Abstract
Purpose
Melanoma represents an important public health problem, due to its high case-fatality rate. Identification of individuals at high risk would be of major interest to improve early diagnosis and ultimately survival. The aim of this study was to evaluate whether MC1R variants predicted melanoma risk independently of at-risk phenotypic characteristics.
Materials and methods
Data were collected within an international collaboration – the M-SKIP project. The present pooled analysis included data on 3,830 single, primary, sporadic, cutaneous melanoma cases and 2,619 controls from seven previously published case–control studies. All the studies had information on MC1R gene variants by sequencing analysis and on hair color, skin phototype, and freckles, ie, the phenotypic characteristics used to define the red hair phenotype.
Results
The presence of any MC1R variant was associated with melanoma risk independently of phenotypic characteristics (OR 1.60; 95% CI 1.36–1.88). Inclusion of MC1R variants in a risk prediction model increased melanoma predictive accuracy (area under the receiver-operating characteristic curve) by 0.7% over a base clinical model (P=0.002), and 24% of participants were better assessed (net reclassification index 95% CI 20%–30%). Subgroup analysis suggested a possibly stronger role of MC1R in melanoma prediction for participants without the red hair phenotype (net reclassification index: 28%) compared to paler skinned participants (15%).
Conclusion
The authors suggest that measuring the MC1R genotype might result in a benefit for melanoma prediction. The results could be a valid starting point to guide the development of scientific protocols assessing melanoma risk prediction tools incorporating the MC1R genotype.
Introduction
Incidence rates of malignant cutaneous melanoma (CM) continue to rise in most European countries, whereas in other countries, rates have become rather stable in recent years.Citation1 CM still represents an important public health problem for its high case-fatality rate,Citation2 and thus, identification of individuals at high risk of developing melanoma would be of major interest to improve early diagnosis and ultimately survival.
Known risk factors for CM include sun sensitivity, sun exposure, light hair and eye color, high number of melanocytic nevi, atypical nevi, and family history of melanoma.Citation3–Citation5 Knowledge of risk factors for CM is the basis for the development of risk prediction tools that may improve understanding and decision-making, leading to favorable behavior change and disease prevention.Citation6–Citation9 In addition to their clinical uses, these tools can assist in planning intervention trials and prevention strategies that target particular risk groups.Citation7–Citation9 Clinical risk prediction models for CM have been previously reviewed:Citation10 their discrimination ranged from fair to very good (area under the receiver-operating characteristic curve [AUC] 0.62–0.86), comparable with those obtained for other cancers.Citation10,Citation11 The US Preventive Services Task Force considered the utility of these tools for population-based screening and concluded that the current evidence was insufficient to assess the balance of benefits and harms of visual skin examination by a primary care clinician or patient self-examination to screen for skin cancer of any type in adults.Citation2,Citation12 An accompanying editorial suggested that the Preventive Services Task Force statement should be viewed as an invitation to the scientific communities “to work together in executing well-designed studies … so future recommendations can be of greater public health benefit”.Citation13 Since melanoma seems to be determined by complex interactions among host characteristics, environmental exposure, and genetic factors,Citation14,Citation15 the inclusion and evaluation of genetic markers in risk models may be warranted and has been considered an important step for further development and testing of prediction tools before they can be used routinely with confidence.Citation10
MC1R is the most important gene found to play a role in predisposition to sporadic CM, and its association with CM has been replicated and confirmed by meta-analyses and genome-wide association studies.Citation16–Citation21 The MC1R gene is located on chromosome 16q24.3 and is a key regulator of skin pigmentation.Citation22 It is highly polymorphic in populations of European origin, with more than 200 coding region variants described to dateCitation23 and a prevalence of any MC1R variant of ~60% in healthy controls.Citation16 Some of these variants have been shown to reduce receptor function,Citation24–Citation26 result in a quantitative shift of melanin synthesis from eumelanin to phaeomelanin,Citation27 and determine the red hair (RH) phenotype, characterized by the co-occurrence of fair skin, RH, freckles, and ultraviolet (UV) radiation sensitivity (poor tanning response and solar lentigines).
Previous melanoma risk prediction models have included MC1R alongside base clinical risk factorsCitation15,Citation28–Citation31 and reported slight improvement in risk prediction with MC1R inclusion. However, because of the strong relationship between MC1R and phenotypic characteristics, their joint inclusion in the same model may generate biased estimates if the effect of MC1R on CM is mediated mainly by pigmentation. Therefore, before inclusion of MC1R in a risk prediction model in addition to phenotypic characteristics, it should be demonstrated that MC1R has a direct effect on CM development through biological pathways that are independent of pigmentation. There is some evidence for a wider biological role, as inherited variation at the MC1R locus has been reported to be associated with better melanoma survival overall,Citation32 but to reduce therapeutic benefit from treatment with BRAF inhibitors.Citation33 A stronger role of MC1R variants in increasing melanoma risk in darker pigmented individuals has been suggested,Citation16,Citation18,Citation34,Citation35 but the extent to which pigmentation and nonpigmentation pathways account for the association between MC1R and CM is still not clear.
Therefore, the aims of this study were 1) to decompose the total risk estimate of MC1R on CM into two different effects: one due to the nonpigmentation pathway (direct effect) and one due to the pigmentation pathway (indirect effect); and 2) to evaluate whether the inclusion of MC1R variants in risk-prediction models increases their ability to predict CM in both the whole population and targeted subgroups of subjects with different phenotypic characteristics.
Materials and methods
Study population
Data were collected within the M-SKIP (melanocortin 1 receptor, skin cancer, and phenotypic characteristics) project, described in detail elsewhere.Citation36 Briefly, we gathered original individual data from studies on MC1R variants and phenotypic characteristics in patients with sporadic CM and nonmelanoma skin cancer and/or in healthy controls. According to familial melanoma definition,Citation37,Citation38 sporadic melanoma cases were defined as subjects with no more than one first-degree relative or two any-degree relatives with melanoma. Since 2009, of 49 investigators contacted, 38 (78%) agreed to participate and sent their data along with a signed statement declaring that their original study was approved by an ethics committee.
For the purpose of the present study, we excluded all the nonmelanoma skin cancer cases and included seven melanoma case–control studiesCitation18,Citation30,Citation34,Citation39–Citation43 according to inclusion criteria of the MC1R gene being sequenced and there being information available on hair color, skin phototype, and freckles, ie, the phenotypic characteristics used to define the RH phenotype. These phenotypic characteristics were those associated with MC1R genetic variants in our previous publication.Citation44 The present study included data on 3,830 CM cases and 2,619 controls ().
Table 1 Description of the studies included in the analysis
Statistical analysis
A complete description of statistical analysis methods is reported in the Supplementary material.
Mediation analysis
To estimate the independent contribution of MC1R variants on CM development, we performed a mediation analysis.Citation45,Citation46 We decomposed the overall risk estimate for CM associated with MC1R into a direct effect due to the nonpigmentation pathway and an indirect effect due to the pigmentation pathway. We estimated the direct effect of MC1R (any variant and the nine single common variants vs wild type [WT] on CM in the presence and in the absence of the RH phenotype (controlled direct effect [CDE]). Following our previous publication,Citation44 RH phenotype was primarily defined as the presence of at least one of the characteristics of RH, freckles, and skin type I/II. Skin type is a measure of sun sensitivity of the skin and was defined in our study according to the known Fitzpatrick classification as type I (always burns, never tans), II (usually burns, tans minimally), III (sometimes mild burns, tans uniformly), and IV (never burns, tans easily). We also estimated the natural direct effect (NDE), which essentially averages CDE over the population and finally the indirect effect of MC1R mediated by RH phenotype (natural indirect effect [NIE]). Mediation analysis was separately applied to each of the seven studies, and ORs with 95% CIs were obtained for total effect (TE), NDE, NIE, and CDE using unconditional logistic regression models with the following covariates (when available) of age, sex, intermittent and chronic sun exposure, lifetime and childhood sunburns, family history of melanoma, common nevi count, and presence of atypical nevi. Following the two-stage analysis approach, we pooled study-specific ORs with a random effects model. We calculated I2-values to assess the percentage of total variation across studies that was attributable to heterogeneity rather than to chance.
Model comparison
We tested the prediction ability to identify CM participants by adding MC1R variants to a clinical base prediction model. Variables included in the base model were age, sex, sunburn, number of common nevi, and RH phenotype. These covariates were available in a subset of 4,390 (68%) participants from six studies. We used unconditional logistic regression to estimate the risk of CM according to the base clinical risk model and to the model including the MC1R gene, defined as the presence of any MC1R variants versus WT, the presence of only r variants and presence of at least one R variant versus WT, and the presence of each of the nine most common MC1R variants or rarer variants. R and r alleles have previously been defined according to their association with RH phenotype.Citation17,Citation22 We compared the predictive ability of the model with MC1R over the base clinical model by receiver-operating characteristic (ROC) curves, net reclassification improvement (NRI), and decision curve analysis. Analysis was carried out with the software SAS (version 9.2) and Stata (version 11.2).
Results
The main characteristics of the studies included are summarized in . Three studies were performed in Italy, two in the US, one in the UK, and one in the Netherlands. All studies included more than 97% Caucasians. Two studies included hospital-based controls,Citation30,Citation31 and five recruited healthy controls. One studyCitation41 included an unpublished group of sporadic melanoma cases. The study approach, control group, and genetic analysis were the same described in the corresponding published paper.
Direct and indirect effects of MC1R on CM development
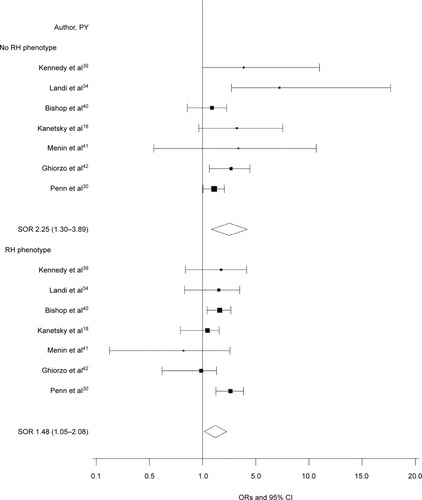
The OR (95% CI) for the TE of any MC1R variants on CM risk was 1.71 (1.46–2.00; I2=0; ). When decomposed, the risk was primarily due to the NDE, independent of phenotypic characteristics (OR 1.60; 95% CI 1.36–1.88; I2=0; ); the NIE, which would be dependent on the pigmentation pathway, was smaller (OR 1.07; 95% CI 1.03–1.11; I2=0; ). When the CDE according to RH phenotype was examined, we found a direct, positive association between MC1R and CM in the absence of RH phenotype (OR 1.75; 95% CI 1.33–2.33; I2=0; ) and a smaller direct association between MC1R and CM in participants with the RH phenotype (OR 1.50; 95% CI 1.19–1.89; I2=37%; ).
Figure 1 Forest plot for NDE, NIE, and TE of any MC1R variant on melanoma risk.
Notes: CDE estimates the direct effect of MC1R on melanoma when the mediator is controlled at level 0 (absent) or 1 (present) uniformly in the population, NDE essentially averages CDE over the population, NIE estimates the indirect effect of MC1R mediated by RH phenotype, and TE is the overall melanoma risk estimate for MC1R carriers and in each study is the product of NDE and NIE.
Abbreviations: CDE, control direct effect; NDE, natural direct effect; NIE, natural indirect effect; PY, publication year; RH, red hair; SOR, summary OR; TE, total effect.
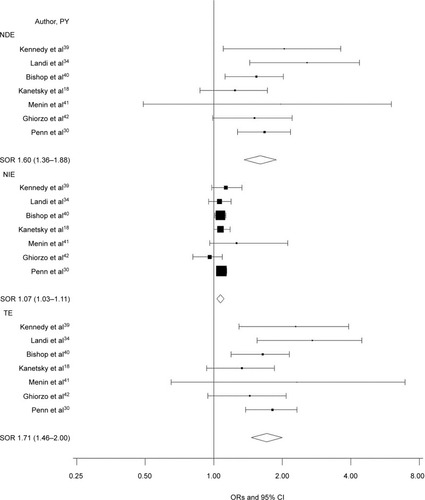
Figure 2 Forest plot for control direct effect of any MC1R variant on melanoma risk according to RH phenotype.*
Notes: *Defined as presence of red hair, freckles, or skin type I/II. Control direct effect estimates the direct effect of MC1R on melanoma when the mediator is controlled at level 0 (absent) or 1 (present) uniformly in the population.
Abbreviations: PY, publication year; RH, red hair; SOR, summary OR.
Looking at each of the nine most common MC1R variants (Table S1), we still found for all of them larger NDE than NIE, with significant NDE found for the R variants R142H, R151C, R160W, and D294K (ranging from OR 2.22; 95% CI 1.33–3.71 to OR 3.55; 95% CI 1.21–10.47) and significant NIE found only for the R variant R151C (OR 1.18; 95% CI 1.00–1.39). Furthermore, CDE was higher for non-RH-phenotype subjects than for RH-phenotype subjects for the most common variants (allele frequency ≥1.5%), while it was opposite for the three rarer MC1R variants D84E, R142H, and I155T (Table S1).
Risk models for CM prediction
reports the ORs and 95% CIs for variables included in the base clinical risk model and for MC1R variants. Having more than 30 common nevi and RH phenotype increased CM risk in our population (). Independent of other risk factors, carriers of any MC1R variant had a higher risk of CM than noncarriers (OR 1.63; 95% CI 1.40–1.90). The OR slightly decreased when the analysis was restricted to RH participants, while it increased for non-RH participants (). When we considered a distinction between MC1R r and R variants, in comparison with WT, carriers of at least one R variant had a higher risk of CM (OR 2.08; 95% CI 1.76–2.46) than carriers of only r variants (OR 1.24; 95% CI 1.04–1.47). For RH participants, carrying only MC1R r variants did not increase CM risk, while the risk was increased for carriers of MC1R R variants. By contrast, both MC1R r and R variants were associated with a higher risk of CM in participants with the non-RH phenotype (). Similar results were found looking at each of the nine MC1R variants separately (Table S2).
Table 2 ORs with 95% CIs for melanoma risk according to a base clinical model and the same model with inclusion of MC1R variants
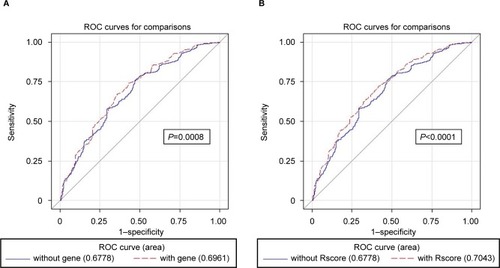
The clinical risk model yielded an AUC of 0.706 (95% CI 0.691–0.721; Table S3). The model including any MC1R variant showed slightly greater discrimination, with an AUC of 0.713 (95% CI 0.698–0.728; P=0.002) and an NRI of 24% (95% CI 20%–30%). Differentiation between r and R variants and considering each single variant further increased diagnostic accuracy by 1.5% and 1.9%, respectively, over the base clinical risk model, with an NRI of 37% (95% CI 32%–43%) and 34% (28%–39%), respectively. Subgroup analysis restricted to participants with the non-RH phenotype revealed that MC1R improved the AUC by 1.8% (from 0.678 to 0.696, P=0.0008; ; Table S3), suggesting a stronger role of MC1R in melanoma prediction for darker pigmented participants compared to RH participants. The NRI due to MC1R inclusion for participants with a non-RH phenotype was 28% (95% CI 19%–37%), while it was 15% (95% CI 9%–22%) for RH participants. The addiction of separate information on r and R MC1R variants and on single specific variants obtained a better model performance for both RH and non-RH participants. Decision curves showed a small increase in net benefit of MC1R testing for non-RH participants over almost the entire range of threshold probabilities (Figure S1), with an average increase in net benefit of 0.003 for the model with any MC1R variant and 0.005 for the model with r or R MC1R variant over the base clinical model.
Figure 3 ROC curve comparison between base clinical model and the same model with inclusion of MC1R variants for patients with no RH phenotype.*
Notes: (A) MC1R defined as the presence or absence of any MC1R variant and (B) as no MC1R variant, only r variants, and ≥1 R variants. *Non-RH patients defined as those without RH and freckles and with skin type III/IV. R and r alleles were respectively defined basing on their stronger or weaker association with the RH phenotype for the most common variantsCitation44,Citation67–Citation70 and on likely pathogenicity using the algorithm proposed by Davies et alCitation32 for the less common variants.
Abbreviations: RH, red hair; ROC, receiver-operating characteristic.
Sensitivity analysis on a subset of 2,472 (38%) participants from four studies with additional information on atypical nevi provided similar results (not shown): having more than 30 common nevi, RH phenotype, and atypical nevi increased CM risk. In this sensitivity analysis, MC1R variants increased CM risk in non-RH participants, but not in RH participants. Sensitivity analysis with different definitions of RH phenotype provided similar results (not shown).
Discussion
Our pooled analysis showed that the presence of any MC1R variant had a direct effect on CM, conferring a 60% higher risk to carriers versus noncarriers. The pigmentation-mediated effect of MC1R on CM was smaller with any MC1R variant and each of the nine most common MC1R variants. This result confirms and expands the previous suggestionCitation16–Citation18,Citation34 of the existence of a nonpigmentation pathway leading MC1R to CM development. Here, we give for the first time an estimate of the magnitude of total effect explained by each of the two (pigmentation and nonpigmentation) pathways. Recent studies and reviewsCitation47 have implicated MC1R signaling in a number of key biological pathways involved in cell-cycle control,Citation48 apoptosis,Citation49 and activation of DNA-repair mechanisms and antioxidant defenses.Citation50 Production of pheomelanin pigments seems associated with increased oxidative DNA damage compared with synthesis of eumelanins.Citation51 Further evidence for pheomelanin-associated increased cellular oxidative stress was obtained in studies of mice carrying a loss-of-function mutation of the Mc1r gene, which provided evidence in support that the pheomelanin-pigment pathway produces UV-independent carcinogenic contributions to melanomagenesis.Citation52 Another recent studyCitation53 found a role of germ-line MC1R variants in influencing the somatic mutational landscape of melanoma, with an expected higher number of somatic C>T mutations in carriers of R alleles than those without R alleles. In this respect, it is worth noting that although the most relevant UV radiation-induced mutations are C>T transitions, highly recurrent mutations in key melanoma-driver genes, such as the V600E mutation in BRAF, are non-C>T changes. Importantly, significant increases in the rate of non-C>T changes, some of which might depend on oxidative DNA damage, have also been found in R allele carriers compared with noncarriers.Citation53,Citation54 Accordingly, associations of MC1R and genes frequently mutated in melanoma, such as BRAF or TERT, have been reported.Citation55–Citation57
We found that MC1R slightly improved risk prediction accuracy over a base clinical model, especially for non-RH participants: CM predictive accuracy increased by 1.8% and the CM risk of 28% of participants was better assessed. If a distinction is used in the model to differently score r and R variants, the benefit for the whole population increased from 24% of participants correctly reclassified with just presence/absence of MC1R variants to 37% of participants correctly reclassified with separate information on r and R variants. Distinction between r and R alleles, however, was more apparent for RH than for non-RH participants. In the study by Cust et al,Citation15 the R variants were responsible for most of the improvement in risk prediction, but separate analysis for RH and non-RH participants was not performed.
Previous melanoma risk prediction models have included MC1R with base clinical risk factors.Citation15,Citation28–Citation30 Whiteman and GreenCitation28 did not report on predictive ability. Stefanaki et alCitation29 found no improvement in AUC with the addition of eight melanoma-associated single-nucleotide polymorphisms (SNPs) to the base model. Both Cust et alCitation15 and Penn et alCitation30 reported slight improvement in AUC with the inclusion of MC1R. However, no previous paper has reported separate results according to fairer or darker phenotypic characteristics. This point seems in fact extremely important, because MC1R seems to have a stronger role in non-RH participants in both the present paper and in previously published stratified analyses.Citation16,Citation18,Citation34 A more precise risk assessment, therefore, in participants with no RH, no freckles, and skin type III/IV could potentially change individual clinical follow-up schedules and perhaps UV-exposure behavior and indoor tanning habits.
The application of risk prediction tools in cancer screening has been widely discussed. In particular, there have been concerns on the impact of genetic screening in clinical decision-making. For example, in a previous review,Citation58 genetic screening was discussed using commercially available SNP panel tests in prostate cancer. Conclusions were that the investigated SNP panels had poor discriminative ability and clinical validity. In our study, adding the MC1R genotype resulted in a small yet significant improvement in predictive ability over the clinical model and a substantial change in the NRI, and it is worth noting that this improvement was based on a single gene, while risk indices for both prostate and breast cancer require several genetic markers to produce increases of similar magnitude.Citation59–Citation62 Decreasing genotyping costs and increasing use of genetic testing is making it more feasible to incorporate genetic risk factors into clinical risk prediction tools, and limiting testing to the non-RH participants with no other risk factors may result in a cost-effective strategy via better allocation of resources. However, translation into routine clinical practice requires several additional steps,Citation63,Citation64 and new studies are needed in order better to assess the clinical utility of these models, taking also into account the small increase in net benefit observed in our decision curve analysis.
Our study has several strengths. We quantified for the first time the amount of total effect of MC1R on CM due to pigmentation and nonpigmentation pathways. Previous stratified analyses, including ours,Citation16 have already suggested that the effect of MC1R was stronger in darker pigmented participants; however, stratified analyses are not conclusive, especially in the presence of genotype–phenotype interaction.Citation46,Citation65 Precise and powerful quantification of the effect of the two pathways was only feasible in the present analysis after inclusion of new studies.Citation30,Citation40,Citation41 The large sample and international collaborative nature of the M-SKIP project make it possible to assess various populations and ancestries, thus providing results that are robust and consistent in different geographical areas. We were also able to create different predictive models according to the RH and non-RH phenotypes, which was not possible in previous studies. In our centralized statistical analysis, we were able to take into account all the available confounders, with a homogeneous plan of analysis and definition of covariables.
Heterogeneity among different populations is a possible limitation of our study; therefore, this tool may require adjustments before being applicable to each specific population.Citation10 However, it is not easy to develop a good and precise tool for each population due to the lack of power of single studies. Moreover, we did not observe any heterogeneity in risk estimates for MC1R and CM, suggesting that information on MC1R improves CM risk prediction in different populations of European origins. Following our previous publication,Citation44 RH participants were defined as participants with either RH, freckles, or skin type I/II, and we are aware that other definitions may modify the results. However, in a sensitivity analysis using RH defined as a score obtained from multiple correspondence analysis,Citation44 the results were similar. Phenotype misclassification is a possibility, although a previous study reported a good correlation between self-defined skin pigmentation and measured melanin density.Citation66 In order to minimize phenotype misclassification, we performed a sensitivity analysis that included only extreme categories of the RH phenotype.Citation5 Although this analysis was underpowered, we observed similar risk estimates to those reported for the main analysis in the present paper (results not shown). Finally, it should be noted that our analyses were performed on sporadic melanoma cases, and thus, generalization to familial melanoma is not appropriate.
Conclusion
We found a direct role of MC1R in melanoma risk independently of RH phenotype and demonstrated that adding the MC1R genotype to classical clinical risk factors results in a benefit for CM prediction. A change in clinical follow-up schedules and UV exposure and sun protection habits of identified at-risk individuals might favor early melanoma diagnosis and prevention. The application of risk prediction tools in cancer screening has been controversial, because of concerns on their impact in clinical decision-making. Our results could be a valid starting point to guide the development of scientific protocols assessing melanoma risk prediction tools incorporating the MC1R genotype, ideally with a prospective design and cost–benefit evaluation.
Acknowledgments
This work was supported by the Italian Association for Cancer Research (grant MFAG 11831). The Melanoma Susceptibility Study (PAK) was supported by the National Cancer Institute (CA75434, CA80700, CA092428). The Genoa study (PG) was supported by AIRC IG 15460. The M-SKIP study group consists of the following members: principal investigator (PI), Sara Raimondi (European Institute of Oncology, Milan, Italy); advisory committee members, Philippe Autier (International Prevention Research Institute, Lyon, France), Maria Concetta Fargnoli (University of L’Aquila, Italy), José C García-Borrón (University of Murcia, Spain), Jiali Han (Indiana University, Indianapo-lis, IN, USA), Peter A Kanetsky (Department of Cancer Epidemiology, H Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA), Maria Teresa Landi (National Cancer Institute, NIH, Bethesda, MD, USA), Julian Little (University of Ottawa, Canada), Julia Newton-Bishop (University of Leeds, Leeds, UK), and Francesco Sera (London School of Hygiene and Tropical Medicine, London, UK); consultants, Saverio Caini (ISPO, Florence, Italy), Sara Gandini, and Patrick Maisonneuve (European Institute of Oncology, Milan, Italy); participant investigators, Albert Hofman, Manfred Kayser, Fan Liu, Tamar Nijsten, and Andre G Uitterlinden (Erasmus MC University Medical Center, Rotterdam, Netherlands), Rajiv Kumar (German Cancer Research Center, Heidelberg, Germany), Tim Bishop, Faye Elliott (University of Leeds, Leeds, UK), Eduardo Nagore (Instituto Valenciano de Oncologia, Valencia, Spain), DeAnn Lazovich (Division of Epidemiology and Community Health, University of Minnesota, MN, USA), David Pol-sky (New York University School of Medicine, New York, NY, USA), Johan Hansson, Veronica Hoiom (Karolinska Institutet, Stockholm, Sweden), Paola Ghiorzo, Lorenza Pastorino (University of Genoa, Italy), Nelleke A Gruis, Jan Nico Bouwes Bavinck (Leiden University Medical Center, Leiden, Netherlands), Ricardo Fernandez-de-Misa (Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain), Paula Aguilera, Celia Badenas, Cristina Carrera, Pol Gimenez-Xavier, Josep Malvehy, Miriam Potrony, Susana Puig, Joan Anton Puig-Butille, Gemma Tell-Marti (Hospital Clinic, IDIBAPS, and CIBERER, Barcelona, Spain), Terence Dwyer (Murdoch Children’s Research Institute, Melbourne, Australia), Leigh Blizzard, Jennifer Cochrane (Menzies Institute for Medical Research, Hobart, Australia), Wojciech Branicki (Institute of Forensic Research, Krakow, Poland), Tadeusz Debniak (Pomeranian Medical University, Szczecin, Poland), Niels Morling, Peter Johansen (University of Copenhagen, Copenhagen, Denmark), Susan Mayne, Allen Bale, Brenda Cartmel, Leah Ferrucci (Yale School of Public Health and Medicine, New Haven, CT, USA), Ruth Pfeiffer (National Cancer Institute, NIH, Bethesda, MD, USA), Giuseppe Palmieri (Istituto di Chimica Biomolecolare, CNR, Sassari, Italy), Gloria Ribas (Fundación Investigación Clínico de Valencia Instituto de Investigación Sanitaria, INCLIVA, Spain), Chiara Menin (Veneto Institute of Oncology, IOV-IRCCS, Padua, Italy), Alexander Stratigos, Katerina Kypreou (Andreas Sygros Hospital, University of Athens, Athens, Greece), Anne Bow-cock, Lynn Cornelius, M Laurin Council (Washington University School of Medicine, St Louis, MO, USA), Tomonori Motokawa (POLA Chemical Industries, Yokohama, Japan), Sumiko Anno (Shibaura Institute of Technology, Tokyo, Japan), Per Helsing, Per Arne Andresen (Oslo University Hospital, Oslo, Norway), Gabriella Guida, Stefania Guida (University of Bari, Bari, Italy), Terence H Wong (University of Edinburgh, Edinburgh, UK), and the GEM study group. Participants in the GEM study group are as follows: coordinating center, Memorial Sloan-Kettering Cancer Center, New York, NY, USA, Marianne Berwick (PI, currently at the University of New Mexico), Colin Begg (co-PI), Irene Orlow (coinvestigator), Urvi Mujumdar (project coordinator), Amanda Hummer (biostatistician), Klaus Busam (dermato-pathologist), Pampa Roy (laboratory technician), Rebecca Canchola (laboratory technician), Brian Clas (laboratory technician), Javiar Cotignola (laboratory technician), and Yvette Monroe (interviewer); study centers; University of Sydney and Cancer Council New South Wales, Sydney (Aus-tralia), Bruce Armstrong (PI), Anne Kricker (co-PI), Melisa Litchfield (study coordinator); Menzies Institute for Medical Research, University of Tasmania, Hobart (Australia), Terence Dwyer (PI), Paul Tucker (dermatopathologist), Nicola Stephens (study coordinator); British Columbia Cancer Agency, Vancouver, BC (Canada), Richard Gallagher (PI), Teresa Switzer (coordinator), Cancer Care Ontario, Toronto, ON (Canada), Loraine Marrett (PI), Beth Theis (coinvesti-gator), Lynn From (dermatopathologist), Noori Chowdhury (coordinator), Louise Vanasse (coordinator), Mark Purdue (research officer), David Northrup (manager for CATI), Centro per la Prevenzione Oncologia Torino, Piedmont (Italy), Roberto Zanetti (PI), Stefano Rosso (data manager), Carlotta Sacerdote (coordinator); University of California, Irvine, CA (USA), Hoda Anton-Culver (PI), Nancy Leighton (coordinator), Maureen Gildea (data manager); University of Michigan, Ann Arbor, MI (USA), Stephen Gruber (PI), Joe Bonner (data manager), Joanne Jeter (Coordinator); New Jersey Department of Health and Senior Services, Trenton, NJ (USA), Judith Klotz (PI), Homer Wilcox (co-PI), Helen Weiss (coordinator); University of North Carolina, Chapel Hill, NC (USA), Robert Millikan (PI), Nancy Thomas (coinvestigator), Dianne Mattingly (coordinator), Jon Player (laboratory technician), Chiu-Kit Tse (data analyst); University of Pennsylvania, Philadelphia, PA (USA), Timothy Rebbeck (PI), Peter Kanetsky (coinvestigator), Amy Walker (laboratory technician), Saarene Panossian (laboratory technician); consultants, Harvey Mohrenweiser, University of California, Irvine, Irvine, CA (USA); Richard Setlow, Brookhaven National Laboratory, Upton, NY (USA).
Disclosure
The authors report no conflicts of interest in this work.
References
- ErdmannFLortet-TieulentJSchuzJInternational trends in the incidence of malignant melanoma 1953–2008: are recent generations at higher or lower risk?Int J Cancer2013132238540022532371
- WernliKJHenriksonNBMorrisonCCNguyenMPocobelliGBlasiPRScreening for skin cancer in adults: updated evidence report and systematic review for the US Preventive Services Task ForceJAMA2016316443644727458949
- GandiniSSeraFCattaruzzaMSMeta-analysis of risk factors for cutaneous melanoma – I: common and atypical naeviEur J Cancer2005411284415617989
- GandiniSSeraFCattaruzzaMSMeta-analysis of risk factors for cutaneous melanoma – II: sun exposureEur J Cancer2005411456015617990
- GandiniSSeraFCattaruzzaMSMeta-analysis of risk factors for cutaneous melanoma – III: family history, actinic damage and phenotypic factorsEur J Cancer200541142040205916125929
- AhmedHNaikGWilloughbyHEdwardsAGCommunicating riskBMJ2012344e399622709962
- FreedmanANSeminaraDGailMHCancer risk prediction models: a workshop on development, evaluation, and applicationJ Natl Cancer Inst2005971071572315900041
- JacksonAWilkinsonCRangerMPillRAugustPCan primary prevention or selective screening for melanoma be more precisely targeted through general practice? A prospective study to validate a self administered risk scoreBMJ1998316712434399451264
- QuereuxGN’GuyenJMCaryMJumbouOLequeuxYDrenoBValidation of the self-assessment of melanoma risk score for a melanoma-targeted screeningEur J Cancer Prev201221658859522555198
- VuongKMcGeechanKArmstrongBKCustAERisk prediction models for incident primary cutaneous melanoma: a systematic reviewJAMA Dermatol2014150443444424522401
- OlsenCMNealeREGreenACIndependent validation of six melanoma risk prediction modelsJ Invest Dermatol201513551377138425548858
- US Preventive Services Task ForceBibbins-DomingoKGrossmanDCScreening for skin cancer: US preventive services task force recommendation statementJAMA2016316442943527458948
- TsaoHWeinstockMAVisual inspection and the US Preventive Services Task Force recommendation on skin cancer screeningJAMA2016316439840027458944
- ChaudruVChompretABressac-de PailleretsBSpatzAAvrilMFDemenaisFInfluence of genes, nevi, and sun sensitivity on melanoma risk in a family sample unselected by family history and in melanoma-prone familiesJ Natl Cancer Inst2004961078579515150307
- CustAEGoumasCVuongKMC1R genotype as a predictor of early-onset melanoma, compared with self-reported and physician-measured traditional risk factors: an Australian case-control-family studyBMC Cancer20131340624134749
- PasqualiEGarcia-BorronJCFargnoliMCMC1R variants increased the risk of sporadic cutaneous melanoma in darker-pigmented Caucasians: a pooled-analysis from the M-SKIP projectInt J Cancer2015136361863124917043
- RaimondiSSeraFGandiniSMC1R variants, melanoma and red hair color phenotype: a meta-analysisInt J Cancer2008122122753276018366057
- KanetskyPAPanossianSElderDEDoes MC1R genotype convey information about melanoma risk beyond risk phenotypes?Cancer2010116102416242820301115
- ChatzinasiouFLillCMKypreouKComprehensive field synopsis and systematic meta-analyses of genetic association studies in cutaneous melanomaJ Natl Cancer Inst2011103161227123521693730
- AmosCIWangLELeeJEGenome-wide association study identifies novel loci predisposing to cutaneous melanomaHum Mol Genet201120245012502321926416
- WilliamsPFOlsenCMHaywardNKWhitemanDCMelanocortin 1 receptor and risk of cutaneous melanoma: a meta-analysis and estimates of population burdenInt J Cancer201112971730174021128237
- Garcia-BorronJCSanchez-LaordenBLJimenez-CervantesCMelanocortin-1 receptor structure and functional regulationPigment Cell Res200518639341016280005
- Garcia-BorronJCAbdel-MalekZJimenez-CervantesCMC1R, the cAMP pathway, and the response to solar UV: extending the horizon beyond pigmentationPigment Cell Melanoma Res201427569972024807163
- BeaumontKAShekarSNNewtonRAReceptor function, dominant negative activity and phenotype correlations for MC1R variant allelesHum Mol Genet200716182249226017616515
- BeaumontKAShekarSNCookALDuffyDLSturmRARed hair is the null phenotype of MC1RHum Mutat2008298E88E9418484624
- DoyleJRFortinJPBeinbornMKopinASSelected melanocortin 1 receptor single-nucleotide polymorphisms differentially alter multiple signaling pathwaysJ Pharmacol Exp Ther2012342231832622547573
- DuffyDLBoxNFChenWInteractive effects of MC1R and OCA2 on melanoma risk phenotypesHum Mol Genet200413444746114709592
- WhitemanDCGreenACA risk prediction tool for melanoma?Cancer Epidemiol Biomarkers Prev200514476176315824139
- StefanakiIPanagiotouOAKodelaEReplication and predictive value of SNPs associated with melanoma and pigmentation traits in a southern European case-control studyPLoS One201382e5571223393597
- PennLAQianMZhangEDevelopment of a melanoma risk prediction model incorporating MC1R genotype and indoor tanning exposure: impact of mole phenotype on model performancePLoS One201497e10150725003831
- DwyerTStankovichJMBlizzardLDoes the addition of information on genotype improve prediction of the risk of melanoma and nonmelanoma skin cancer beyond that obtained from skin phenotype?Am J Epidemiol2004159982683315105175
- DaviesJRRanderson-MoorJKukalizchKInherited variants in the MC1R gene and survival from cutaneous melanoma: a BioGenoMEL studyPigment Cell Melanoma Res201225338439422325793
- GuidaMStrippoliSFerrettaADetrimental effects of melanocortin-1 receptor (MC1R) variants on the clinical outcomes of BRAF V600 metastatic melanoma patients treated with BRAF inhibitorsPigment Cell Melanoma Res201629667968727540956
- LandiMTKanetskyPATsangSMC1R, ASIP, and DNA repair in sporadic and familial melanoma in a Mediterranean populationJ Natl Cancer Inst20059713998100715998953
- GuidaSBartolomeoNZannaPTSporadic melanoma in southeastern Italy: the impact of melanocortin 1 receptor (MC1R) polymorphism analysis in low-risk people and report of three novel variantsArch Dermatol Res2015307649550325736238
- RaimondiSGandiniSFargnoliMCMelanocortin-1 receptor, skin cancer and phenotypic characteristics (M-SKIP) project: study design and methods for pooling results of genetic epidemiological studiesBMC Med Res Methodol20121211622862891
- BergmanWGruisNAManagement of melanoma familiesCancers (Basel)20102254956624281082
- de SnooFGruisNFamilial melanoma [webpage on the Internet]2005 Available from: http://atlasgeneticsoncology.org/Kprones/Famil-ialMelanomID10088.htmlAccessed March 10, 2018
- KennedyCter HuurneJBerkhoutMMelanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair colorJ Invest Dermatol2001117229430011511307
- BishopDTDemenaisFIlesMMGenome-wide association study identifies three loci associated with melanoma riskNat Genet200941892092519578364
- MeninCVecchiatoAScainiMCContribution of susceptibility gene variants to melanoma risk in families from the Veneto region of ItalyPigment Cell Melanoma Res201124472873021672182
- GhiorzoPBonelliLPastorinoLMC1R variation and melanoma risk in relation to host/clinical and environmental factors in CDKN2A positive and negative melanoma patientsExp Dermatol201221971872022804906
- PastorinoLCusanoRBrunoWNovel MC1R variants in Ligurian melanoma patients and controlsHum Mutat2004241103
- TagliabueEGandiniSGarcia-BorronJCAssociation of melanocortin-1 receptor variants with pigmentary traits in humans: a pooled analysis from the M-SKIP projectJ Invest Dermatol201613691914191727251790
- VanderweeleTJVansteelandtSOdds ratios for mediation analysis for a dichotomous outcomeAm J Epidemiol2010172121339134821036955
- ValeriLVanderweeleTJMediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macrosPsychol Methods201318213715023379553
- HorrellEMBoulangerMCD’OrazioJAMelanocortin 1 receptor: structure, function, and regulationFront Genet201679527303435
- AprilCSBarshGSDistinct pigmentary and melanocortin 1 receptor-dependent components of cutaneous defense against ultraviolet radiationPLoS Genet200731e917222061
- HauserJEKadekaroALKavanaghRJMelanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytesPigment Cell Res200619430331416827749
- KadekaroALChenJYangJAlpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytesMol Cancer Res201210677878622622028
- WongSSAingerSALeonardJHSturmRAMC1R variant allele effects on UVR-induced phosphorylation of p38, p53, and DDB2 repair protein responses in melanocytic cells in cultureJ Invest Dermatol201213251452146122336944
- MitraDLuoXMorganAAn ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin backgroundNature2012491742444945323123854
- Robles-EspinozaCDRobertsNDChenSGermline MC1R status influences somatic mutation burden in melanomaNat Commun201671206427403562
- JohanssonPAPritchardALPatchAMMutation load in melanoma is affected by MC1R genotypePigment Cell Melanoma Res201730225525828024115
- FargnoliMCPikeKPfeifferRMMC1R variants increase risk of melanomas harboring BRAF mutationsJ Invest Dermatol2008128102485249018368129
- LandiMTBauerJPfeifferRMMC1R germline variants confer risk for BRAF-mutant melanomaScience2006313578652152216809487
- NagoreEReyes-GarciaDHeidenreichBGarcia-CasadoZRequenaCKumarRTERT promoter mutations associate with MC1R variants in melanoma patientsPigment Cell Melanoma Res201730227327527930874
- LittleJWilsonBCarterRMultigene panels in prostate cancer risk assessment: a systematic reviewGenet Med201618653554426426883
- MealiffeMEStokowskiRPRheesBKPrenticeRLPettingerMHindsDAAssessment of clinical validity of a breast cancer risk model combining genetic and clinical informationJ Natl Cancer Inst2010102211618162720956782
- LindströmSSchumacherFRCoxDCommon genetic variants in prostate cancer risk prediction: results from the NCI Breast and Prostate Cancer Cohort Consortium (BPC3)Cancer Epidemiol Biomarkers Prev201221343744422237985
- MacinnisRJAntoniouACEelesRAA risk prediction algorithm based on family history and common genetic variants: application to prostate cancer with potential clinical impactGenet Epidemiol201135654955621769933
- ChatterjeeNParkJHCaporasoNGailMHPredicting the future of genetic risk predictionCancer Epidemiol Biomarkers Prev20112013821212066
- PearsonTAManolioTAHow to interpret a genome-wide association studyJAMA2008299111335134418349094
- CollinsFSGreenEDGuttmacherAEGuyerMSA vision for the future of genomics researchNature2003422693483584712695777
- HernanMAClaytonDKeidingNThe Simpson’s paradox unraveledInt J Epidemiol201140378078521454324
- CargillJLucasRMGiesPValidation of brief questionnaire measures of sun exposure and skin pigmentation against detailed and objective measures including vitamin D statusPhotochem Photobiol201389121922622891914
- Garcia-BorronJCSanchez-LaordenBLJimenez-CervantesCMelanocortin-1 receptor structure and functional regulationPigment Cell Res200518639341016280005
- DuffyDLBoxNFChenWInteractive effects of MC1R and OCA2 on melanoma risk phenotypesHum Mol Genet200413444746114709592
- BoxNFWyethJRO’GormanLEMartinNGSturmRACharacterization of melanocyte stimulating hormone receptor variant alleles in twins with red hairHum Mol Genet1997611189118979302268
- SturmRADuffyDLBoxNFGenetic association and cellular function of MC1R variant alleles in human pigmentationAnn N Y Acad Sci200399434835812851335
