Abstract
Background
Cytotoxic T lymphocyte antigen 4 (CTLA-4) is an inhibitory regulator of the T-cell immune response against tumor cells. Ipilimumab is a monoclonal antibody directed against CTLA-4.
Objective
This review describes the basic mechanism of ipilimumab and discusses data available to date with regards to its safety and efficacy profile.
Methods
Data from clinical trials including abstracts was reviewed using the PubMed Database, as well as the American Society of Clinical Oncology Abstract Database.
Conclusion
CTLA-4 inhibition with a monoclonal antibody is usually well tolerated and has efficacy as a therapeutic agent in a variety of cancers. The classical response interpretation has changed because of the delayed mechanism of action. The toxicities are autoimmune events and guidelines for treatment of these effects are discussed. Therapy with ipilimumab leads to durable responses. The first two Phase III randomized studies showed an improvement of survival at 1, 2, and 3 years. Other studies are currently underway to better understand the optimal treatment administration of ipilimumab in melanoma.
Introduction
The 1-year survival of patients with metastatic melanoma is 25%, with a median survival of around 7–9 months.Citation1–Citation3 Chemotherapy has been widely used to treat patients with unresectable disease, but most of the time the disease fails to respond.Citation4 Non-specific immunotherapy has induced durable responses in very few patients. The Food and Drug Administration approved therapies for metastatic melanoma are dacarbazine and interleukin 2 (IL-2), and more recently, ipilimumab (March 2011) and vemurafenib (September 2011).
The rationale for studying immunotherapies is based on evidence of spontaneous remissions and observed response to biological agents that stimulate the immune system. Cytotoxic T lymphocyte antigen 4 (CTLA-4) exerts an inhibitory control on T-cell activation. Therefore, blockade of CTLA-4 is a unique way of enhancing patients’ immune response against tumors. Ipilimumab is a humanized monoclonal antibody against CTLA-4, recently approved for the treatment of metastatic melanoma. This review concentrates on the recent development of melanoma treatments with ipilimumab.
Mechanism of action of ipilimumab
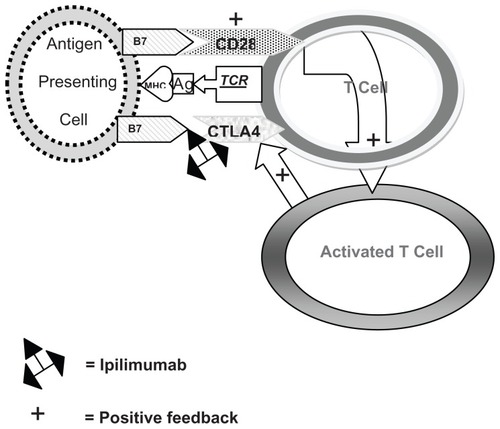
CTLA-4 is important in immune homeostasis and in the induction of tolerance to self-antigens.Citation5,Citation6 T-cell activation requires at least two signals: the presentation of an antigen to the T-cell receptor by a major histocompatibility complex molecule on an antigen presenting cell, and interaction of the T-cell with other receptors that either enhance or inhibit the T-cell response.Citation7 CD28 and CTLA-4 competitively interact with the B7-1 and B7-2 ligands located on the antigen presenting cell, with antagonistic effects.Citation8,Citation9 CD28 enhances T-cell activation and IL-2 production and CTLA-4 antagonizes T-cell activation by interfering with IL-2 secretion and IL-2 receptor expression.Citation10 IL-2 stimulates T-cell growth, but has also been implicated in the expansion of regulatory T-cells that express CTLA-4. CTLA-4 binds to B7-1 and B7-2 ligands with greater affinity than CD28.Citation11 Although CD28 is constitutively expressed on naive T-cells, CTLA-4 becomes functional only after T-cell activation.Citation12 This temporal delay in CTLA-4 upregulation allows for initial T-cell activation by CD28, followed by a regulatory feedback loop mediated by CTLA-4. Ipilimumab binds to CTLA-4 and allows the T-cell immune response to persist ().
Figure 1 When an antigen (Ag) is presented in the context of the major histocompatibility complex (MHC) to the T cell receptor (TCR), binding of B7 with CD28 occurs which activates the T cell. Slightly later, the activated T cell stimulates CTLA4 which also binds to B7 to down-regulate the T cell. Ipilimumab inactivates the binding of CTLA4 with B7, allowing the T cell to remain activated.
Abbreviations: Ag, antigen; MHC, major histocompatibility complex; TCR, T cell receptor.
Preclinical data
In cynomolgus macaques, administration of a CTLA-4 antibody resulted in enhanced antibody responses to hepatitis surface antigen and a human melanoma cell vaccine.Citation13 The injection of anti-CTLA-4 antibodies in mouse models stimulates the rejection of murine tumors such as colon, ovarian, and fibrosarcoma models, but can also lead to lymphoproliferative disease and autoimmunity in other organs. The effects of these antibodies can be potentiated by chemotherapy. CTLA-4 knockout mice develop fatal autoimmunity resulting from unopposed T-cell activation and reaction to self-antigens.Citation14
Pharmacodynamics and immunity stimulation
There are two mechanisms by which CTLA-4 blockade can create anti-tumor responses.Citation15 The first mechanism is by interfering with tumor-specific effector cells such as CD8 cells where CTLA-4 blockade causes increased clonal expansion.Citation16,Citation17 Studies using flow cytometry to compare cell surface marker expression on peripheral blood mononuclear cells before and after treatment with ipilimumab, in combination with interleukin-2 therapy, showed that HLA-DR, a marker of T-cell activation, increased after treatment on CD3+CD4+ and CD3+CD4− (likely CD8+) cells.Citation18 The second mechanism is via depletion of tumor-induced CD4+CD25+ regulatory T-cell (Tregs), which inhibit immune response to tumor-associated antigens. Tregs are a suppressive CD4 T-cell population that expresses CD25 (the high affinity IL-2α receptor subunit). Tregs also produce immunosuppressive cytokines such as IL-10.Citation19 Additionally, an increase in T helper 17 (Th17) cells, a distinct lineage of CD4+ T-cells producing specific cytokines (IL-17 and IL-22),Citation20 was associated with fewer relapses in a Phase II study of 75 patients treated with ipilimumab (3 or 10 mg/kg) alone or in combination with melanoma-specific peptides.Citation21 However, IL17 secretion is also linked to the development of colitis (see below).
Melanoma-specific tumor antigens have been studied in the context of ipilimumab treatment. Ipilimumab enhances immunity to NY-ESO-1, a cancer/testis antigen expressed in a subset of patients with melanoma. In 140 ipilimumab-treated patients, 16% were seropositive at baseline and 22% following treatment. These NY-ESO-1-seropositive patients are more responsive to ipilimumab than NY-ESO-1-seronegative patients. Furthermore, NY-ESO-1-seropositive patients with associated CD8(+) T-cells experienced greater clinical benefit (77%) than those with undetectable CD8(+) T-cell response (14%).Citation22 Inducible costimulator (ICOS), a member of the immunoglobulin gene family, is expressed on CD4+ and CD8+ T-cells following activation. ICOS functions as a costimulatory molecule on activated T-cells and increases effector T-cell survival.Citation23 An increase in the frequency of CD4+ ICOS-high cells is seen in patients treated with ipilimumab, compared to untreated melanoma patients or healthy volunteers. This increase may be associated with a clinical benefit at week 24 and an increased survival.Citation24 Another antigens associated to melanocyte differentiation, the melanoma inhibitor of apoptosis protein (ML-IAP or livin) is overexpressed in melanoma and contributes to disease progression and to treatment resistance. CD4(+) and CD8(+) cellular responses have been observed against livin and are associated with novel class I and class II epitopes. Some patients treated with ipilimumab develop humoral immune responses to livin, which seems associated with clinical benefit.Citation25
There is no early predictive marker of tumor response identified in clinical studies of CTLA-4 inhibition. Various studies are searching for pharmacodynamic biomarkers. In 35 patients treated with ipilimumab (10 mg/kg every 3 weeks for four doses with maintenance therapy as clinically indicated), lymphocyte counts and flow cytometry for CD8/CD4/CD25+ populations showed increases in the absolute lymphocytic count related to an increase of CD8+ T-cells between weeks 1 and 7. There was a statistically significant increase in CD8+ T-cells in responding patients, but not in CD4+ T-cells. Changes in CD4+/CD25+ T-cells did not correlate with clinical response.Citation26 However, in a study of patients with advanced localized melanoma (stage IIIB–C) treated with neoadjuvant ipilimumab (10 mg/kg every 3 weeks for two doses preoperatively and for two doses postoperatively, a significant increase in the frequency of circulating Treg cells was observed from baseline to 6 weeks.Citation27 These nonaligned observations require more study to really understand the modulation of the immune system in this patient population.Citation28 Furthermore, most clinical responses occur after 12 weeks of treatment, and it is possible that the immune system has not fully mounted proper anti-melanoma activity in the first few weeks following ipilimumab administration. However, to avoid a costly treatment that would not help a patient, predictive markers of response should ideally be identified.
There are no specific clinical predictors of response either. The variable most closely related is the presence of autoimmune toxicities. In 50 patients who had a grade 3 or 4 autoimmune toxicity, 28% achieved an objective response (P = 0.0004). Some autoimmune events required treatment with steroids. In 23 patients who responded to CTLA-4 anti-bodies and received steroids for adverse effects, the covariate analysis revealed that administration of steroids had no effect on the duration of response (P = 0.23).Citation29 Disease burden, as diagnosed by elevated LDH and/or brain metastases, does not correlate with an absence of response. There was no association between baseline LDH levels and disease control in previously treated patients with M1c-stage melanoma, who received ipilimumab (10 mg/kg every 3 weeks). Of 123 patients with M1c-stage disease, 81 had elevated LDH. Seventeen (21.0%) experienced disease control, compared to 28.6% of patients with normal LDH levels. Ipilimumab appears to induce clinical benefit even in patients with M1c-stage melanoma and elevated LDH levels.Citation30 The compassionate expanded access program was initially an open-label study of ipilimumab 10 mg/kg every 3 weeks for four doses (CA184-045). There were 165 asymptomatic patients with stable brain metastases at baseline who were enrolled at this dose. The overall survival of this patient population at 1 year was 20%. This compared to a controlled study of patients with stable brain metastases, who had a 1-year survival of 31% (CA184-042).Citation31 Thus, it appears that ipilimumab is active in some patients at any stage of the disease and it is not possible to determine upfront who will respond and who will not.
Pharmacokinetics and metabolism
After a single dose of ipilimumab, the plasma concentration decay over time could be monoexponential (four subjects) or bioexponential (ten subjects).Citation32 Tmax occurred at the end of the ipilimumab infusion in all subjects. The mean Cmax was 155.94 ± 64.5 μg/mL. The mean terminal elimination half-life was 299.4 ± 126.9 hours or 12.5 days, consistent with antibody pharmacokinetics, such that a single dose would give levels of over 10 μg/mL for at least 60 days. The mean apparent volume of distribution at steady state was 4.07 ± 1.3 L. The mean total body clearance was 0.01 ± 0.004 L/hour. In another study of ipilimumab (3 mg/kg), the mean peak concentration after the first dose was 72 ± 33 μg/mL, and the trough before the second dose was 12 ± 7 μg/mL. After therapy was completed, the mean plasma concentration was 99 ± 41 μg/mL, with a trough of 17 ± 10 μg/mL after 3 weeks. There was no correlation between plasma concentrations or clearance and tumor responses or toxicity. In addition, clearance increases with body weight, but as expected for a monoclonal antibody, is not affected by renal or hepatic functions,Citation33 or by steroid use.Citation34
Clinical efficacy
The first randomized study to show a benefit of ipilimumab treatment in patients previously treated for metastatic melanoma tested the monoclonal antibody (3 mg/kg every 3 weeks for four doses) with or without a glycoprotein 100 (gp100) peptide vaccine. The control was gp100 alone. All patients were HLA-A*0201–positive and had unresectable stage III or IV melanoma progressing after therapy for meta-static disease. Patients were randomly assigned to receive ipilimumab plus gp100 (403 patients), ipilimumab alone (137 patients), or gp100 alone (136 patients).Citation35 The primary end point was overall survival. The median overall survival was 10 months among patients receiving ipilimumab compared to 6.4 months among patients receiving gp100 alone. This study led to the Food and Drug Administration approval of ipilimumab. The second randomized study compared dacarbazine (850 mg/m2) plus ipilimumab (10 mg/kg every 3 weeks for four doses) or placebo, followed by dacarbazine alone every 3 weeks through to week 22.Citation36 Patients with clinical benefit received ipilimumab or placebo every 12 weeks as maintenance therapy. The primary end point was overall survival. The median overall survival was 11.2 months among patients receiving ipilimumab compared to 9.1 months among patients receiving dacarbazine alone. However, because of the immune mechanism of action, long-term survival rates are observed; at 1 year, it was 47.3% versus 36.3%; at 2 years, 28.5% versus 17.9%; and at 3 years, 20.8% versus 12.2%.
Other studies are testing various combinations. A study of 36 patients treated with ipilimumab and IL-2 demonstrated an objective response rate of 22%.Citation18 Twenty-seven patients were treated with ipilimumab (10 mg/kg on day 1) and oral temozolomide (200 mg/m2 on days 1–4) every 3 weeks for four courses, followed by maintenance. By immune-related response criteria (see below), there were six (22%) confirmed partial responses and twelve (44%) patients experienced stable disease for an overall disease control rate of 67%.Citation37 In a Phase I study of ipilimumab (fixed dose of 10 mg/kg) and bevacizumab (starting dose of 7.5 mg/kg), the overall response rate was 38% for partial responses, with stable disease seen in 28%. All responses lasted longer than 6 months. Post-treatment biopsies showed activated vessel endothelium with extensive T-cell trafficking and marked increase in CD4/CCR7/CD45RO central memory cells in the peripheral blood of most patients, not seen with ipilimumab alone.Citation38
An international randomized double-blind study of adjuvant ipilimumab versus placebo has recently completed accruals. The study has not yet matured and results will be reported in the next few years (CA184-029).
New insights for response evaluation
The modification of native immune processes by ipilimumab extends observation of benefits weeks to months after treatment administration. Hence, classical chemotherapy response criteria do not measure appropriately the immunotherapeutic effects. An initial increase in tumor burden or the appearance of new lesions could precede immunotherapy-induced tumor regression. Novel criteria for the evaluation of antitumor responses with immunotherapeutic agents have been proposed after a detailed analysis of the Phase II clinical trial program with ipilimumab. Ipilimumab monotherapy results in four distinct response patterns: (1) shrinkage in baseline lesions, without new lesions; (2) durable stable disease (in some patients followed by a slow, steady decline in total tumor burden over a period of months); (3) response after an increase in total tumor burden; and (4) response in the presence of new lesions. All patterns could be associated with favorable survival. Response patterns (1) and (2) may be captured using standard methods, but response patterns (3) and (4) would be classified as progressive disease using Response Evaluation Criteria in Solid Tumors or the World Health Organization criteria. Because patients on ipilimumab may have delayed responses or durable stable disease even after apparent disease progression, these new immune-related response criteria are now evaluated to capture additional response patterns observed with immune therapy in advanced melanoma beyond those described by conventional assessments.Citation39,Citation40 Examples of radiologic immune-related response criteria and toxicity in patients with advanced melanoma treated with ipilimumab can be seen in the pictorial assay published by O’Regan et al.Citation41
Safety and tolerability
Characteristic side effects from inhibition of CTLA-4 are called immune (or inflammation)-related adverse events (IRAEs). A retrospective review of 14 completed Phase I–III trials of ipilimumab in patients with advanced melanoma looked at safety data pooled from 1498 patients. IRAEs occur in 64.2% and result in death in <1% of patients. Most common IRAEs are skin rash, hepatitis, colitis, endocrinopathies, particularly hypopituitarism, and hepatic inflammation or neurological impairment. Other organ affections are seen in <1% of patients and include uveitis, pneumonitis, pancreatitis, autoimmune nephritis, myasthenia gravis, and others. Most IRAEs occurred during induction (initial four doses given once every 3 weeks).Citation42 There is an association with the development of IRAEs and tumor regression in patients with metastatic melanoma or renal cell carcinoma, as well as a prolonged time to relapse in those with resected high-risk melanoma, similar to other data.Citation43 The timing of IRAEs is variable and might depend on peak dosing and area under the curve of the drug. IRAEs may occur after the first cycle and may require aggressive, prompt management with corticosteroids. Treating the adverse autoimmune effects with corticosteroids does not affect anti-tumor activity.Citation29
Rash
This is by far the most common side effect of ipilimumab. The rash can be very itchy and is treated first with emollient skin lotion, anti-histamine, or in difficult cases, local steroid application.
Colitis
The occurrence of autoimmune enterocolitis is about 20%, with adverse events graded 3 and 4.Citation44 There is a 5% mortality rate in patients who developed autoimmune colitis and a significant risk for colonic perforation if untreated. Complaints include watery stools, abdominal pain, fever, nausea and vomiting, and anal pain. Colon biopsies show neutrophilic and/or lymphocytic infiltrates and even granulomas. When serum IL-17 levels were measured in patients treated with ipilimumab (10 mg/kg), comparing patients who developed colitis to those without IRAEs, significantly higher serum IL-17 levels were observed in patients with colitis (n = 13) versus patients with no IRAEs (n = 16). Serum IL-17 levels showed no difference at baseline, but in patients with colitis paralleled the course of the inflammation.Citation45 Diarrhea usually responds to treatment by restriction of oral intake and steroid use. When enterocolitis is refractory to high-dose steroids, the treatment should add a dose of infliximab. Guidelines for the treatment of diarrhea have been developed and validated based on the above clinical experience.Citation46 Safety data from studies conducted by Medarex (which were generally conducted before the implementation of diarrhea guidelines) were compared with those of Bristol Meyers Squibb (conducted after the guideline implementation). The dose of ipilimumab in the Medarex studies was generally 3 mg/kg, while that of the subsequent studies was 10 mg/kg every 3 weeks for four doses. There was a 50% reduction in gastrointestinal perforation or colectomy rate after guideline implementation, despite the higher dose of ipilimumab.Citation46
Prophylactic budesonide does not provide clinical benefit. Citation47 Patients with metastatic melanoma given ipilimumab 10 mg/kg every 3 weeks for four doses also received prophylactic budesonide or placebo. Of the 115 patients treated with placebo or budesonide, the rate of grade 2 or higher diarrhea was 35.0% and 32.7%, the best overall response rate 15.8% and 12.1% and the median overall survival 17.7 and 19.3 months, respectively. Again, the disease control rate was higher in patients with grade 3 to 4 IRAEs than in patients with grade 0 to 2 IRAEs, although some patients with grade 1 to 2 IRAEs also experienced clinical benefit. Budesonide should not be used prophylactically to prevent colitis-induced ipilimumab therapy.
Guidelines for the management of colitis
Guidelines were developed on the basis of 687 episodes of diarrhea or colitis in 511 subjects enrolled in five studies of ipilimumab (3 or 10 mg/kg every 3 weeks for four doses). Rapid institution of steroid therapy (within 5 days) leads to faster resolution of symptoms (see –).Citation46
Table 1 Treatment outcome with diarrhea
Table 2 Immunosuppressive guidelines
Table 3 Doses of immunosuppressive agents
Hypophysitis
A review of patients who developed autoimmune hypophysitis after treatment with ipilimumab showed that eight of the nine patients had correlative increase in pituitary size on imaging. Citation48 These patients all had symptoms and laboratory findings consistent with this diagnosis. A sudden headache could be the herald of pituitary involvement and should trigger consideration of this IRAE.Citation49 Therefore, patients should be monitored with baseline TSH; other laboratory investigations such as cortisol, thyroxine, testosterone or LH/FSH levels should be monitored to rule out a suspicion of hypophysitis. A short course of high-dose steroids may improve pituitary function. Patients with evidence of autoimmune hypophysitis should stop ipilimumab and be treated with physiological hormone replacements with hydrocortisone and thyroxine, with or without sexual hormones.
Conclusions
The discovery of cytotoxic T-lymphocyte antigen-4 (CTLA-4) and its role as a key negative regulator for T-cells have led to the approval of ipilimumab as a new approach to treat unresectable melanoma. Ipilimumab inhibits CTLA-4 and prolongs immune response. Consequently, antitumor response has been associated with a higher incidence of autoimmunity reactions, which can be severe but are commonly manageable with steroids. There is currently no evidence that the use of steroids reduces the response mechanism fueled by ipilimumab. Ipilimumab yields prolonged antitumor effects in 20%–30% of patients with melanoma; however, the effect of this immunotherapy takes many weeks to peak. This delay response time has engendered a new definition of response criteria for immunotherapy, because the classical Response Evaluation Criteria in Solid Tumors criteria are inadequate to measure efficacy. Responses can occur later and may be more durable with ipilimumab than with traditional chemotherapy. The current 12-week benchmark may not be long enough to determine response to ipilimumab and patients should be observed longer. The immune response generated in the first few weeks of therapy, may be wrongly interpreted as progressive disease on radiological imaging. In this instance, the T-cell infiltration and inflammation cause the increased tumor size on radiological images. The appearance of new lesions may also be related to immune-mediated inflammation in previous areas of subclinical micro-metastases. Observing patients for 20 weeks after the first dose of ipilimumab before making the decision to modify therapy, as long as the cancer is not life threatening, might be worthwhile. The current approved dose is 3 mg/kg every 3 weeks for four doses. Maintenance treatment is not approved, but patients who are progressing after an initial clinical benefit may be retreated if clinically indicated. Studies of higher dosing (10 mg/kg) are underway. An individualized approach with a predictive marker of early response would be crucial, especially since many patients have delayed responses. Increases in the absolute lymphocyte count may be a crude marker of immune stimulation.Citation50 Apparition of autoimmune reactions may be an early sign of effectiveness. Monitoring immunologic changes to better understand the role of ipilimumab has not been established. Ipilimumab is also under investigation in combination with other agents, and in a smaller cohort of patients with various cancers.
Disclosure
The author reports no conflicts of interest in this work.
References
- BedikianAYMillwardMPehambergerHBcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study GroupJ Clin Oncol200624294738474516966688
- KornELLiuPYLeeSJMeta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trialsJ Clin Oncol200826452753418235113
- MiddletonMRGrobJJAaronsonNRandomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanomaJ Clin Oncol200018115816610623706
- AtkinsMBInterleukin-2: clinical applicationsSemin Oncol2002293 Suppl 7121712068383
- BluestoneJAIs CTLA-4 a master switch for peripheral T cell tolerance?J Immunol19971585198919939036940
- DavisSJIkemizuSEvansEJFuggerLBakkerTRvan der MerwePAThe nature of molecular recognition by T cellsNat Immunol20034321722412605231
- FujiwaraHHamaokaTRegulatory mechanisms of antitumor T cell responses in the tumor-bearing stateImmunol Res19951442712918722044
- BrunetJFDenizotFLucianiMFA new member of the immunoglobulin superfamily – CTLA-4Nature198732861272672703496540
- ChambersCAKuhnsMSEgenJGAllisonJPCTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapyAnnu Rev Immunol20011956559411244047
- KrummelMFAllisonJPCD28 and CTLA-4 have opposing effects on the response of T cells to stimulationJ Immunol20101877459465
- LinsleyPSGreeneJLBradyWBajorathJLedbetterJAPeachRHuman B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptorsImmunity1994197938017534620
- LindstenTLeeKPHarrisESCharacterization of CTLA-4 structure and expression on human T cellsJ Immunol19931517348934998397258
- KelerTHalkEVitaleLActivity and safety of CTLA-4 blockade combined with vaccines in cynomolgus macaquesJ Immunol2003171116251625914634142
- LeachDRKrummelMFAllisonJPEnhancement of antitumor immunity by CTLA-4 blockadeScience19962715256173417368596936
- CallahanMKWolchokJDAllisonJPAnti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapySemin Oncol201037547348421074063
- PhanGQYangJCSherryRMCancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanomaProc Natl Acad Sci U S A2003100148372837712826605
- SandersonKScotlandRLeePAutoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanomaJ Clin Oncol200523474175015613700
- MakerAVPhanGQAttiaPTumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II studyAnn Surg Oncol200512121005101616283570
- Von BoehmerHMechanisms of suppression by suppressor T cellsNat Immunol20056433834415785759
- HirotaKMartinBVeldhoenMDevelopment, regulation and functional capacities of Th17 cellsSemin Immunopathol201032131620107806
- WeberJSO’DaySUrbaWPhase I/II study of ipilimumab for patients with metastatic melanomaJ Clin Oncol200826365950595619018089
- YuanJAdamowMGinsbergBAIntegrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumabProc Natl Acad Sci U S A201110840167231672821933959
- HutloffADittrichAMBeierKCICOS is an inducible T-cell costimulator structurally and functionally related to CD28Nature199939767162632669930702
- LiakouCIKamatATangDNCTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patientsProc Natl Acad Sci U S A200810539149871699218818309
- ZhouJYuenNKZhanQImmunity to the melanoma inhibitor of apoptosis protein (ML-IAP; livin) in patients with malignant melanomaCancer Immunol Immunother2011 Epub date 2011/10/29
- YangAKendleRGinsbergBCTLA-4 blockade with ipilimumab increases peripheral CD8+ T cells: Correlation with clinical outcomesJ Clin Oncol20102816SA2555
- TarhiniAAEdingtonHButterfieldLHNeoadjuvant ipilimumab in patients with stage IIIB/C melanoma: Immunogenicity and biomarker analysisJ Clin Oncol20112916S8536
- GabrielEMLattimeECAnti-CTL-associated antigen 4: are regulatory T cells a target?Clin Cancer Res200713378578817289867
- DowneySGKlapperJASmithFOPrognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockadeClin Cancer Res20071322 Pt 16681668817982122
- SmylieMFrancisSNeystBEffect of ipilimumab at 10 mg/kg on disease control in patients (pts) with M1c-stage melanoma in relation to baseline lactate dehydrogenase (LDH) levelsJ Clin Oncol20092715sA9041
- HellerKNPavlickACHodiFSSafety and survival analysis of ipilimumab therapy in patients with stable asymptomatic brain metastasesJ Clin Oncol20112917SA8581
- SmallEJTchekmedyianNSRiniBIFongLLowyIAllisonJPA pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancerClin Cancer Res20071361810181517363537
- DaiDWuCParkerSMModel-based evaluation of ipilimumab dosage regimen in patients with advanced melanomaJ Clin Oncol20082616SA9073
- ThompsonJABermanDSiegalJEffect of prior treatment status on the efficacy and safety of ipilimumab monotherapy in treatment-naive and previously treated patients with advanced melanomaJ Clin Oncol20082616SA9055
- HodiFSO’DaySJMcDermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- RobertCThomasLBondarenkoIIpilimumab plus dacarbazine for previously untreated metastatic melanomaN Engl J Med2011364262517252621639810
- PatelSPBedikianAYPapadopoulosNEIpilimumab plus temozolomide in metastatic melanomaJ Clin Oncol20112917SA8579
- HodiFSFriedlanderPAAtkinsMBA phase I trial of ipilimumab plus bevacizumab in patients with unresectable stage III or stage IV melanomaJ Clin Oncol20112918SA8511
- PennockGKWaterfieldWWolchokJDPatient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: how different are these from conventional treatment responses?Am J Clin Oncol2011 [Epub ahead of print.]
- WolchokJDHoosAO’DaySGuidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteriaClin Cancer Res200915237412742019934295
- O’ReganKNJagannathanJPRamaiyaNHodiFSRadiologic aspects of immune-related tumor response criteria and patterns of immune-related adverse events in patients undergoing ipilimumab therapyAJR Am J Roentgenol20111972W241W24621785048
- IbrahimRABermanDMDePrilVIpilimumab safety profile: summary of findings from completed trials in advanced melanomaJ Clin Oncol201129158583
- WeberJReview: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse eventsOncologist200712786487217673617
- BeckKEBlansfieldJATranKQEnterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4J Clin Oncol200624152283228916710025
- CallahanMKYangATandonSEvaluation of serum IL-17 levels during ipilimumab therapy: Correlation with colitisJ Clin Oncol2011292505
- O’DaySWeberJSWolchokJDEffectiveness of treatment guidance on diarrhea and colitis across ipilimumab studiesJ Clin Oncol2011298554
- WeberJThompsonJAHamidOA randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanomaClin Cancer Res200915175591559819671877
- DillardTYedinakCGAlumkalJFleseriuMAnti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypesPituitary2010131293819639414
- BarnardZRWalcottBPKahleKTNahedBVCoumansJVHyponatremia associated with Ipilimumab-induced hypophysitisMed Oncol2011 [Epub ahead of print.]
- KuGYYuanJPageDBSingle-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survivalCancer201011671767177520143434