Abstract
Shoulder morbidity is a well-documented sequela of breast cancer treatment, which includes various manifestations such as pain, reduced range of motion, and lymphedema, among others. The multifactorial nature of such morbidities has long been appreciated, and research on reliable risk predictors of development thereof still continues. Previous studies have demonstrated the potential of different types of physical therapy to treat such shoulder problems, and the integration of such interventions into routine care for breast cancer survivors is a requirement in most high-income countries. Although patients at risk for developing shoulder problems would most likely benefit from posttreatment physical therapy, currently, there is no gold standard for identifying this patient group. This is particularly important in low- and middle-income countries where scarce monetary resources need to be directed specifically to those most in need. Modulators of the angiogenesis pathway have been implicated in noncancer shoulder conditions such as rotator cuff disease, adhesive capsulitis, and tendon injuries. The present review summarizes the role of angiogenesis in the development of shoulder morbidity among breast cancer survivors and sets forth the rationale for our belief that angiogenesis signaling may help explain a proportion of the reported clinical variability noted in the development of shoulder pain and dysfunction and upper-limb lymphedema after breast cancer treatment.
Introduction
Among women, breast cancer is both the most common cause of cancer-related deaths and the most frequently diagnosed type of cancer (~25% of all cancers) worldwide.Citation1 The International Agency for Research on Cancer estimated 1.7 million new breast cancer cases among women worldwide in 2012,Citation1,Citation2 while in South Africa, an age-standardized incidence rate of 31.4 per 100,000 was estimated in 2011 as provided by the National Cancer Registry.Citation3 More recent prevalence or incidence rates have not yet been established, either globally or in South Africa.
High 5-year survival rates of up to 89.2% have been reported following breast cancer treatment.Citation4 Although generally lower in low-income countries,Citation4 survival rates will most likely continue to rise due to advances in treatment, increased awareness, and early detection. Nevertheless, a significant proportion of breast cancer survivors experience upper extremity problems after treatment, which include pain, tightness, numbness, lymphedema, and limited range of motion, among others.Citation5–Citation9 Moreover, previous studies have suggested that shoulder morbidity after breast cancer treatment may persist longer than is commonly reported.Citation5,Citation10,Citation11 Although substantial variability exists among previous reports, 20%–32% of breast cancer survivors report arm lymphedema and pain or dysfunction in the shoulder, arm, or breast at least 1 year after primary treatment.Citation5,Citation12–Citation17 These side effects, especially at the shoulder or arm, reduce the functional use of the upper limb of affected patients, limiting their quality of life and ability to return to work.Citation5,Citation18,Citation19 The shoulder girdle comprises joints (glenohumeral, acromioclavicular, and sternoclavicular joints), associated muscles, and connective tissue connecting the upper arm and shoulder area, providing a mechanism for upper-limb motion. Breast cancer treatment is believed to place survivors at risk of developing impaired resting shoulder girdle alignment.Citation20 The altered shoulder movement patterns observed in breast cancer survivors mimic those seen in known general shoulder conditions such as rotator cuff disease and adhesive capsulitis.Citation21 In fact, such diagnoses have been used to describe shoulder and arm morbidities in breast cancer survivors and have been associated with pain.Citation20,Citation22,Citation23 It is believed that breast cancer treatment-related impairments of the shoulder complex place breast cancer survivors at risk of developing symptomatic rotator cuff disease.Citation20
There is substantial evidence supporting the effectiveness of different types of physical therapy in reducing upper-limb pain and lymphedema and improving shoulder range of motion (and, thus, shoulder function) in breast cancer survivors experiencing upper-limb morbidity.Citation24–Citation28 Nonetheless, the complex etiology of shoulder morbidity in breast cancer survivors has long been appreciated, with both treatment-related and patient-related factors implicated.Citation10,Citation12,Citation15,Citation17,Citation20,Citation29–Citation37 These factors include treatment type (e.g., type of surgery and the extent of lymph node surgery), time after treatment, disease characteristics, age, genetic factors, and the presence of comorbidities and other phenotypes such as high BMI. However, a large proportion of the variation in developing pain, and perhaps other morbidities as well, following breast cancer treatment still remains unexplained.Citation31,Citation38 For instance, Wang et al reviewed that absolute risk increases of only 3%–21% in developing persistent pain following breast cancer treatment can be explained from a total of 77 factors.Citation31 Previous findings suggest that shoulder morbidity after breast cancer treatment is bilateral,Citation10,Citation30,Citation36 and it has been shown that “structures unrelated to direct surgery and/or radiotherapy treatment are affected,”Citation39 suggesting a systemic cause. These collective findings suggest the need to focus on molecular signaling pathways.
Fibrogenic and inflammatory molecular signaling pathways have been implicated in the development of morbidity following breast cancer treatment as well as other forms of cancer.Citation40–Citation46 Although such signaling pathways constitute normal healing and repair processes, aberrant expression of signaling factors may underlie pathological processes such as persistent pain, swelling, and dysfunction of the shoulder complex reported among breast cancer survivors. According to the central dogma of molecular biology, it would follow that genetics may also be an important factor. Indeed, evidence suggesting specific single-nucleotide polymorphism (SNP)-modulated variation in inflammatory gene expression has been reported in studies on musculoskeletal pain following breast cancer treatment with aromatase inhibitors.Citation47,Citation48 Signaling pathways of inflammation, fibrosis, and angiogenesis have several links. In addition to fibrosis and inflammation, angiogenesis has been implicated in noncancer shoulder conditions such as rotator cuff diseaseCitation49–Citation51 and adhesive capsulitis.Citation52 Angiogenesis has also been implicated in healing and adaptation pathways in non-cancer musculoskeletal conditions such as tendon injuriesCitation53 and in the physiological response to mechanical loading.Citation54,Citation55 Evaluation of angiogenesis signaling factors in breast cancer survivors may, therefore, increase our understanding of the pathophysiology of upper-limb morbidities in this context and, perhaps, help explain a proportion of the variability in the development of such morbidities. In this review, we discuss the aspects of angiogenesis-related signaling factors as potential markers of risk for the development of shoulder morbidity after breast cancer treatment.
Ethical approval
The current review is part of an ongoing study approved by the Human Research Ethics Committee at the University of Cape Town, South Africa.
Literature search and selection
Database searches for articles and reviews were conducted in the following databases: PubMed, Scopus, Web of Science, MEDLINE, and CINAHL for articles published from January 1990 up to July 2016 using the following search terms: (“breast cancer” OR “breast carcinoma” OR “breast neoplasm*” OR “breast tumor”) AND (“treatment” OR “therapy”) AND (“shoulder pain” OR “shoulder dysfunction” OR “lymphoedema” OR “lymphedema” OR “range of motion”) AND (“angiogenesis” OR “inflammat*”). A search was also conducted using the same terms excluding (“breast cancer” OR “breast carcinoma” OR “breast neoplasm*” OR “breast tumor”) and (“treatment” OR “therapy”) to capture articles implicating angiogenesis in general shoulder conditions independent of breast cancer treatment. The articles were narrowed down by analyzing the titles and then the abstracts. We retained articles and reviews on the pathophysiology of shoulder pain and dysfunction both in breast cancer survivors and in noncancer patients. Trials on the efficacy of treatment of shoulder pathology were excluded.
Angiogenesis
Angiogenesis, the formation of new blood vessels from the endothelium of existing vasculature, is a fundamental process that occurs in both physiological and pathophysiological situations.Citation56 It is a complex process that results in the transition of an endothelial cell (EC) from a quiescent to angio-active phenotype, through the activation of vital cellular events including EC activation, proliferation, migration, survival, and extracellular matrix remodelingCitation57 (). Angiogenesis regulation involves two main transcription factors, nuclear factor κB (NF-κB) and hypoxia-inducible factors (HIFs), in separate molecular pathways that induce expression of angiogenesis-associated signaling factors (cytokines, growth factors, and receptors).Citation56 The transcription factor NF-κB links angiogenesis and inflammation processes.Citation56 Furthermore, inflammatory cells participate in the regulation of angiogenesis by secreting proinflammatory cytokines that directly or indirectly influence the activity of ECs.Citation58
Figure 1 A schematic representation of angiogenesis signaling and suggested links to breast cancer therapy, inflammatory signaling, and matrix remodeling.
Notes: Breast cancer treatments, especially radiotherapy and chemotherapy, are known to induce an inflammatory response and resultant hypoxia in the tissue microenvironment. This response activates the two main transcription factors involved in angiogenesis regulation, NFκB and HIF1-α, which in turn activate angiogenesis signaling. Angiogenesis is largely regulated by the main factor VEGF-A and its receptor KDR, which induce the activation, proliferation, migration, survival, and maturation of endothelial cells.
Abbreviations: ECM, extracellular matrix; eNOS, endothelial nitric oxide synthase; HIF, hypoxia-inducible factor; ICAM, intercellular adhession molecule; MMPs, matrix metalloproteinases; NF-κB, nuclear factor κB; PDGF, platelet-derived growth factor; THBS, thrombospondins; TIMPs, tissue inhibitors of metalloproteinases; TNF-α, tumor necrosis factor-alpha; VCAM, vascular cell adhesion molecule; VEGF-A, vascular endothelial growth factor-A.
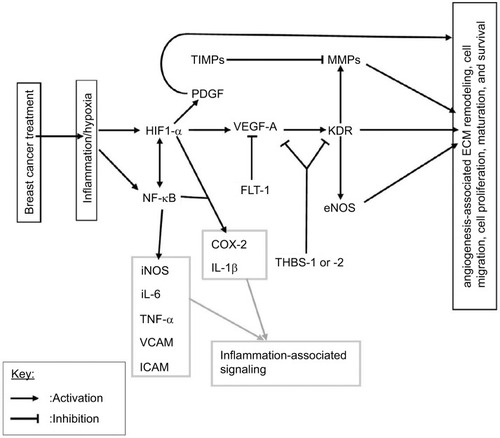
Vascular endothelial growth factor-A (VEGF-A), the main angiogenesis signaling factor, stimulates EC proliferation, survival, and migration, mainly through its receptor VEGF-R2 (also known as KDR; ).Citation56,Citation57 Matrix metalloproteinases (MMPs) catalyze the proteolytic degradation of the basement membrane, which precedes EC migration, and their activity is regulated by tissue inhibitors of metalloproteinases (TIMPs).Citation57 Thrombospondins (THBS-1 or -2) mediate antiangiogenic effects through the suppression of VEGF-A bioavailability and the inhibition of its activity through KDR.Citation59 VEGF-A signaling through KDR upregulates some MMPs and endothelial nitric oxide synthase (eNOS), and the later catalyzes nitric oxide (NO) production which stimulates vascular permeability, an essential event in angiogenesis.Citation60,Citation61 The adhesion molecules vascular cell adhesion molecules (VCAMs) and intercellular adhesion molecules, which mediate leukocyte infiltration, are also important components of angiogenesis.Citation62,Citation63 VEGFR-1 also known as FLT-1, with its relatively higher affinity for VEGF-A and weak tyrosine kinase activity, is believed to reduce VEGF-A bioavailability to the main angiogenesis signaling receptor, KDR, and is therefore antiangiogenic.Citation57,Citation64 Even though the links to angiogenesis are not included, for simplicity, it is noteworthy that the proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) also possess proangiogenic effects and can transcriptionally be activated by HIF or NF-κBCitation56,Citation58 ().
The direct determination of angiogenesis involves investigation of changes in microvessel density, which may be done using ultrasound examination or immunohistochemistry of tissue biopsies. However, angiogenesis-related factors may provide useful information regarding angiogenic potential. The occurrence of angiogenesis depends on the balance between proangiogenic and antiangiogenic molecules in the tissue microenvironmentCitation65 (). Upregulation of proangiogenic factors promotes angiogenesis by facilitating the migration, proliferation, and differentiation of ECs into newly formed capillaries that can subsequently develop into more mature vessels.Citation57,Citation65 The HIF and NF-κB signaling pathways crossregulate each other,Citation56,Citation57 and in effect, the HIF pathway can be induced in nonhypoxic conditions such as in response to reactive oxygen species (ROS) and NO.Citation56 Angiogenesis is also regulated by microRNAs, which either upregulate or downregulate specific components of the angiogenesis pathway.Citation56,Citation66
Table 1 Examples of proangiogenic and antiangiogenic factors
Role of angiogenesis in response to breast cancer treatment
Angiogenesis is, at least to some extent, involved in the physiological response of the tumor microenvironment, or surrounding tissue, to breast cancer treatment, especially the common adjuvant treatments radiotherapy and chemotherapy. Moreover, an imbalance of or changes in inflammatory angiogenesis-related cytokines (ARCs) have been demonstrated in breast cancer patients after treatment.Citation67,Citation68 For example, De Sanctis et al reported significant increases in levels of interleukins (IL[s]) IL-1β, IL-2, and IL-6 and TNF-α 4 weeks after adjuvant radiation therapy, particularly in patients presenting with fatigue.Citation67 Although some angiogenesis signaling factors are immobilized by various means such as binding to extracellular matrix components, some are soluble and thus capable of mediating systemic effects. This may particularly be important in the development of morbidity among breast cancer survivors since it has been shown that structures outside the direct treatment field are also affected.Citation39
Radiotherapy and angiogenesis
Radiotherapy involves exposure of the tumor site to a specific dose of ionizing radiation, usually given in fractions. More than 50% of early stage breast cancer patients receive radiation therapy either alone or as an adjuvant treatment.Citation69 Ionizing radiation damages DNA, which induces an intracellular response that leads to death by apoptosis in susceptible cells.Citation70 Rapid proliferating cells, such as tumor cells, are particularly sensitive to ionizing radiation but DNA damage is also induced in normal tissue, including ECs, due to the indiscriminate nature of the treatment.Citation41 Ionizing radiation also acts indirectly through the generation of ROS and reactive nitrogen species which, in addition to damaging DNA, inhibit cellular enzymes.Citation70,Citation71 Consequently, when the cell’s antioxidant and DNA repair capacities are overwhelmed, cell death by apoptosis occurs.Citation70,Citation71
The DNA damage from irradiation elicits a biological response in the tumor microenvironment, which involves the release of chemokines, cytokines, and adhesion molecules leading to altered cell–cell interactions and the influx of inflammatory cells, particularly in ECs of tumor and/or normal tissue.Citation41,Citation72,Citation73 These mediators are involved in various molecular pathways including angiogenesis, and they may participate in the development of normal tissue damage characteristic of either early (occurring in <6 months after irradiation) or late (occurring in ≥6 months after irradiation) radiation toxicity effects.Citation74,Citation75 Radiation also induces soft tissue fibrosis, which can cause stiffness in the shoulder girdle leading to reduced range of motion.Citation20,Citation40 Fibrosis is also linked to angiogenesis through its main signaling factor, transforming growth factor-beta (TGF-β), which can influence angiogenesis.Citation57
One of the late toxicity effects of radiation therapy is damage to the vascular system in the form of lesions, which may occur months to several years after treatment.Citation42 The microvessels (i.e., capillaries) appear to be the most sensitive to lesions, and the damage culminates in thrombosis, necrosis, capillary rupture, or even loss of entire capillary segments.Citation42 The physiological response to vascular damage is the activation of the angiogenesis pathway. There are several ways in which radiotherapy-induced vascular damage can promote angiogenesis. The resultant hypoxia is, in itself, a trigger for angiogenesis. Inflammatory cells attracted to the site of radiation damage can potentiate angiogenesis by secreting inflammatory cytokines that have proangiogenic signals such as TNF-α, IL-1β, and IL-6 or angiogenesis factors such as VEGF. For example, macrophages have been shown to enhance their expression of VEGF-A and release NO, a stabilizer of HIF-1, in response to irradiation.Citation76 Radiation has also been shown to reduce the expression of antiangiogenic factors at low doses.Citation76 As a result, there is ongoing research on the co-use of antiangiogenic drugs to increase effectiveness of radiotherapy in cancer treatment.Citation72,Citation76
Clearly, there are conflicting findings on the effect of irradiation on angiogenesis,Citation41,Citation73,Citation77 and it has been suggested that the effects are dose and time dependent.Citation76 Sofia Vala et al reported that low-dose ionizing radiation promotes EC migration and, in harmony with their findings, they demonstrated that low-dose ionizing radiation promotes tumor growth and metastasis using murine experimental models of leukemia and orthotopic breast cancer.Citation73 Plausibly, the same effect on angiogenesis can be expected given the low doses that characterize conventional fractionated radiotherapy.
Chemotherapy and angiogenesis
Many different types of chemotherapy drugs administered in various combinations are used to treat breast cancer. In a curative setting, cytotoxic drugs are usually given as an adjuvant (i.e., in addition to surgery and/or radiotherapy) for primary breast cancer to reduce the risk of recurrence. Chemotherapy can also be given in a neoadjuvant setting to shrink the tumor. Although there are many different types of mechanistically distinct chemotherapy drugs, there are general side effects associated with most of them such as fatigue, joint pain, depression, mouth ulcers, hair loss, and myalgia.Citation45 However, there appears to be an involvement of cytokines in the development of these symptoms, although cytokine profiles may vary with the type of drug.Citation45,Citation68,Citation78
Taxanes have been shown to have intratumoral antiangiogenic effects in murine models of breast cancer.Citation79 Although intratumoral effects on angiogenesis are less relevant to breast cancer survivors, after tumor resection, this demonstrates the potential of some chemotherapy drugs to influence angiogenesis. Furthermore, given the integrated approach that characterizes conventional breast cancer treatment, there may be augmentation of angiogenesis stimuli. Wood et al suggested that several mechanistically distinct chemotherapy drugs share a common ability to activate the enzyme p38 mitogen-activated protein kinase (p38 MAPK), which induces expression of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6.Citation45 Elevated levels of cytokines such as TNF-α, IL-6, IL-8, and IL-10 have been reported in cancer patients undergoing conventional chemotherapy.Citation80–Citation82 It is noteworthy that these cytokines are also associated with angiogenesis.
Potential role in healing and adaptation pathways
Owing to their roles in cellular proliferation, survival, migration, chemotaxis, matrix remodeling, blood vessel formation, and links to other molecular pathways such as inflammation, ARCs and growth factors may play a role in healing and adaptation pathways after treatment. Angiogenesis-related growth factors such as basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and insulin-like growth factor-1 are believed to participate in rotator cuff tendon healing.Citation53 Genes encoding angiogenesis-related signaling factors such as VEGF have been shown to be involved in the response of tendon cells and cartilage discs to mechanical loading.Citation54,Citation55 Previous findings suggest a compensatory angiogenesis in lymphedema.Citation83 It would follow that the development of shoulder/arm pain, lymphedema, and dysfunction after treatment may, perhaps, be a result of failed healing. Aberrant angiogenesis signaling may lead to abnormal cell–cell interactions, nonoptimal angiogenesis, and impairments in matrix remodeling, which collectively compromises tissue healing.
Potential role in pain, inflammation, and dysfunction
Angiogenesis-related signaling factors such as IL-1β, IL-6, TNF-α, and COX-2 are known to contribute to pain hyper-sensitivity by inducing the production of prostaglandins and other proalgesic agents, which activate nociceptors.Citation84 Lymph node dissection can lead to lymphedema by compromising the lymphatic system’s ability to return interstitial fluid to main circulation, which is important in the resolution of inflammation. However, pro-inflammatory ARCs such as IL-1β, IL-6, and TNF-α have the potential to aggravate lymphedema by enhancing inflammation. Some angiogenesis-related factors are involved in extracellular matrix remodeling (). Abnormal collagen and matrix production can potentially contribute to stiffness in the shoulder joint capsule,Citation85 which can lead to reductions in the range of motion.
Implication in noncancer general shoulder conditions
Previous studies suggest the importance of angiogenesis in noncancer musculoskeletal conditions of the shoulder such as tendon injuries,Citation53 adhesive capsulitis,Citation52 and rotator cuff disease.Citation51,Citation86–Citation88 For example, such studies have demonstrated altered vascularity or potential to heal as determined from fibroblast cellularity, vascularity, and the presence of a significant inflammatory component in rotator cuff tears.Citation86–Citation88 Moreover, previous studies have demonstrated altered expression of ARCs in such noncancer general musculoskeletal conditions of the shoulder.Citation51,Citation52,Citation85,Citation89,Citation90 For example, altered levels of cytokines including IL-1β, IL-8, and VEGF have been shown in rotator cuff disease,Citation51 whereas IL-1β, TNF-α, PDGF, and TGF-β were implicated in the pathogenesis of adhesive capsulitis.Citation52
Genetic aspects
Evidence of a potential genetic component in the development of posttreatment morbidity in breast cancer survivors has been explored and reported in several studies.Citation34,Citation35,Citation44,Citation47,Citation91–Citation93 Most of these studies involved associations between gene polymorphisms with clinical outcomes such as lymph-edema, breast pain, fatigue, and anxiety, covering a total of 17 SNPs and 4 haplotypes from 14 genesCitation34,Citation35,Citation44,Citation47,Citation91–Citation93 (). However, replication of these findings has not been achieved, largely due to methodological issues among the studies which include, but are not limited to, variations in population structure and size, and heterogeneity in follow-up periods (or period after surgery) and outcome measures. Moreover, to date, these studiesCitation34,Citation35,Citation44,Citation47,Citation91–Citation93 have largely focused on inflammatory gene polymorphisms, with one exception.Citation34 Clearly, angiogenesis is unexplored even though evidence shows it is linked to inflammation. Associations were reported for both increased and decreased risks of morbidity (). For example, one study reported an association between persistent breast pain following breast cancer surgery and a polymorphism within IL-1 receptor type 2 (IL1-R2 rs1,16,74,595 T>C) and also highlighted a haplotype, implicating the 3′ untranslated region within IL-10.Citation35 In another study, an association between a polymorphism in NFKB2 gene and the risk of developing lymphedema after breast cancer treatment was reported.Citation44 With the exception of lymphedema, the clinical outcomes these studies focused on were not shoulder complex-specific outcomes such as movement-related pain or dysfunction, which are known to reduce quality of life.Citation34,Citation35,Citation44,Citation47,Citation91–Citation93 There are indications of a genetic component in the development of noncancer musculoskeletal shoulder conditions, evident from the predisposition of siblings to develop rotator cuff disease or rotator cuff tears.Citation49,Citation94,Citation95 In support of this, associations between several polymorphisms and risk of developing rotator cuff disease have been reported.Citation96 Furthermore, previous studies have reported associations between polymorphisms in various genes including genes encoding collagen and angiogenesis-related factors and risks of musculoskeletal injuries in sport.Citation97–Citation100 These factors are components of matrix remodeling, which is linked to angiogenesis.
Table 2 Genes and SNPs that have been reported to be significantly associated with morbidity after breast cancer treatment
Conclusion and knowledge gaps
Angiogenesis is potentially involved in the etiology of shoulder pain, lymphedema, and dysfunction and may help explain a proportion of the interindividual variability in the development of such morbidity among breast cancer survivors. It is involved in the response of the tissue microenvironment to adjuvant cancer therapies and has potential roles in pain, lymphedema, and dysfunction pathways. Furthermore, angiogenesis has been shown to play a role in noncancer shoulder conditions such as rotator cuff disease.Citation86–Citation88 However, there is a paucity of relevant studies investigating its role in morbidity after breast cancer treatment. Although several studies have explored expression profiles of cytokines and growth factors in breast cancer patients, most of these have focused on a few clinical symptoms such as cognitive impairments, fatigue, and breast pain or general pain.Citation67,Citation68,Citation78,Citation80,Citation81,Citation101,Citation102 Clearly, most of these symptoms do not reside at the shoulder complex, the site most associated with limitations in daily activities. In addition, most focus has been on inflammatory cytokines with one exception.Citation34 Similarly, with one exception,Citation34 candidate genetic associations have largely focused on polymorphisms in inflammatory cytokine genes. Indeed, the role of angiogenesis signaling in the development of shoulder complex morbidity after breast cancer treatment is unexplored despite that it is linked to inflammation. Therefore, there is a need to explore and characterize the potential of angiogenesis-associated signaling factors in explaining interindividual variability in the development of posttreatment morbidity in breast cancer survivors. It is noteworthy that most of the studies that explored signaling factors in posttreatment morbidities,Citation67,Citation68,Citation78,Citation80,Citation81,Citation101,Citation102 with one exception,Citation101 involved a relatively short follow-up period (or period after surgery), up to 6 months after treatment, whereas current evidence suggests that shoulder/arm morbidity in breast cancer patients can persist for up to 6 years or more after surgery.Citation10,Citation11 Molecular signaling pathways or symptoms may evolve over time, and there is a need for longer follow-up periods (or period after surgery) to investigate this phenomenon.
Acknowledgments
We acknowledge the financial assistance of the South African National Research Foundation (NRF) toward this research. Opinions expressed and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the NRF.
Disclosure
The authors report no conflicts of interest in this work.
References
- WHOLatest World Cancer StatisticsGenevaWorld Health Organization 201312122013 Available from: https://www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdfAccessed December 07, 2017
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- National Cancer Registry Report 2011Cancer in South Africa 2011 Full ReportNational Health Laboratory Service201643 Available from: http://www.cansa.org.za/files/2016/08/NCR-2011-cancer-tables.pdfAccessed May 15, 2017w
- YouldenDRCrambSMDunnNAMullerJMPykeCMBaadePDThe descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortalityCancer Epidemiol201236323724822459198
- HayesSCJohanssonKStoutNLUpper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of careCancer20121188 Suppl2237224922488698
- HayesSCRyeSBattistuttaDDiSipioTNewmanBUpper-body morbidity following breast cancer treatment is common, may persist longer-term and adversely influences quality of lifeHealth Qual Life Outcomes2010892
- DeanLTDeMicheleALeBlancMBlack breast cancer survivors experience greater upper extremity disabilityBreast Cancer Res Treat2015154111712526420404
- FeitenSDunnebackeJHeymannsJBreast cancer morbidity: questionnaire survey of patients on the long term effects of disease and adjuvant therapyDtsch Arztebl Int20141113132537544
- JohansenSFossaKNesvoldILMalinenEFossaSDArm and shoulder morbidity following surgery and radiotherapy for breast cancerActa Oncol201453452152924495044
- ShamleyDLascurain-AguirrebenaIOskrochiRSrinaganathanRShoulder morbidity after treatment for breast cancer is bilateral and greater after mastectomyActa Oncol20125181045105322731831
- SchmitzKHSpeckRMRyeSADiSipioTHayesSCPrevalence of breast cancer treatment sequelae over 6 years of follow-up: the Pulling Through StudyCancer20121188 Suppl2217222522488696
- TogawaKMaHSullivan-HalleyJRisk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort studyBreast Cancer Res: BCR201416441425145603
- YenTWFanXSparapaniRLaudPWWalkerAPNattingerABA contemporary, population-based study of lymphedema risk factors in older breast cancer womenAnn Surg Oncol200916497998819194754
- PeuckmannVEkholmORasmussenNKChronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in DenmarkEur J Pain200913547848518635381
- MiaskowskiCPaulSMCooperBIdentification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgeryEur J Oncol Nurs201418324225324485012
- GartnerRJensenMBNielsenJEwertzMKromanNKehletHPrevalence of and factors associated with persistent pain following breast cancer surgeryJAMA2009302181985199219903919
- HiddingJTBeurskensCHvan der WeesPJvan LaarhovenHWNijhuis-van der SandenMWTreatment related impairments in arm and shoulder in patients with breast cancer: a systematic reviewPLoS One201495e9674824816774
- NesvoldILReinertsenKVFossaSDDahlAAThe relation between arm/shoulder problems and quality of life in breast cancer survivors: a cross-sectional and longitudinal studyJ Cancer Surviv201151627220972640
- NesvoldILFossaSDHolmINaumeBDahlAAArm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of lifeActa Oncol201049334735319842790
- EbaughDSpinelliBSchmitzKHShoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivorsMed Hypotheses201177448148721764521
- ShamleyDSrinaganathanROskrochiRLascurain-AguirrebenaISugdenEThree-dimensional scapulothoracic motion following treatment for breast cancerBreast Cancer Res Treat2009118231532218998205
- StubblefieldMDCustodioCMUpper-extremity pain disorders in breast cancerArch Phys Med Rehabil2006873 Suppl 1S96S9916500198
- StubblefieldMDKeoleNUpper body pain and functional disorders in patients with breast cancerPMR201462170183
- TathamBSmithJCheifetzOThe efficacy of exercise therapy in reducing shoulder pain related to breast cancer: a systematic reviewPhysiother Can201365432133024396158
- GalantinoMLStoutNLExercise interventions for upper limb dysfunction due to breast cancer treatmentPhys Ther201393101291129723907077
- McNeelyMLCampbellKOspinaMExercise interventions for upper-limb dysfunction due to breast cancer treatmentCochrane Database Syst Rev20106CD005211
- LohSYMusaANMethods to improve rehabilitation of patients following breast cancer surgery: a review of systematic reviewsBreast Cancer (Dove Med Press)20157819825792854
- De GroefAVan KampenMDieltjensEEffectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic reviewArch Phys Med Rehabil20159661140115325595999
- NesvoldILDahlAALokkevikEMarit MengshoelAFossaSDArm and shoulder morbidity in breast cancer patients after breast-conserving therapy versus mastectomyActa Oncol200847583584218568481
- SagenAKaaresenRSandvikLThuneIRisbergMAUpper limb physical function and adverse effects after breast cancer surgery: a prospective 2.5-year follow-up study and preoperative measuresArch Phys Med Rehabil201495587588124389401
- WangLGuyattGHKennedySAPredictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studiesCMAJ201618814E352E36127402075
- JohansenSFossKNesvoldILMalinenEFossSDArm and shoulder morbidity following surgery and radiotherapy for breast cancerActa Oncol201453452152924495044
- DahlAANesvoldILReinertsenKVFossaSDArm/shoulder problems and insomnia symptoms in breast cancer survivors: cross-sectional, controlled and longitudinal observationsSleep Med201112658459021645872
- MiaskowskiCDoddMPaulSMLymphatic and angiogenic candidate genes predict the development of secondary lymphedema following breast cancer surgeryPLoS One201384e6016423613720
- StephensKCooperBAWestCAssociations between cytokine gene variations and severe persistent breast pain in women following breast cancer surgeryJ Pain201415216918024411993
- AdriaenssensNVinh-HungVMiedemaGEarly contralateral shoulder-arm morbidity in breast cancer patients enrolled in a randomized trial of post-surgery radiation therapyBreast Cancer (Auckl)20126799322904635
- DiSipioTRyeSNewmanBHayesSIncidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysisLancet Oncol201314650051523540561
- BellRJRobinsonPJNazeemFPersistent breast pain 5 years after treatment of invasive breast cancer is largely unexplained by factors associated with treatmentJ Cancer Surviv2014811823975613
- ShamleyDRSrinanaganathanRWeatherallRChanges in shoulder muscle size and activity following treatment for breast cancerBreast Cancer Res Treat20071061192717221154
- StraubJMNewJHamiltonCDLominskaCShnayderYThomasSMRadiation-induced fibrosis: mechanisms and implications for therapyJ Cancer Res Clinical Oncol2015141111985199425910988
- BaetenCICastermansKLammeringGEffects of radiotherapy and chemotherapy on angiogenesis and leukocyte infiltration in rectal cancerInt J Radiat Oncol Biol Phys20066641219122717145537
- FajardoLFThe pathology of ionizing radiation as defined by morphologic patternsActa Oncol2005441132215848902
- PanisCPavanelliWRCytokines as mediators of pain-related process in breast cancerMediators Inflamm2015201512903426635447
- LeungGBaggottCWestCCytokine candidate genes predict the development of secondary lymphedema following breast cancer surgeryLymphat Re Biol20141211022
- WoodLJNailLMGilsterAWintersKAElseaCRCancer chemotherapy-related symptoms: evidence to suggest a role for proinflammatory cytokinesOncol Nurs Forum200633353554216676010
- Di MaggioFMMinafraLForteGIPortrait of inflammatory response to ionizing radiation treatmentJ Inflamm (Lond)2015121425705130
- IngleJNSchaidDJGossPEGenome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitorsJ Clin Oncol201028314674468220876420
- LiuMWangLBongartzTAromatase inhibitors, estrogens and musculoskeletal pain: estrogen-dependent T-cell leukemia 1A (TCL1A) gene-mediated regulation of cytokine expressionBreast Cancer Res2012142R4122405131
- LongoUGBertonAPapapietroNMaffulliNDenaroVEpidemiology, genetics and biological factors of rotator cuff tearsMed Sport Sci2012571921986040
- HegedusEJCookCBrennanMWylandDGarrisonJCDriesnerDVascularity and tendon pathology in the rotator cuff: a review of literature and implications for rehabilitation and surgeryBr J Sports Med2010441283884719293165
- SavitskayaYAIzaguirreASierraLThe effect of angiogenesis-related cytokine profiles on rotator cuff disease: discovery of new biomarkers of early tendon degeneration (SS-19)J Arthroscopic Relat Surg2011275 Supple39
- RodeoSAHannafinJATomJWarrenRFWickiewiczTLImmunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulderJ Orthop Res19971534274369246090
- OlivaFViaAGMaffulliNRole of growth factors in rotator cuff healingSports Med Arthrosc201119321822621822105
- PufeTLemkeAKurzBMechanical overload induces VEGF in cartilage discs via hypoxia-inducible factorAm J Pathol2004164118519214695332
- MousavizadehRDuronioVMcCormackBKhosraviSScottAMechanical loading modulates angiogenic factors in tendon cellsBr J Sports Med2013479e2
- SzadeAGrochot-PrzeczekAFlorczykUJozkowiczADulakJCellular and molecular mechanisms of inflammation-induced angiogenesisIUBMB Life201567314515925899846
- CarmelietPJainRKMolecular mechanisms and clinical applications of angiogenesisNature2011473734729830721593862
- NaldiniACarraroFRole of inflammatory mediators in angiogenesisCurr Drug Targets Inflamm Allergy2005413815720228
- LawlerPRLawlerJMolecular basis for the regulation of angiogenesis by thrombospondin-1 and -2Cold Spring Harbor Perspect Med201225a006627
- FunahashiYShawberCJSharmaAKanamaruEChoiYKKitajew-skiJNotch modulates VEGF action in endothelial cells by inducing matrix metalloprotease activityVasc Cell201131221349159
- KrollJWaltenbergerJVEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR)Biochem Biophys Res Commun199825237437469837777
- SchlesingerMBendasGVascular cell adhesion molecule-1 (VCAM-1)–an increasing insight into its role in tumorigenicity and metastasisInt J Cancer2015136112504251424771582
- HuangMTMasonJCBirdseyGMEndothelial intercellular adhesion molecule (ICAM)-2 regulates angiogenesisBlood200510651636164315920013
- ShibuyaMVascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapiesGenes Cancer20112121097110522866201
- EichhornMEKleespiesAAngeleMKJauchKWBrunsCJAngiogenesis in cancer: molecular mechanisms, clinical impactLangenbecks Arch Surg2007392337137917458577
- BuysschaertISchmidtTRoncalCCarmelietPLambrechtsDGenetics, epigenetics and pharmaco-(epi)genomics in angiogenesisJ Cell Mol Med2008126B2533255119210754
- De SanctisVAgolliLViscoVCytokines, fatigue, and cutaneous erythema in early stage breast cancer patients receiving adjuvant radiation therapyBiomed Res Int2014201452356824800238
- JanelsinsMCMustianKMPaleshOGDifferential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment researchSupport Care Cancer201220483183921533812
- DeSantisCELinCCMariottoABCancer treatment and survivorship statistics, 2014CA Cancer J Clin201464425227124890451
- BaskarRDaiJWenlongNYeoRYeohKWBiological response of cancer cells to radiation treatmentFront Mol Biosci201412425988165
- MiaoLHolleyAKZhaoYClairWHStClairDKStRedox-mediated and ionizing-radiation-induced inflammatory mediators in prostate cancer development and treatmentAntioxid Redox Signal20142091481150024093432
- MartinBJInhibiting vasculogenesis after radiation: a new paradigm to improve local control by radiotherapySemin Radiat Oncol201323428128724012342
- Sofia ValaIMartinsLRImaizumiNLow doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesisPLoS One201056e1122220574535
- BentzenSMPreventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathologyNat Rev Cancer20066970271316929324
- BarnettGCWestCMDunningAMNormal tissue reactions to radiotherapy: towards tailoring treatment dose by genotypeNat Rev Cancer20099213414219148183
- KleibeukerEAGriffioenAWVerheulHMSlotmanBJThijssenVLCombining angiogenesis inhibition and radiotherapy: a double-edged swordDrug Resist Updat201215317318222561672
- ImaizumiNMonnierYHegiMMirimanoffRORüeggCRadiotherapy suppresses angiogenesis in mice through TGF-βRI/ALK5-dependent inhibition of endothelial cell sproutingPLoS One201056e1108420552031
- CheungYTNgTShweMAssociation of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort studyAnn Oncol20152671446145125922060
- KlauberNParangiSFlynnEHamelED’AmatoRJInhibition of angiogenesis and breast cancer in mice by the microtubule inhibitors 2-methoxyestradiol and taxolCancer Res199757181868988045
- PusztaiLMendozaTRReubenJMChanges in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapyCytokine20042539410214698135
- TsavarisNKosmasCVadiakaMKanelopoulosPBoulamatsisDImmune changes in patients with advanced breast cancer undergoing chemotherapy with taxanesBr J Cancer2002871212712085250
- RovatiBMariucciSDelfantiSSimultaneous detection of circulating immunological parameters and tumor biomarkers in early stage breast cancer patients during adjuvant chemotherapyCell Oncol (Dordr)201639321122826769126
- MellorRHStantonAWMenadueLLevickJRMortimerPSEvidence for dermal angiogenesis in breast cancer related lymphedema demonstrated using dual-site fluorescence angiographyMicrocirculation20029320721912080418
- BasbaumAIBautistaDMScherrerGJuliusDCellular and molecular mechanisms of painCell2009139226728419837031
- BunkerTDReillyJBairdKSHamblenDLExpression of growth factors, cytokines and matrix metalloproteinases in frozen shoulderJ Bone Joint Surg Br200082576877310963182
- KarthikeyanSGriffinDRParsonsNMicrovascular blood flow in normal and pathologic rotator cuffsJ Shoulder Elbow Surg201524121954196026412209
- LevyORelwaniJZamanTEvenTVenkateswaranBCopelandSMeasurement of blood flow in the rotator cuff using laser Doppler flowmetryJ Bone Joint Surg Br200890789389818591599
- MatthewsTJHandGCReesJLAthanasouNACarrAJPathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increasesJ Bone Joint Surg Br200688448949516567784
- BediAMaakTWalshCCytokines in rotator cuff degeneration and repairJ Shoulder Elbow Surg201221221822722244065
- LakemeierSReicheltJJPatzerTFuchs-WinkelmannSPalettaJRSchoferMDThe association between retraction of the torn rotator cuff and increasing expression of hypoxia inducible factor 1α and vascular endothelial growth factor expression: an immunohistological studyBMC Musculoskelet Disor2010111230
- Collado-HidalgoABowerJEGanzPAIrwinMRColeSWCytokine gene polymorphisms and fatigue in breast cancer survivors: early findingsBrain, Behav Immun20082281197120018617366
- BowerJEGanzPAIrwinMRCastellonSArevaloJColeSWCytokine genetic variations and fatigue among patients with breast cancerJ Clin Oncol201331131656166123530106
- MiaskowskiCElboimCPaulSMPolymorphisms in tumor necrosis factor-α are associated with higher anxiety levels in women after breast cancer surgeryClin Breast Cancer20161616371.e325813148
- GwilymSEWatkinsBCooperCDGenetic influences in the progression of tears of the rotator cuffJ Bone Joint Surg Br200991791591719567856
- HarviePOstlereSJTehJGenetic influences in the aetiology of tears of the rotator cuff. Sibling risk of a full-thickness tearJ Bone Joint Surg Br200486569670015274266
- Motta GdaRAmaralMVRezendeEEvidence of genetic variations associated with rotator cuff diseaseJ Shoulder Elbow Surg201423222723524129055
- MaffulliNMargiottiKLongoUGLoppiniMFazioVMDenaroVThe genetics of sports injuries and athletic performanceMuscles Ligaments Tendons J20133317318924367777
- PosthumusMSeptemberAVO’CuinneagainDvan der MerweWSchwellnusMPCollinsMThe COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participantsAm J Sports Med200937112234224019654427
- GibbonAPHobbsHvan der MerweWPolymorphisms of the matrix metalloproteinase-3 gene and the risk of musculoskeletal soft tissue injuriesJ Sci Med Sport201418e42
- RahimMGibbonAHobbsHThe association of genes involved in the angiogenesis-associated signaling pathway with risk of anterior cruciate ligament ruptureJ Orthop Res201432121612161825111568
- Collado-HidalgoABowerJEGanzPAColeSWIrwinMRInflammatory biomarkers for persistent fatigue in breast cancer survivorsClin Cancer Res20061292759276616675568
- GeorgiouGKIgglezouMSainisIImpact of breast cancer surgery on angiogenesis circulating biomarkers: a prospective longitudinal studyWorld J Surg Oncol20131121323981902
