Abstract
Background
We undertook a meta-analysis to evaluate the clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) for the detection of distant metastases in patients with non-small cell lung cancer (NSCLC) at initial staging.
Materials and methods
All topic-related studies were comprehensively searched in the MEDLINE and Embase databases. We obtained the summary estimates and constructed the summary receiver operating characteristic curve for 18F-FDG PET/CT using the bivariate regression model.
Results
Across 10 studies (1333 patients), the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio for 18F-FDG PET/CT were 0.81 (95% confidence interval [CI] = 0.63–0.92), 0.96 (95% CI = 0.94–0.98), 22.9 (95% CI = 13.3–39.5), and 0.20 (95% CI = 0.09–0.42), respectively. Overall weighted area under the curve was 0.97 (95% CI = 0.96–0.98).
Conclusion
18F-FDG PET/CT has a good diagnostic performance for distant metastasis staging in patients with NSCLC at initial staging.
Introduction
Lung cancer is the leading cause of cancer mortality worldwide.Citation1,Citation2 Non-small cell lung cancer (NSCLC) accounts for 80% of lung cancer.Citation1,Citation2 Distant metastasis is one of the most critical factors guiding treatment decisions in NSCLC patients. NSCLC patients with locoregional disease are generally treated with curative surgery or radiotherapy with/without chemotherapy.Citation1 In contrast, palliative chemotherapy or molecule-targeted treatment is the standard treatment for NSCLC patients with metastasis.Citation1
Conventional imaging procedure for distant metastasis staging of lung cancer is the combination of computed tomography, bone scan, and magnetic resonance imaging (MRI), and its accuracy is still unsatisfactory.Citation3–Citation8 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) can detect the increase in glucose metabolism in tumor cells and offer anatomical details at the same time, enabling whole-body examination in a single procedure.Citation3 Although many previous studies about 18F-FDG PET/CT have been reported for distant metastasis staging in NSCLC patients at initial staging,Citation4–Citation10 the results among studies are still controversial. Here, we undertook a meta-analysis to evaluate the diagnostic performance of 18F-FDG PET/CT for detecting distant metastasis in NSCLC patients at initial staging.
Materials and methods
Search strategy
We searched for studies evaluating 18F-FDG PET/CT for the detection of distant metastasis in NSCLC patients at initial staging. Articles were identified with a search of the MEDLINE and Embase databases through October 29, 2017. We used a search algorithm that was based on a combination of text words: PET/CT, positron emission tomography/computed tomography, PET-CT, positron emission tomography-computed tomography, non-small cell lung cancer, NSCLC, staging, and distant metastasis. We had no language restrictions for searching and selecting relevant studies. References of pertinent articles (the retrieved articles, meta-analysis, reviews, and editorials) and guidelines were also screened manually to retrieve additional eligible studies. Authors of eligible studies were contacted and asked to supplement additional data when key information was missing.
Study selection
We included studies based on the following criteria: 1) 18F-FDG PET/CT was used as a diagnostic tool for the detection of distant metastases in NSCLC patients at initial staging; 2) studies can provide sufficient data of the true-positive, false-negative, false-positive, and true-negative values to reconstruct a 2×2 table; 3) studies with a minimal sample size of 10 patients were included; 4) studies were based on a per-patient analysis; and 5) pathological analysis and/or imaging follow-up data served as the reference standard.
We excluded studies based on the following criteria: 1) studies enrolled patients with recurrent NSCLC; 2) studies enrolled patients with NSCLC and small cell lung cancer, if relevant data regarding the NSCLC patients could not be obtained; 3) studies came from the same study group; and 4) non-original articles (case reports, conference abstracts, and reviews).
Data extraction
Two reviewers extracted data from eligible studies independently and resolved discrepancies by discussion. For each report, we recorded the author names, publication time, country of origin, sample size, technical protocols of PET–CT (technical parameters, interpreters, criteria for defining positive PET/CT results), reference standards, and follow-up time. For each study, we also recorded the number of true-positive, false-positive, true-negative, and false-negative values for 18F-FDG PET/CT.
Quality assessment
To evaluate the quality, two reviewers independently evaluated the methodological quality of all included studies using the Quality Assessment of Diagnostic Accuracy Studies version 2 (QUADAS-2) tool.Citation11 It consists of four key domains covering patient selection, index test, reference standard, and flow and timing.
Statistical analyses
All analyses were conducted with Stata version 12.0 (Stata-Corp LP, College Station, TX, USA). We used the bivariate regression model to obtain weighted overall estimates of the sensitivity and specificity as the main outcome measures and to construct summary receiver operating characteristic (SROC) curves for 18F-FDG PET/CT.Citation9,Citation10 By using the pooled sensitivity and specificity, we also calculated diagnostic odds ratio (DOR), positive likelihood ratios (PLRs), and negative likelihood ratios (NLRs) for 18F-FDG PET/CT.Citation12–Citation14
We used the summary estimates of sensitivity and specificity to calculate the negative predictive values of 18F-FDG PET/CT when the prevalence of distant metastasis in NSCLC was assumed to be 10%, 20%, and 30%, respectively.
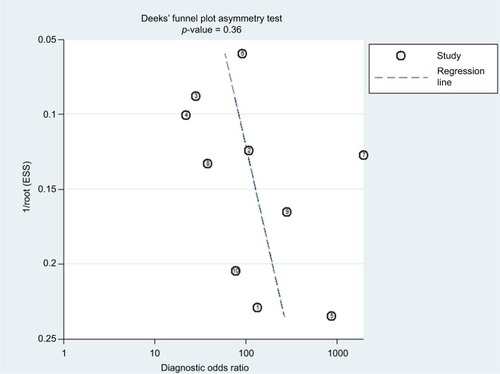
The Deeks’ funnel plot was used to assess publication bias. An asymmetric Deeks’ funnel plot (p < 0.05) would suggest that a publication bias is present.
Results
Eligible studies
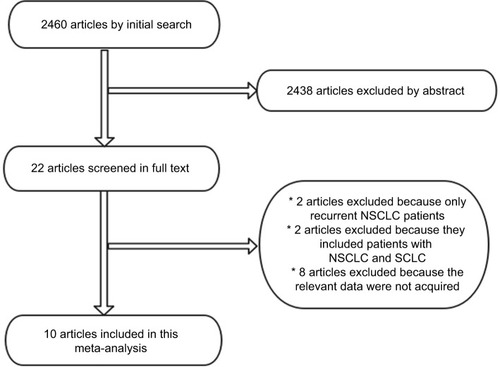
The electronic search yielded 2460 articles; 2438 were excluded upfront by reading the abstract because they did not present any diagnostic information. We screened in full text 22 articles and rejected 12; 10 articlesCitation4–Citation10,Citation14–Citation17 were eligible for meta-analysis. Reasons for exclusion are listed in . Ten of the 10 (100%) studies stated that they were prospective (Prosp). A total of 10 studies (1333 patients) were analyzed for the accuracy of 18F-FDG PET/CT to detect distant metastases (). In four studies, 18F-FDG PET/CT was interpreted in a qualitative (QL) manner, whereas in six studies, it was interpreted in a quantitative and QL manner.
Table 1 Clinical characteristics and study quality of the included studies
Quality assessment
The results of the quality assessment using the QUADAS-2 tool are shown in . The risk of bias and applicability concerns with regard to patient selection were unclear or low in three of all 10 studies.Citation4,Citation10,Citation17 Risk of bias with regard to the index test was low in all 10 studies because the PET–CT results were consistently interpreted without knowledge of the reference standard results. However, the risk of bias for the reference standard was high in all 10 studies because the reference standard was not executed without knowledge of the PET–CT results.
Table 2 The results of the quality assessment using the QUADAS-2 tool
Results of meta-analysis
Summary estimates of sensitivity, specificity, DOR, PLR, and NLR
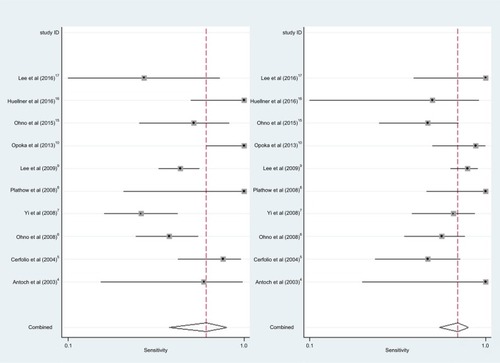
shows the forest plot of sensitivity and specificity for 18F-FDG PET/CT in NSCLC patients at initial M staging. When considering 10 studies (1333 patients), the pooled sensitivity, specificity, DOR, PLR, and NLR for 18F-FDG PET/CT were 0.81 (95% confidence interval [CI] = 0.63–0.92), 0.96 (95% CI = 0.94–0.98), 117 (95% CI = 38–355), 22.9 (95% CI = 13.3–39.5), and 0.20 (95% CI = 0.09–0.42), respectively.
SROC curve
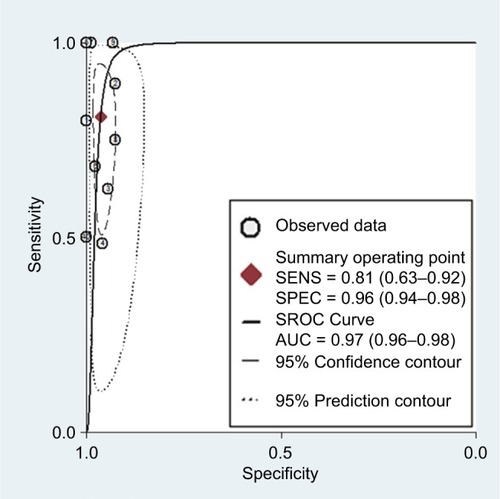
The SROC curve for 18F-FDG PET/CT in NSCLC patients at initial M staging is shown in . The results showed that the overall weighted area under the SROC curve was 0.97 (95% CI = 0.96–0.98).
Figure 3 The SROC curve for 18F-FDG PET/CT for the detection of NSCLC patients at initial M staging.
Abbreviations: SROC, summary receiver operating characteristic; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; NSCLC, non-small cell lung cancer; AUC, area under the curve.
Negative predictive values
Assuming a prevalence of distant metastases of 10%, 20%, and 30% in NSCLC patients, the negative predictive values for 18F-FDG PET-CT were 0.98, 0.95, and 0.92, respectively.
Publication bias
The publication bias for 18F-FDG PET/CT (p = 0.38 > 0.05) was insignificant. The funnel plot for 18F-FDG PET/CT was symmetrical (). These results indicated that the publication bias was not statistically significant.
Discussion
Distant metastasis staging is one of the major prognostic factors of survival in NSCLC patients. Accurate staging of distant metastasis is crucial, as the treatment strategy is directly dependent on tumor stage.Citation1 In this meta-analysis of 10 studies, distant metastasis involves 239 of 1333 (17.9%) eligible patients. 18F-FDG PET/CT has a sensitivity and a specificity of 0.81 (95% CI = 0.63–0.92) and 0.96 (95% CI = 0.94–0.98), respectively. This meta-analysis documents that 18F-FDG PET/CT has a good diagnostic performance for NSCLC patients at initial M staging. With the application of PET–CT in initial M staging of NSCLC, distant metastases may be detected earlier. This would have a considerable impact on patient staging of distant metastasis and the execution of more reliable treatment protocols.
As one of the best indicators of diagnostic meta-analysis, the likelihood ratio is widely considered to be more important than sensitivity and specificity.Citation12–Citation14 In this meta-analysis, the PLR value of 22.9 suggested that the correct probability of testing the positivity of the PET–CT assay in NSCLC patients with distant metastasis would be ~23 times higher than that in patients without distant metastasis. The NLR value of 0.09 indicated that the incorrect probability of testing the negativity of the PET–CT assay in NSCLC patients with distant metastasis would be 0.20 times lower than that in patients without distant metastasis. In other words, the correct probability of testing the negativity of the PET–CT assay in NSCLC patients without distant metastasis would be approximately five (1/0.20) times higher than that in patients with distant metastasis.
The SROC curve has been recommended to represent the performance of a diagnostic meta-analysis, which indicates the balance between sensitivity and specificity to compare diagnostic values from different diagnostic tests.Citation18,Citation19 The SROC curve showed that the overall weighted area under the curve was 0.97 (95% CI = 0.96–0.98), suggesting that 18F-FDG PET/CT has an excellent accuracy for the initial M staging of NSCLC patients.
Nevertheless, the present study has several limitations that should be considered. First, the exclusion of unpublished studies and meeting abstracts was probably a cause of publication bias. Second, the reference standard of the pathological analysis was not obtained from all distant metastatic lesions, which may affect the accuracy of 18F-FDG PET/CT. Third, we did not perform subgroup analyses according to clinical stage and subtype of pathology because this would have required individual patient data. Clinical stage and subtype of pathology may influence the probability of distant metastases, which may also affect the diagnostic accuracy of 18F-FDG PET/CT. Fourth, there is a unified protocol of PET/CT (technical parameters, interpreters, criteria for defining positive PET/CT results). The optimal accuracy of 18F-FDG PET/CT for distant metastasis staging in NSCLC patients is still unclear.
Conclusion
18F-FDG PET/CT has excellent diagnostic performance for the detection of distant metastases in NSCLC patients. Large and Prosp studies with a standardized protocol of 18F-FDG PET/CT are needed to investigate the optimal value of 18F-FDG PET/CT and could help establish 18F-FDG PET/CT as an accurate tool for distant metastasis staging in NSCLC.
Acknowledgments
This meta-analysis has been financially supported by the National Natural Science Foundation of China (81760544 and 81360343), and the Key Laboratory of High-Incidence-Tumor Prevention and Treatment, Independent Research Project (Contract number: GKZ201605 and GJZ201605).
Disclosure
The authors report no conflicts of interest in this work.
References
- PostmusPEKerrKMOudkerkMESMO Guidelines CommitteeEarly and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201728suppl_4iv1iv2128881918
- FerlayJAutierPBoniolMHeanueMColombetMBoylePEstimates of the cancer incidence and mortality in Europe in 2006Ann Oncol200718358159217287242
- XuGZhaoLHeZPerformance of whole-body PET/CT for the detection of distant malignancies in various cancers: a systematic review and meta-analysisJ Nucl Med201253121847185423073605
- AntochGStattausJNematATNon-small cell lung cancer: dual-modality PET/CT in preoperative stagingRadiology2003229252653314512512
- CerfolioRJOjhaBBryantASRaghuveerVMountzJMBartolucciAAThe accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancerAnn Thorac Surg200478310171023 discussion 1017–102315337041
- OhnoYKoyamaHOnishiYNon-small cell lung cancer: whole-body MR examination for M-stage assessment – utility for whole-body diffusion-weighted imaging compared with integrated FDG PET/CTRadiology2008248264365418539889
- YiCAShinKMLeeKSNon-small cell lung cancer staging: efficacy comparison of integrated PET/CT versus 3.0-T whole-body MR imagingRadiology2008248263264218552311
- PlathowCAschoffPLichyMPPositron emission tomography/computed tomography and whole-body magnetic resonance imaging in staging of advanced nonsmall cell lung cancer – initial resultsInvest Radiol200843529029718424949
- LeeHYLeeKSKimBTDiagnostic efficacy of PET/CT plus brain MR imaging for detection of extrathoracic metastases in patients with lung adenocarcinomaJ Korean Med Sci20092461132113819949671
- OpokaLKunikowskaJPodgajnyZStaging of non-small cell lung cancer using CT and integrated PET-CTPneumonol Alergol Pol201381151523258466
- WhitingPFRutjesAWWestwoodMEQUADAS-2 GroupQUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studiesAnn Intern Med2011155852953622007046
- ChuHColeSRBivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approachJ Clin Epidemiol2006591213311332 author reply 1332–133317098577
- ReitsmaJBGlasASRutjesAWScholtenRJBossuytPMZwindermanAHBivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviewsJ Clin Epidemiol2005581098299016168343
- JaeschkeRGuyattGHSackettDLUsers’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working GroupJAMA199427197037078309035
- OhnoYKoyamaHYoshikawaTThree-way comparison of whole-body MR, coregistered whole-body FDG PET/MR, and integrated whole-body FDG PET/CT imaging: TNM and stage assessment capability for non-small cell lung cancer patientsRadiology2015275384986125584709
- HuellnerMWde Galiza BarbosaFHusmannLTNM staging of non-small cell lung cancer: comparison of PET/MR and PET/CTJ Nucl Med2016571212626471696
- LeeSMGooJMParkCMPreoperative staging of non-small cell lung cancer: prospective comparison of PET/MR and PET/CTEur Radiol201626113850385726883332
- WalterSDProperties of the summary receiver operating characteristic (SROC) curve for diagnostic test dataStat Med20022191237125612111876
- RosmanASKorstenMAApplication of summary receiver operating characteristics (sROC) analysis to diagnostic clinical testingAdv Med Sci200752768218217394