Abstract
Colorectal cancer is one of the most common human malignant tumors. Recent research has shown that colorectal cancer is a dysbacteriosis-induced disease; however, the role of intestinal bacteria in colorectal cancer is unclear. This review explores the role of intestinal flora in colorectal cancer. In total, 57 articles were included after identification and screening. The pertinent literature on floral metabolites in colorectal cancer from three metabolic perspectives – including carbohydrate, lipid, and amino acid metabolism – was analyzed. An association network regarding the role of intestinal flora from a metabolic perspective was constructed by analyzing the previous literature to provide direction and insight for further research on intestinal flora in colorectal cancer.
Introduction
Colorectal cancer is the third leading cancer in humans and the fourth most common cause of cancer-related death.Citation1 The causes of the occurrence and development of colorectal cancer are unclear, but it is thought to result from a combination of genetic and environmental factors.Citation2 Intestinal flora and their metabolites, as environmental factors, play important roles in colorectal cancer by regulating related genes.Citation3
The main function of the colorectum is to store feces while under siege from complex intestinal flora. Several probiotics,Citation4–Citation6 including Lactobacillus acidophilus, Bifidobacterium, Lactobacillus rhamnosus, and Streptococcus thermophilus, as well as pathogenic bacteria,Citation7–Citation9 including Enterococcus faecalis, Enterotoxigenic bacteroides fragilis, Streptococcus bovis, Salmonella, Clostridia, and Fusobacterium nucleatum, comprise the diverse intestinal flora. This intestinal flora, mucosal epithelial cells, foodborne probiotic components, and small molecules – including hormones, enzymes, mucus, and bile salts – constitute a complex intestinal micro-ecosystem.Citation10 Although individual substances vary, the intestinal micro-ecosystem is relatively stable under physiological conditions. Multiple diseases may result if changes occur beyond the ability of compensatory adjustment.Citation11,Citation12 Studies have shown that the micro-ecosystem equilibrium in patients with colorectal cancer is disrupted.Citation13 Various intestinal flora and metabolites are closely related to colorectal cancer.Citation14
With advanced developments in microbiome and microbial metabolomics, especially rapid advancements in high-throughput sequencing technology, increasing attention has been given to studying intestinal flora and intestinal microecology in recent years.Citation15 Current research has focused on the relationship between intestinal flora and colorectal cancer; however, the specific mechanism of the intestinal flora in causing colorectal cancer is unclear.Citation16,Citation17 Microbial primary metabolites, including amino acids, nucleotides, polysaccharides, lipids, and vitamins, are necessary to sustain intestinal flora growth and reproduction.Citation18 Microbial primary metabolites are similar in most microbial cells. The synthesis of primary metabolites is a constant process, and synthetic obstacles affect normal microbial activities.Citation19,Citation20 Microbial secondary metabolites, including alkaloids, phenols, antibiotics, and pigments, determine the specificity and function of the flora. Microbes are valuable in maintaining the balance of the intestinal microecology;Citation21,Citation22 however, the significance of microbial metabolites in colorectal cancer is unclear. Given that the metabolism of three substances, including carbohydrate, lipid, and amino acid metabolism, is the general metabolic mechanism among all creatures, we tried to build a link between the intestinal flora and colorectal cancer from this angle.
In this review, we comprehensively analyzed and classified the pertinent literature on microflora metabolites in colorectal cancer from three metabolic perspectives, including carbohydrate, lipid, and amino acid metabolism. An association network of intestinal flora, their metabolites, and colorectal cancer was built that may provide direction and insight for further research on intestinal flora in colorectal cancer.
Methods
Literature search
We searched the “PubMed”, “Embase”, and “Cochrane” databases for literature published up to August 11, 2017. To achieve maximum sensitivity of the search strategy and identify all studies, the following terms were combined: (“colorectal or colon or rectal, large intestine or large bowel or intestinum crassum” and “neoplasms or tumor or carcinoma or cancer” and “flora or microflora or microorganism or microbiome or microbiota or microbe or microbiology or germ or bacteria or bacterium or fungus”) and (“glucose or adenosine triphosphate or lactic acid or mitochondria or galactose or sucrase or amylase or hexokinase or glucokinase or pyruvate kinase or glucuronidase” OR “triglyceride or fat or aliphatic acid or lipoprotein or cholesterol or cholesterin or bile acid or lithocholic acid or vitamin d or dehydroxylase” OR “amino acid or ammonia or amine or urea or carbamide or ureophil or mucin or mucoprotein or nitrosamines or nitroguanidine or nitrosourea or aromatic amines or mycotoxin or endotoxin or exotoxin or sulfuretted hydrogen or hydrogen sulfide or hydrothion”). All relevant abstracts were retrieved independently by two authors, and articles with available information for the present systematic review were fully reviewed. In total, 42 articles were included. To present a more comprehensive role of flora in colorectal cancer, flora appearing in the 42 articles were used as the medical subject headings (MeSH) and the pertinent literature was retrieved. Finally, 15 articles were added after identification and screening. Moreover, pertinent literature from the searched studies was analyzed. A detailed search strategy is presented in .
Figure 1 Literature search strategy.
Notes: The databases “PubMed”, “Embase”, and “Cochrane” were searched for literature published up to August 11, 2017. Forty-two articles were assessed for eligibility after identification and screening. To present a more comprehensive role of flora in colorectal cancer, the flora appearing in the 42 articles were used as the medical subject headings (MeSH) and the pertinent literature was retrieved. A further 15 articles were added after identification and screening.
Study selection
Studies catering to the following criteria were considered for inclusion: 1) studies that were published in English and 2) studies that involved intestinal flora and intestinal flora metabolism in colorectal cancer, both in vivo and in vitro. Exclusion criteria were as follows: 1) letters, case reports, reviews, or conference reports; 2) predominant studies that were not on intestinal flora metabolism in colorectal cancer; and 3) correlation studies did not involve flora metabolism.
Role of flora metabolites in colorectal cancer
Intestinal flora and carbohydrate metabolism in colorectal cancer
Carbohydrate metabolism refers to a series of complex chemical reactions in vivo. The tricarboxylic acid cycle, as the principal pathway of carbohydrate metabolism, is the final metabolic pathway and metabolic hub of the three major nutrients, including carbohydrates, lipids, and amino acids.Citation23,Citation24 Carbohydrate metabolism is important for intestinal flora and colorectal cancer. First, oxygen plays a decisive role in choosing the carbohydrate metabolic pathway. Both anaerobic and aerobic bacteria coexist in the intestinal tract. Superoxide, oxygen radicals, and oxygen molecules are closely related to the development of colorectal cancer.Citation25,Citation26 Second, carbon dioxide and water are the primary producers in carbohydrate metabolism.Citation27 Various bacteria decompose glucose and lactose and produce acid.Citation28 Intestinal micro-ecology is regulated by maintaining the acid–base balance and regulating osmotic pressure. Third, adenosine triphosphate (ATP) is produced during carbohydrate metabolism and is an important compound that supplies energy to all living cells. Phosphoribose produced during the metabolism of pentose phosphate is necessary to synthesize DNA and RNA, and they are especially important for rapidly reproducing bacteria and infinitely replicating cancer cells.Citation29 Fourth, nicotinamide adenine dinucleotide phosphate (NADPH) is the intermediate metabolite in carbohydrate metabolism, and it participates in phosphorylating proteins and genes. It may be involved in microbial variation and epigenetic regulation of colorectal cancer.Citation30 Finally, mitochondria are the key location for carbohydrate metabolism, and mitochondrial dysfunction is one the most important features in colorectal cancer and intestinal flora imbalance.Citation31,Citation32
Intestinal flora and lipid metabolism in colorectal cancer
Lipids include triglycerides, phospholipids, cholesterol, and glycolipid. Triglycerides provide energy for living organisms by emulsifying bile acid salts and catalyzing lipase in the small intestine.Citation33,Citation34 Phospholipids and sugar esters maintain biomembrane structure and function.Citation35 Cholesterol can transform into vitamins, bile acid, or steroid hormones.Citation36 Many studies indicate that a high-fat diet can induce colorectal cancer, and imply that intestinal flora play irreplaceable roles; however, their specific mechanisms remain unclear.Citation37,Citation38 In this review, we searched for clues on tumorigenesis by summarizing the pertinent literature. High-fat diets can increase bile and bile acid secretion in the colorectum, and some clostridia can accelerate transformation of bile acid into secondary bile acid by participating in the synthesis of various enzymes during fatty acid metabolism.Citation39,Citation40 Secondary bile acid, as a carcinogenic substance, promotes colorectal cancer by multiple molecular mechanisms – synthesizing oxygen free radicals, fracturing DNA strands, making chromosomes unstable, and forming cancer stem cells.Citation41,Citation42 Interactions between fatty acids, bile acids, and intestinal flora can produce diacylglycerol, prostaglandin, and leukotriene, leading to tumorigenesis by activating immune or inflammatory responses.Citation43–Citation45
Intestinal flora and amino acid metabolism in colorectal cancer
Amino acid metabolism involves two parts. Amino acids can be used to synthesize proteins, peptides, and other nitrogenous substances and, moreover, they can be decomposed into α-ketonic acid, amines, and carbon dioxide through deamination, transamination, and decarboxylation. Many toxic substances such as sulfur, nitrates, hydrogen sulfide, ammonia, and amines are involved in the metabolic process, and these toxic substances can lead to colorectal cancer.Citation46 Food residue with high protein content can stimulate sulfate-reducing bacterial growth. Hydrogen sulfide is a product of sulfate-reducing bacteria as well as an intermediate product of amino acid metabolism.Citation47 Hydrogen sulfide elicits several pathogenic events, including cell proliferation, differentiation, apoptosis, and inflammation – ultimately leading to malignant enterocyte transformation.Citation48,Citation49 Nitrate is not toxic, but easily reduces to nitrite due to the intestinal flora. Nitrite combines with nitrogenous compounds such as amines, amino compounds, and methyl urea to form carcinogenic nitroso compounds.Citation50,Citation51 Furthermore, mucin as an intermediate product of amino acid metabolism is a mutagenic agent with cooperation from the intestinal flora.Citation52 Many enzymes, peptides, and other nitrogenous substances secreted by the intestinal flora are involved in activating and regulating important signal molecules and signaling pathways in tumorigenesis.Citation53,Citation54
Results and discussion
The intestinal flora and host maintain a dynamic balance under physiological conditions. When this balance is disrupted, the entire micro-ecological system is significantly altered.Citation55,Citation56 The synergistic effect among intestinal flora, metabolites, and the host plays a pivotal role in the occurrence and development of colorectal cancer. First, microbes are the initial factors in colorectal cancer. Changes to the intestinal flora distribution and abundance contributes to inflammatory and immunological responses and induces malignant transformation of the intestinal mucosal cells.Citation57 Second, epidemiological surveys have indicated that the balance of intestinal flora in patients with precancerous lesions, including inflammatory bowel disease (IBD) and intestinal polyps, were altered significantly.Citation58,Citation59 Third, various metabolic products of the intestinal flora can, directly or indirectly, promote development and progression of colorectal cancer.Citation60 Fourth, micro-ecology helps to prevent tumorigenesis by reestablishing the intestinal micro-ecological balance.Citation61,Citation62 In conclusion, colorectal cancer is a dysbacteriosis-induced disease, and the understanding of this disease has changed in the molecular age.
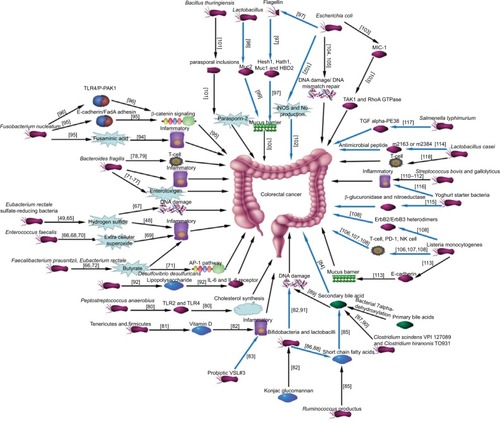
Researchers have increasingly focused on determining the specific bacteria or microbial community structural changes in colorectal cancer by sequencing 16S rRNA and bioinformatics analysis in recent years. Many researchers support that Streptococcus bovisCitation63 and Streptococcus gallolyticusCitation64 are the specific bacteria involved in colorectal cancer; however, there are some lacunae in this research. Sequencing of the 16s rRNA variable region only identifies the bacterial species, and the intra-individual variability of the bacteria was not considered. In addition, a better scientific method for studying the intestinal flora in colorectal cancer is to explore its relationship with the intestinal micro-ecological system. The intestinal micro-ecological system is complex and integral, with individual differences. Establishing an association network for the intestinal micro-ecological system in colorectal cancer may offer an approach to solving this dilemma. As shown in , the association network for the intestinal flora and microbial metabolites in colorectal cancer, from a metabolic perspective, was constructed by analyzing the previous literature. Although we tried to search all pertinent literature, mistakes of omission inevitably occurred because of the complexity of microbial metabolism and the many compounds involved in it. Research on the relationship between microbial metabolites and colorectal cancer were relatively insufficient.
Figure 2 Network for the intestinal flora and microbial metabolites in colorectal cancer.
Notes: An association network of the intestinal flora and microbial metabolites in colorectal cancer from a metabolic perspective was constructed by analyzing the previous literature. The black and blue arrows represent pathogenic bacteria as well as probiotics, respectively. References are located within the square parentheses.
Future directions
There appears to be a complex relationship between colorectal cancer and intestinal flora. Microbial metabolites may play vital roles in balancing the intestinal micro-ecology and in developing colorectal cancer. The intestinal flora is insufficiently understood, intestinal micro-ecology is complex, and intestinal flora show significant intra-individual variability; thus, evaluating all of these interactions is challenging. With the great progress of integrated systems, molecular biology, and bioinformatics, we urgently call for a synthesis of the existing research to establish a comprehensive database that focuses on individual relationships among the intestinal flora, microbial metabolites, and colorectal cancer.
It is plausible that the intestinal flora and microbial metabolites in colorectal cancer are related to the immune system and inflammatory abnormalities. Although much effort has been expended, many bottlenecks must be addressed before stepping from the imbalanced intestinal micro-ecological system to immune system and inflammatory abnormalities to genesis and development of colorectal cancer. The clinical correlation of the intestinal flora, microbial metabolites, and colorectal cancer remains unknown; thus, it is essential to conduct further functional assays on pathogenesis such as the microbiome, microbial metabolomics, and peptidome assays. Multiple probiotics have been applied clinically for some time, and preclinical trials involving intestinal flora transfusion are also underway. Prospective and retrospective studies on the incidence of colorectal cancer after clinical interventions with microbial preparations should be scheduled.
Several avenues are available to pursue translational applications. First, microchip arrays or metabonomic technologies can assess the risk and monitor the curative effects of a bacterial species or specific microbial metabolite in colorectal cancer. Second, probiotics, microbial metabolites, and fecal microbial transfusions can all be used to recover the intestinal micro-ecological system to prevent colorectal cancer; however, this requires further clinical testing. Third, intestinal micro-ecology is influenced by many factors, including the endocrine system, diet, sleep, and stress. Testing the intestinal flora and microbial metabolites in feces can guide the adjustment of dietary structure or living habits to prevent colorectal cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- MármolISánchez-de-DiegoCPradilla DiesteACerradaERodriguez YoldiMJColorectal Carcinoma: a general overview and future perspectives in colorectal cancerInt J Mol Sci2017181 pii:E197
- Ibáñez-SanzGDíez-VillanuevaAAlonsoMHRisk model for colorectal cancer in Spanish population using environmental and genetic factors: results from the MCC-Spain studySci Rep201774326328233817
- Manzat-SaplacanRMMirceaPABalacescuLChiraRIBerindan-NeagoeIBalacescuOCan we change our microbiome to prevent colorectal cancer development?Acta Oncol20155481085109526073561
- DodooCCWangJBasitAWStapletonPGaisfordSTargeted delivery of probiotics to enhance gastrointestinal stability and intestinal colonisationInt J Pharm20175301–222422928764983
- QuagliarielloAAloisioIBozzi CionciNEffect of Bifidobacterium breve on the intestinal microbiota of coeliac children on a gluten free diet: a pilot studyNutrients2016810 pii:E660
- YoonJSSohnWLeeOYEffect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trialJ Gastroenterol Hepatol2014291525923829297
- AdesidaSAEzentaCCAdagbadaAOAladesokanAACokerAOCarriage of multidrug resistant Enterococcus faecium and Enterococcus faecalis among apparently healthy humansAfr J Infect Dis2017112838928670644
- FukugaitiMHIgnacioAFernandesMRRibeiro JúniorUNakanoVAvila-CamposMJHigh occurrence of Fusobacterium nucleatum and Clostridium difficile in the intestinal microbiota of colorectal carcinoma patientsBraz J Microbiol20154641135114026691472
- ParitskyMPastukhNBrodskyDIsakovichNPeretzAAssociation of Streptococcus bovis presence in colonic content with advanced colonic lesionWorld J Gastroenterol201521185663566725987793
- GomezAPetrzelkovaKYeomanCJGut microbiome composition and metabolomic profiles of wild western lowland gorillas (Gorilla gorilla gorilla) reflect host ecologyMol Ecol201524102551256525846719
- NiJWuGDAlbenbergLTomovVTGut microbiota and IBD: causation or correlation?Nat Rev Gastroenterol Hepatol2017141057358428743984
- SommerFAndersonJMBhartiRRaesJRosenstielPThe resilience of the intestinal microbiota influences health and diseaseNat Rev Microbiol2017151063063828626231
- SheflinAMWhitneyAKWeirTLCancer-promoting effects of microbial dysbiosisCurr Oncol Rep2014161040625123079
- WangXWangJRaoBDengLGut flora profiling and fecal metabolite composition of colorectal cancer patients and healthy individualsExp Ther Med20171362848285428587349
- ShafferMArmstrongAJSPhelanVVReisdorphNLozuponeCAMicrobiome and metabolome data integration provides insight into health and diseaseTransl Res2017189516428764956
- CalistriDRengucciCCasadei GardiniAFecal DNA for noninvasive diagnosis of colorectal cancer in immunochemical fecal occult blood test-positive individualsCancer Epidemiol Biomarkers Prev201019102647265420929882
- SinhaRAhnJSampsonJNFecal microbiota, fecal metabolome, and colorectal cancer interrelationsPLoS One2016113e015212627015276
- SinghRKumarMMittalAMehtaPKMicrobial metabolites in nutrition, healthcare and agriculture3 Biotech20177115
- YapIKLiJVSaricJMetabonomic and microbiological analysis of the dynamic effect of vancomycin-induced gut microbiota modification in the mouseJ Proteome Res2008793718372818698804
- PattersonECryanJFFitzgeraldGFRossRPDinanTGStantonCGut microbiota, the pharmabiotics they produce and host healthProc Nutr Soc201473447748925196939
- ShiYPanCWangKSynthetic multispecies microbial communities reveals shifts in secondary metabolism and facilitates cryptic natural product discoveryEnviron Microbiol20171993606361828714207
- Narsing RaoMPXiaoMLiWJFungal and bacterial pigments: secondary metabolites with wide applicationsFront Microbiol20178111328690593
- Ribel-MadsenARibel-MadsenRBrønsCNewgardCBVaagAAHellgrenLIPlasma acylcarnitine profiling indicates increased fatty acid oxidation relative to tricarboxylic acid cycle capacity in young, healthy low birth weight menPhysiol Rep2016419 pii:e12977
- PatkovaAJoskovaVHavelEEnergy, protein, carbohydrate, and lipid intakes and their effects on morbidity and mortality in critically ill adult patients: a systematic reviewAdv Nutr20178462463428710148
- AzzolinVFCadonáFCMachadoAKSuperoxide-hydrogen peroxide imbalance interferes with colorectal cancer cells viability, proliferation and oxaliplatin responseToxicol In Vitro20163281526674755
- TsuruyaAKuwaharaASaitoYMajor anaerobic bacteria responsible for the production of carcinogenic acetaldehyde from ethanol in the colon and rectumAlcohol Alcohol201651439540126755640
- VinkePCEl AidySvan DijkGThe role of supplemental complex dietary carbohydrates and gut microbiota in promoting cardiometabolic and immunological health in obesity: lessons from healthy non-obese individualsFront Nutr201743428791292
- LiuZLiuTProduction of acrylic acid and propionic acid by constructing a portion of the 3-hydroxypropionate/4-hydroxybutyrate cycle from Metallosphaera sedula in Escherichia coliJ Ind Microbiol Biotechnol201643121659167027722922
- DeanBMPerrettDStudies on adenine and adenosine metabolism by intact human erythrocytes using high performance liquid chromatographyBiochim Biophys Acta1976437115949498
- LiYKunduPSeowSWGut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ miceCarcinogenesis20123361231123822461519
- ShuwenHXiYYuefenPCan mitochondria DNA provide a novel biomarker for evaluating the risk and prognosis of colorectal cancer?Dis Markers20172017518980328408773
- Saint-Georges-ChaumetYEdeasMMicrobiota-mitochondria inter-talk: consequence for microbiota-host interactionPathog Dis2016741ftv09626500226
- RyanMKochunovPRowlandLMLipid metabolism, abdominal adiposity, and cerebral health in the AmishObesity (Silver Spring)201725111876188028834322
- GiangTMGaucelSBrestazPDynamic modeling of in vitro lipid digestion: individual fatty acid release and bioaccessibility kineticsFood Chem20161941180118826471670
- ShenCXueMQiuHGuoWInsertion of neurotransmitters into a lipid bilayer membrane and its implication on membrane stability: a molecular dynamics studyChemphyschem201718662663328054433
- BuitenwerfEDullaartRPFMuller KoboldACCholesterol delivery to the adrenal glands estimated by adrenal venous sampling: an in vivo model to determine the contribution of circulating lipoproteins to steroidogenesis in humansJ Clin Lipidol201711373373828461157
- WangCZHuangWHZhangCFRole of intestinal microbiome in American ginseng-mediated colon cancer protection in high fat diet-fed AOM/DSS miceClin Transl Oncol Epub2017814
- WeberCNutrition. Diet change alters microbiota and might affect cancer riskNat Rev Gastroenterol Hepatol2015126314
- RidlonJMWolfPGGaskinsHRTaurocholic acid metabolism by gut microbes and colon cancerGut Microbes20167320121527003186
- O’KeefeSJLiJVLahtiLFat, fibre and cancer risk in African Americans and rural AfricansNat Commun20156634225919227
- FarhanaLNangia-MakkerPArbitEBile acid: a potential inducer of colon cancer stem cellsStem Cell Res Ther20167118127908290
- AjouzHMukherjiDShamseddineASecondary bile acids: an under-recognized cause of colon cancerWorld J Surg Oncol20141216424884764
- SavariSVinnakotaKZhangYSjölanderACysteinyl leukotrienes and their receptors: bridging inflammation and colorectal cancerWorld J Gastroenterol201420496897724574769
- WangDDuBoisRNAn inflammatory mediator, prostaglandin E2, in colorectal cancerCancer J201319650251024270349
- KaiMYamamotoESatoAEpigenetic silencing of diacylglycerol kinase gamma in colorectal cancerMol Carcinog20175671743175228218473
- MaNTianYWuYMaXContributions of the interaction between dietary protein and gut microbiota to intestinal healthCurr Protein Pept Sci201718879580828215168
- CarboneroFBenefielACAlizadeh-GhamsariAHGaskinsHRMicrobial pathways in colonic sulfur metabolism and links with health and diseaseFront Physiol2012344823226130
- Attene-RamosMSNavaGMMuellnerMGWagnerEDPlewaMJGaskinsHRDNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 Int cellsEnviron Mol Mutagen201051430431420120018
- YaziciCWolfPGKimHRace-dependent association of sulfidogenic bacteria with colorectal cancerGut201766111983199428153960
- Espejo-HerreraNGràcia-LavedanEBoldoEColorectal cancer risk and nitrate exposure through drinking water and dietInt J Cancer2016139233434626954527
- BinghamSAPignatelliBPollockJRDoes increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer?Carcinogenesis19961735155238631138
- RokhsefatSLinAComelliEMMucin-microbiota interaction during postnatal maturation of the intestinal ecosystem: clinical implicationsDig Dis Sci20166161473148626792279
- YangTOwenJLLightfootYLKladdeMPMohamadzadehMMicrobiota impact on the epigenetic regulation of colorectal cancerTrends Mol Med2013191271472524051204
- KumarMNagpalRVermaVProbiotic metabolites as epigenetic targets in the prevention of colon cancerNutr Rev2013711233423282249
- OkeSMartinAInsights into the role of the intestinal microbiota in colon cancerTherap Adv Gastroenterol2017105417428
- GagnièreJRaischJVeziantJGut microbiota imbalance and colorectal cancerWorld J Gastroenterol201622250151826811603
- García-CastilloVSanhuezaEMcNerneyEOnateSAGarcíaAMicrobiota dysbiosis: a new piece in the understanding of the carcinogenesis puzzleJ Med Microbiol201665121347136227902422
- PascalVPozueloMBorruelNA microbial signature for Crohn’s diseaseGut201766581382228179361
- BrimHYoosephSZoetendalEGMicrobiome analysis of stool samples from African Americans with colon polypsPLoS One2013812e8135224376500
- WeirTLManterDKSheflinAMBarnettBAHeubergerALRyanEPStool microbiome and metabolome differences between colorectal cancer patients and healthy adultsPLoS One201388e7080323940645
- GaoZGuoBGaoRZhuQWuWQinHProbiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancerMol Med Rep20151246119612726238090
- Dos ReisSAda ConceiçãooLLSiqueiraNPRosaDDda SilvaLLPeluzioMDReview of the mechanisms of probiotic actions in the prevention of colorectal cancerNutr Res20173711928215310
- TsaiCEChiuCTRaynerCKAssociated factors in Streptococcus bovis bacteremia and colorectal cancerKaohsiung J Med Sci201632419620027185602
- Andres-FranchMGalianaASanchez-HellinVStreptococcus gallolyticus infection in colorectal cancer and association with biological and clinical factorsPLoS One2017123e017430528355283
- NavaGMOuJO;KeefeSJGaskinsHRDiversity of colonic Archaea and sulfate reducing bacteria populations in native Africans differs from those in Caucasian and African AmericansGastroenterology20091365A410
- BalamuruganRRajendiranEGeorgeSSamuelGVRamakrishnaBSReal-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancerJ Gastroenterol Hepatol2008238Pt 11298130318624900
- Attene-RamosMSWagnerEDGaskinsHRPlewaMJHydrogen sulfide induces direct radical-associated DNA damageMol Cancer Res20075545545917475672
- MooreDRKotakeYHuyckeMMEffects of iron and phytic acid on production of extracellular radicals by Enterococcus faecalisExp Biol Med (Maywood)2004229111186119515564446
- DeplanckeBGaskinsHRHydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cellsFASEB J200317101310131212738807
- WeirTMarschkeRFBrownRJFecal metabolome and micro-flora differences between colorectal cancer patients and healthy adultsJ Clin Oncol20133115 Suppl11050
- ChmelařDHájekMJanečkováJVobejdováJMartinekováPKašíkováAThe effect of oxygen on endotoxin production in bacteria of the Bacteroides fragilis group isolated from patients with colorectal carcinomaEpidemiol Mikrobiol Imunol2016652129135 Czech27467330
- PurcellRVPearsonJAitchisonADixonLFrizelleFAKeenanJIColonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasiaPLoS One2017122e017160228151975
- Thiele OrbergEFanHTamAJThe myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesisMucosal Immunol201710242143327301879
- IrrazabalTMartinAT regulatory cells gone bad: an oncogenic immune response against Enterotoxigenic B. fragilis infection leads to colon cancerCancer Discov20155101021102326429936
- TsoiHChuESHZhangXPeptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in miceGastroenterology2017152614191433.e528126350
- MeekerSMPaikJHsuCCThe gut microbiome is modulated by dietary vitamin dJ Am Assoc Lab Anim Sci2015545643644
- WuWTChengHCChenHLAmeliorative effects of konjac glucomannan on human faecal β-glucuronidase activity, secondary bile acid levels and faecal water toxicity towards Caco-2 cellsBr J Nutr2011105459360021144106
- AppleyardCBCruzMLIsidroAAArthurJCJobinCDe SimoneCPretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancerAm J Physiol Gastrointest Liver Physiol20113016G1004G101321903764
- XuMQCaoHLWangSCaoXCYanFWangBMThe influence of intestinal microbiota on the secondary bile acid-induced colorectal carcinogenesisJ Dig Dis201415130
- MinamidaKKanekoMOhashiMEffects of difructose anhydride III (DFA III) administration on bile acids and growth of DFA III-assimilating bacterium Ruminococcus productus on rat intestineJ Biosci Bioeng200599654855416233830
- ZampaASilviSFabianiRMorozziGOrpianesiCCresciAEffects of different digestible carbohydrates on bile acid metabolism and SCFA production by human gut micro-flora grown in an in vitro semi-continuous cultureAnaerobe2004101192616701496
- WellsJEWilliamsKBWhiteheadTRHeumanDMHylemonPBDevelopment and application of a polymerase chain reaction assay for the detection and enumeration of bile acid 7alpha-dehydroxylating bacteria in human fecesClin Chim Acta20033311–212713412691873
- FaddenKOwenRWFaecal steroids and colorectal cancer: the effect of lactulose on faecal bacterial metabolism in a continuous culture model of the large intestineEur J Cancer Prev1992121131271463973
- ZhengZYBernsteinCBile salt/acid induction of DNA damage in bacterial cells: effect of taurine conjugationNutr Cancer19921821571641437652
- MurrayWRBlackwoodATrotterJMCalmanKCMacKayCFaecal bile acids and clostridia in the aetiology of colorectal cancerBr J Cancer19804169239287426316
- Pool-ZobelBLNeudeckerCDomizlaffILactobacillus- and bifidobacterium-mediated antigenotoxicity in the colon of ratsNutr Cancer19962633653808910918
- CholewaKWęglarzLParfiniewiczBLodowskaJJaworska-KikMThe influence of desulfovibrio desulfuricans endotoxin on IL-6 and IL-6 receptor genes expression in colon cancer Caco-2 cellsFarmaceutyczny Przeglad Naukowy2010732732
- KosticADChunERobertsonLFusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironmentCell Host Microbe201314220721523954159
- ChenYPengYYuJInvasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascadeOncotarget2017819318023181428423670
- BeckerSOelschlaegerTAWullaertABacteria regulate intestinal epithelial cell differentiation factors both in vitro and in vivoPLoS One201382e5562023418447
- Burger-van PaassenNBoumaJBoehmGvan GoudoeverHVan SeuningenIRenesIBLactobacillus GG stimulates mucin MUC2 synthesis in the human colon cancer cell line LS174TGastroenterology20091365A267
- JohanssonMEPhillipsonMPeterssonJVelcichAHolmLHanssonGCThe inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteriaProc Natl Acad Sci U S A200810539150641506918806221
- FemiaAPGianniniAFaziMIdentification of mucin depleted foci in the human colonCancer Prev Res (Phila)20081756256719139006
- AkibaTAbeYKitadaSCrystal structure of the parasporin-2 Bacillus thuringiensis toxin that recognizes cancer cellsJ Mol Biol2009386112113319094993
- WitthöftTEckmannLKimJMKagnoffMFEnteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cellsAm J Physiol19982753 Pt 1G564G5719724270
- YoshimuraKLairdLSChiaCYLive attenuated Listeria monocytogenes effectively treats hepatic colorectal cancer metastases and is strongly enhanced by depletion of regulatory T cellsCancer Res20076720100581006617942940
- OliveiraMJLauwaetTDe BruyneGMareelMLeroyAListeria monocytogenes produces a pro-invasive factor that signals via ErbB2/ErbB3 heterodimersJ Cancer Res Clin Oncol20051311495915480783
- BoleijAMuytjensCMBukhariSINovel clues on the specific association of Streptococcus gallolyticus subsp gallolyticus with colorectal cancerJ Infect Dis201120381101110921451000
- TsaiTLLiACChenYCLiaoYSLinTHAntimicrobial peptide m2163 or m2386 identified from Lactobacillus casei ATCC 334 can trigger apoptosis in the human colorectal cancer cell line SW480Tumor Biol201536537753789
- de Moreno de LeBlancAPerdigónGReduction of beta-glucuronidase and nitroreductase activity by yoghurt in a murine colon cancer modelBiocell2005291152415954463
- de Moreno de LeBlancAPerdigónGMechanisms involved in the antitumor activity exerted by yoghurt in an experimental colon cancer modelInt J Cancer Prev200523181193
- LimDKimKSKimHAnti-tumor activity of an immunotoxin (TGFα-PE38) delivered by attenuated Salmonella typhimuriumOncotarget2017823375503756028473665
- LenoirMDel CarmenSCortes-PerezNGLactobacillus casei BL23 regulates Treg and Th17 T-cell populations and reduces DMH-associated colorectal cancerJ Gastroenterol201651986287326749362
- SzemesTVlkovaBMinarikGOn the origin of reactive oxygen species and antioxidative mechanisms in Enterococcus faecalisRedox Rep201015520220621062535
- NepelskaMCultroneABéguet-CrespelFButyrate produced by commensal bacteria potentiates phorbol esters induced AP-1 response in human intestinal epithelial cellsPLoS One2012712e5286923300800
- LvYYeTWangHPSuppression of colorectal tumorigenesis by recombinant Bacteroides fragilis enterotoxin-2 in vivoWorld J Gastroenterol201723460361328216966
- DeStefano ShieldsCEVan MeerbekeSWHousseauFReduction of murine colon tumorigenesis driven by enterotoxigenic Bacteroides fragilis using cefoxitin treatmentJ Infect Dis2016214112212926908749
- GeisALFanHWuXRegulatory T-cell response to enterotoxigenic Bacteroides fragilis colonization triggers IL17-dependent colon carcinogenesisCancer Discov20155101098110926201900
- HanYWFusobacterium nucleatum: a commensal-turned pathogenCurr Opin Microbiol20152314114725576662
- RubinsteinMRWangXLiuWHaoYCaiGHanYWFusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesinCell Host Microbe201314219520623954158
- ChoiHJKimJDoKHParkSHMoonYEnteropathogenic Escherichia coli-induced macrophage inhibitory cytokine 1 mediates cancer cell survival: an in vitro implication of infection-linked tumor disseminationOncogene201332414960496923503457
- Cuevas-RamosGPetitCRMarcqIBouryMOswaldENougayrèdeJPEscherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cellsProc Natl Acad Sci U S A201010725115371154220534522
- MaddocksODShortAJDonnenbergMSBaderSHarrisonDJAttaching and effacing Escherichia coli downregulate DNA mismatch repair protein in vitro and are associated with colorectal adenocarcinomas in humansPLoS One200945e551719436735
- OlinoKWadaSEdilBHTumor-associated antigen expressing Listeria monocytogenes induces effective primary and memory T-cell responses against hepatic colorectal cancer metastasesAnn Surg Oncol201219Suppl 3S597S60721979110
- PanZKIkonomidisGLazenbyAPardollDPatersonYA recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumoursNat Med1995154714777585097
- BiarcJNguyenISPiniACarcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S.bovis)Carcinogenesis20042581477148414742316
- EllmerichSSchöllerMDurantonBPromotion of intestinal carcinogenesis by Streptococcus bovisCarcinogenesis200021475375610753212
- LecuitMUnderstanding how Listeria monocytogenes targets and crosses host barriersClin Microbiol Infect200511643043615882192
