counts and ratios between different response groups was performed using a nonpaired t-test or Mann–Whitney U-test as appropriate. Multivariate analysis using logistic regression was performed to determine independent factors impacting the tumor response. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corporation, Armonk, NY, USA). A two-sided P-value <0.05 was considered as statistically significant.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Results
Patient characteristics
A total of 92 patients who underwent nCRT followed by curative resection were included in this analysis. Among them, 62 (67.4%) were male and 30 (32.6%) were female, with a median age of 56 years (range 25–79 years). All the patients received long-term radiotherapy (50 Gy/25) and concurrent chemotherapy with Xeloda (1,000 mg/mm2 of body surface area) and oxaliplatin (130 mg/mm2). The chemotherapy cycles received varied according to patient tolerance, with 41 (44.6%), 31 (33.7%), 18 (19.6%) and 2 (2.2%) patients receiving four, three, two and one cycle(s), respectively. Total mesorectal excision was performed within 6–8 weeks after radiation. According to the TRG system, pCR was achieved in 24 (26.1%) patients.
Correlation of pCR with clinicopathological characteristics
As shown in , pCR was associated with the received number of cycles of preoperative chemotherapy. Patients who received three or four cycles of chemotherapy were more likely to achieve pCR than patients who received two cycles (31.9% vs. 5%, P=0.015). There were no differences in gender, age, tumor location, pretreatment carcinoembryonic antigen (CEA) and CA19-9 levels, surgical procedure, tumor differentiation and clinical T stage or N stage between the pCR and non-pCR groups.
Table 1 Correlation of pCR with clinicopathological characteristics (n=92)
Regarding hematological factors, patients who achieved pCR showed a higher ratio of lymphocytes than those who did not (0.31 vs. 0.27, P=0.0031). However, the absolute counts of lymphocytes, WBCs, neutrophils, lymphocytes and the NLR were not significantly different between the two groups (). When the NLR and LYMR were classified as “high” or “low,” pCR was observed in more patients with high LYMR and TIL–LYMR and low NLR (P=0.039, P=0.038 and P=0.043, respectively) ( and ).
Table 2 Correlations of pCR with hematological and immunological factors (n=92Table Footnotea)
Figure 1 WBCs, neutrophils, lymphocytes, NLR, LYMR in circulating blood and CD8+ TIL counts in pretreatment biopsy samples taken before CRT in 68 non-pCR and 24 pCR cases.
Abbreviations: WBC, white blood cell; NLR, neutrophil–lymphocyte ratio; LYMR, lymphocyte ratio; TIL, tumor-infiltrating lymphocyte; CRT, chemoradiotherapy; pCR, pathologic complete response.
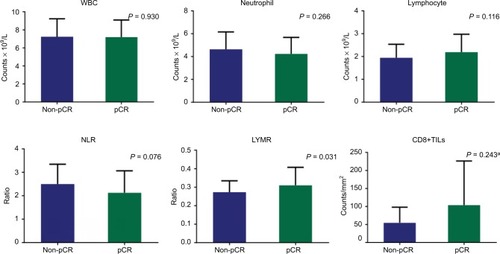
Figure 2 Immunostaining of CD8+ TILs in biopsy samples before CRT showed a high density (A) and a low density (B) of infiltration.
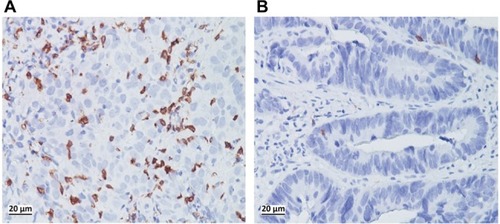
CD8+ TILs were clearly immunostained in tumor nests using specific antibodies (). Counts of CD8+ TILs in pCR patients were higher than those in non-pCR patients, and tumors with a high density of CD8+ TILs tended to achieve better regression than tumors with a low density, although statistical significance was not reached in either case (P=0.243 and P=0.10, respectively). However, when CD8+ TILs were combined with the LYMR (TIL–LYMR), a significant difference in the pCR rate was observed among the different groups (55.6% vs. 31.4% vs. 17.0%, P=0.038).
Multivariate analysis of pCR predictors
Variables with P-values <0.1 in the univariate analysis, which included CD8+ TILs, chemo cycles, NLR, LYMR and TIL–LYMR, were included in a multivariate analysis. To avoid colinearity, two similar multivariate analyses were performed separately, with one including CD8+ TILs, chemo cycles, NLR and LYMR, and the other including chemo cycles, NLR and TIL–LYMR. The results showed that when CD8+ TILs, chemo cycles, NLR and LYMR were analyzed, only chemo cycles remained significant (odds ratio [OR]=8.918, 95% confidence interval [CI]=1.124–70.747, P=0.038). However, when chemo cycles, NLR and TIL–LYMR were analyzed, both chemo cycles and TIL–LYMR remained significant (OR=8.595, 95% CI=1.036–71.305, P=0.046 and OR=1.833, 95% CI=1.069–3.142, P=0.028, respectively; ).
Table 3 Multivariate analyses for predictors associated with a pCR
Correlation of LYMR/NLR with clinicopathologic parameters
There was no correlation of LYMR or NLR with gender, age, CEA or CD8+ TILs (P=0.370, P=0.918, P=0.878 and P=0.467, respectively, and P=0.353, P=0.698, P=0.616 and P=0.926, respectively). Patients with a high LYMR and a low NLR tended to be more tolerant to chemotherapy, although statistical significance was not reached (P=0.096 and P=0.057, respectively; ).
Table 4 Correlation of LYMR/NLR with clinicopathological factors (n=92)
Discussion
In this study, we showed that the LYMR and NLR in circulating blood were associated with pCR after nCRT for rectal cancer and that the density of CD8+ TILs in biopsy samples combined with the circulating lymphocyte ratio (TIL–LYMR) was an independent predictor of pCR. Patients with a high density of CD8+ TILs and a high ratio of lymphocytes were more likely to achieve pCR. These results are generally consistent with those of previous studies,Citation16–Citation18 showing that the systemic status and local immune status are positively associated with the tumor response to nCRT. However, to our knowledge, our study is the first to evaluate the combination of the systemic status and local immune status for predicting the response to nCRT in rectal cancer.
The tumor microenvironment represents the front line of tumor–host interactions, and many inflammatory cells, immune cells and cytokines in this environment are involved in the process of tumor development.Citation19–Citation21 Among them, T lymphocytes (CD4+ and CD8+ lymphocytes) are generally considered to be antitumoral. Galon et al found that a high density of T cells in primary tumors was linked to improved clinical outcome and defined a scoring system called the immune score to predict survival based on lymphocyte infiltration in different sites of the tumor.Citation22,Citation23 In the nCRT setting, Yasuda et alCitation12 studied the correlation of the density of CD4+ and CD8+ TILs in pre-chemoradiotherapy (CRT) biopsy samples with the tumor response and found that CD4+ and CD8+ TILs were strongly associated with tumor shrinkage. These findings revealed that intratumoral T-cell infiltration had a positive impact on radiosensitivity in rectal cancer. However, in our study, although a higher density of CD8+TILs was observed in the pCR group than in the non-PCR group, the difference was not statistically significant (103.8 vs. 55.8, P=0.243). A similar negative result was obtained when CD8+ TILs were analyzed by groups (P=0.100). Given that previous studies in this field included relatively small sample sizes and did not follow uniform nCRT protocols, it is not surprising that different results have been reported.
The systemic immune status, as measured by the NLR and LYMR, is another factor affecting nCRT efficacy. An elevated NLR and a decreased LYMR before nCRT were suggested as predictors of poor tumor response in previous studies.Citation24,Citation13 In our study, the correlation of NLR and LYMR with pCR was also observed in the univariate analysis (P=0.043 and P=0.039, respectively), although this correlation failed to reach significance in the multivariate analysis. However, when LYMR was combined with CD8+ TILs, the new indicator, TIL–LYMR, showed a positive association with pCR (P=0.038) and remained an independent factor in the multivariate analysis (P=0.028). This finding suggests that the combination of the systemic status and local immune status might perform better than either status alone in predicting the tumor response to treatment.
The relationship between the systemic immune status and the tumor microenvironment has not been fully clarified. It was previously believed that circulating immune cells exerted their antitumor effect by penetrating and infiltrating into the tumor environment. This assumption was supported by the study of Grimm et al,Citation25 which evaluated circulating peripheral blood lymphocytes and TILs in tumor specimens of oral squamous cell carcinoma and found that circulating T-cell subsets (CD3+, CD4+ and CD8+) were significantly associated with their corresponding TIL subsets. In contrast, different results were derived in ovarian carcinoma, as Emerson el alCitation26 suggested that intratumoral immune cells were distinct from those circulating in the blood and represented a population of T cells specific to the tumor microenvironment. Here, we showed that both the LYMR and the NLR at baseline were not associated with CD8+ TILs in rectal cancer (P=0.467 and P=0.926, respectively). Patients with a high LYMR or a low NLR in the blood did not necessarily show dense infiltration of CD8+ TILs. Such inconsistency between the local and systemic immune reactions has also been observed in terms of the therapeutic response, as some studies have indicated that lymphocytes in the peripheral blood and the tumor nest respond differently to nCRT, with the circulating LYMR decreasing and the density of TILs increasing after nCRT.Citation14,Citation16
Other factors, such as the platelet count, serum albumin and CEA level, have been used to predict treatment responses in rectal cancer.Citation27–Citation29 In our study, we assessed the association between the CEA and CA 19-9 levels with pCR, but no statistical significance was found (P=0.590 and P=0.231, respectively). Patient compliance with concurrent chemotherapy should also be noted. In a Phase II trial conducted by Garcia-Aguilar et al,Citation30 patients who received chemotherapy with mFOLFOX6 between chemoradiation and surgery achieved a higher rate of pCR than those who did not, and this rate grew as the cycles of chemotherapy received increased from two to six. In our study, all the patients received combined chemotherapy with oxaliplatin, and patients who received three to four cycles achieved a higher rate of pCR than those who received one to two cycles (46.9% vs. 5.3%, P=0.015). Multivariate analysis revealed that the number of preoperative chemotherapy cycles was an independent factor for pCR (P=0.038 and P=0.046, respectively). Therefore, it is reasonable to assume that part of the predictive capacity of the NLR or LYMR, as reported in many studies, can be attributed to chemotherapy tolerance, as patients with a higher level of pre-CRT lymphocytes tend to tolerate more cycles of chemotherapy before surgery; however, in our study, statistical significance was not reached (61.4% vs. 17.6%, P=0.096).
There are some limitations to this study. First, the sample size of our study was small, and many cutoff values yielded from the dataset are different from those of previous studies, which may compromise comparability. Second, circulating lymphocytes and neutrophils can be affected by many other conditions, such as infectious and cardiovascular diseases,Citation31,Citation32 which were not evaluated in this study. Additionally, our evaluation of CD8+ TILs was performed using biopsy samples from one site of the tumor, not from the center and margin of the tumor, as previous studies have done.Citation17
Conclusion
Taken together, our study showed that the NLR, LYMR and combination represented by TIL–LYMR were predictive of pCR after chemoradiation for rectal cancer. TIL–LYMR, in particular, reflecting both the local and systemic immune reactions, may serve as an independent predictor of pCR and help to identify patients to receive “watch and wait” treatment instead of surgery. More studies with larger samples should be performed to confirm the value of this combined marker.
Acknowledgments
We thank professors Peirong Ding, Zhenhai Lu, Gong Chen and Liren Li for their generosity in providing study patients and offering insightful guidance for this study. A special thanks is due to Sherry Young, without whose unselfish support and encouragement this paper would never be completed. The study was funded by National Natural Science Foundation of China (No. 81502459 and No. 81772595), Science and Technology Project in Guangdong Province (No. 2013B021800146) and Sun Yat-sen University Clinical Research 5010 Program (No. 2014010).
Disclosure
The authors report no conflicts of interest in this work.
References
- BossetJFColletteLCalaisGEORTC Radiotherapy Group Trial 22921Chemotherapy with preoperative radiotherapy in rectal cancerN Engl J Med200635511111416971718
- Habr-GamaAPerezRONadalinWOperative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term resultsAnn Surg20042404717718
- LambregtsDMJMaasMBakersFCHLong-term follow-up features on rectal MRI during a wait-and-see approach after a clinical complete response in patients with rectal cancer treated with chemo-radiotherapyDis Colon Rectum201154121521152822067180
- SmithJDRubyJAGoodmanKANonoperative management of rectal cancer with complete clinical response after neoadjuvant therapyAnn Surg2012256696597223154394
- CarlomagnoCPepeSD’ArmientoFPPredictive factors of complete response to neoadjuvant chemoradiotherapy in patients with rectal cancerOncology2010785–636937520798559
- KaladyMFde CamposlobatoLFStocchiLPredictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancerAnn Surg2009250458219710605
- ZengWLiangJWangZClinical parameters predicting pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancerChin J Cancer2015341046847426268466
- JungSWParkIJOhSHAssociation of immunologic markers from complete blood counts with the response to preoperative chemoradiotherapy and prognosis in locally advanced rectal cancerOncotarget2017835597575976528938679
- FreyBRubnerYKulzerLAntitumor immune responses induced by ionizing irradiation and further immune stimulationCancer Immunol Immunother2014631293624052136
- LorimoreSACoatesPJScobieGEMilneGWrightEGInflammatory- type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects?Oncogene200120487085709511704832
- PerezCAFuAOnishkoHHallahanDEGengLRadiation induces an antitumour immune response to mouse melanomaInt J Radiat Biol200985121126113619995238
- YasudaKNireiTSunamiENagawaHKitayamaJDensity of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancerRadiat Oncol2011614921575175
- DouXWangRYanHCirculating lymphocytes as predictors of sensitivity to preoperative chemoradiotherapy in rectal cancer casesAsian Pac J Cancer Prev20131463881388523886201
- KitayamaJYasudaKKawaiKSunamiENagawaHCirculating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancerRadiat Oncol2010514720525293
- MandardAMDalibardFMandardJCPathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlationsCancer19947311268026868194005
- TengFMuDMengXTumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancerAm J Cancer Res2015562064207426269765
- GalonJType, density, and location of immune cells within human colorectal tumors predict clinical outcomeScience200631357951960196417008531
- MalietzisGGiacomettiMAskariAA preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgeryAnn Surg2014260228729224096764
- LewisCEPollardJWDistinct role of macrophages in different tumor microenvironmentsCancer Res200666260561216423985
- HoughtonMGRzymkiewiczDMJiHNeutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growthNat Med201016221922320081861
- SerugaBZhangHBernsteinLJCytokines and their relationship to the symptoms and outcome of cancerNat Rev Cancer200881188789918846100
- MlecnikBBindeaGAngellHKIntegrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instabilityImmunity201644369871126982367
- MlecnikBTosoliniMKirilovskyAHistopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reactionJ Clin Oncol201129661061821245428
- KimIYYouSHKimYWNeutrophil-lymphocyte ratio predicts pathologic tumor response and survival after preoperative chemoradiation for rectal cancerBMC Surg2014149425406793
- GrimmMFeyenOHofmannHImmunophenotyping of patients with oral squamous cell carcinoma in peripheral blood and associated tumor tissueTumor Biol201637338073816
- EmersonROSherwoodAMRiederMJHigh-throughput sequencing of T-cell receptors reveals a homogeneous repertoire of tumour-infiltrating lymphocytes in ovarian cancerJ Pathol2013231443344024027095
- ParkJWLimSBKimDYCarcinoembryonic antigen as a predictor of pathologic response and a prognostic factor in locally advanced rectal cancer patients treated with preoperative chemoradiotherapy and surgeryInt J Radiat Oncol Biol Phys200974381081719101093
- WanSLaiYMyersREPreoperative platelet count associates with survival and distant metastasis in surgically resected colorectal cancer patientsJ Gastrointest Cancer201344329330423549858
- YasudaKSunamiEKawaiKNagawaHKitayamaJLaboratory blood data have a significant impact on tumor response and outcome in preoperative chemoradiotherapy for advanced rectal cancerJ Gastrointest Cancer201243223624321365477
- Garcia-AguilarJChowOSSmithDDTiming of Rectal Cancer Response to Chemoradiation ConsortiumEffect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trialLancet Oncol201516895796626187751
- FarahRKhamisy FarahRAssociation of neutrophil to lymphocyte ratio with presence and severity of gastritis due to Helicobacter pylori infectionJ Clin Lab Anal201428321922324478129
- BhatTTeliSRijalJNeutrophil to lymphocyte ratio and cardiovascular diseases: a reviewExpert Rev Cardiovasc Ther20131115523259445
