Abstract
Background
About 5%–10% of breast cancer and 10%–15% of ovarian cancer are hereditary. BRCA1 and BRCA2 are the most common germline mutations found in both inherited breast and ovarian cancers. Once these mutations are identified and classified, a course of action to reduce the risk of developing either ovarian or breast cancer – including surveillance and surgery – is carried out.
Purpose
The purpose of the current research is to characterize the gene expression differences between healthy cells harboring a mutation in BRCA1/2 genes and normal cells. This will allow detection of candidate genes and help identify women who carry functional BRCA1/2 mutations, which cannot always be detected by the available sequencing methods, for example, carriers of mutations found in regulatory sequences of the genes.
Materials and methods
Our cohort consisted of 50 healthy women, of whom 24 were individuals with BRCA1 or BRCA2 heterozygous mutations and 26 were non-carrier controls. RNA purified from non-irradiated lymphocytes of nine BRCA1/2 mutation carriers versus four control mutation-negative individuals was utilized for RNA-Seq analysis. The selected RNA-Seq transcripts were validated, and the levels of spleen tyrosine kinase (SYK) mRNA were measured by using real-time quantitative polymerase chain reaction.
Results
Differences in gene expression were found when comparing untreated lymphocytes of BRCA1/2 mutation carriers and controls. Among others, the SYK gene was identified as being differently expressed for BRCA1/2 mutation carriers. The expression level of SYK was significantly higher in untreated healthy lymphocytes of BRCA1 heterozygote carriers compared with controls, regardless of irradiation. In contrast to normal tissues, in cancerous breast tissues, the expression levels of the BRCA1 and SYK genes were not intercorrelated.
Conclusion
Collectively, our observations demonstrate that SYK may prove to be a good candidate for better diagnosis, treatment, and prevention of BRCA1 mutation-associated breast cancer.
Introduction
The presence of mutations in BRCA1 or BRCA2 genes increases a woman’s susceptibility to develop breast and ovarian cancers by the age of 70, with the incidence ranging between 47% and 66%, and 40% and 57%, respectively.Citation1,Citation2 Additionally, women with a BRCA mutation have an elevated risk of developing other malignancies, such as gastrointestinal cancers (e.g., gall bladder, bile duct, colon, stomach, pancreas) and melanoma, and when this mutation appears in males, it increases the risk for breast and prostate cancers.Citation3,Citation4
The BRCA1 and BRCA2 proteins are involved in different cellular processes, including homologous recombination of DNA repair,Citation5,Citation6 ubiquitination, chromosomal segregation,Citation7 cell cycle arrest, apoptosis, and gene transcription. In addition, they are required for the S phase and G2/M checkpoint arrest in response to DNA damage.Citation8–Citation10 Abnormal BRCA1 and BRCA2 expression interferes with routine cell cycle events such as cell division, death, or life span. Therefore, these genes are recognized as “gatekeeper” genes, whose mutation stimulates the progression of cancer.Citation11
Although the BRCA1 and BRCA2 genes were identified a long time ago, their specific biological mechanisms and the way disruptions of their functions promote breast and ovarian carcinogenesis remain unclear. According to Knudson’s widely accepted “two-hit” hypothesis,Citation12 inheriting one de novo germline copy of a mutated BRCA1 or BRCA2 gene (i.e., the so-called first hit) is insufficient to enable the development of breast cancer and other hereditary cancer syndromes. The acquisition of a “second hit” to the remaining “healthy” copy of the gene is required for cancer development. This mutation can occur somatically, leading to the loss of both copies of the normal tumor suppressor gene. Notwithstanding, several studies support the idea that haploinsufficiency itself can interrupt normal cell function by contributing to genome instability, which leads to additional cancer driver mutations. For example, mice carrying a heterozygous mutation in the BRCA1 gene have shortened life spans and are more susceptible to ovarian cancer following ionizing irradiation without losing the other BRCA1 allele.Citation13 Such findings suggest that women carrying BRCA1 mutations may be more susceptible to ovarian tumor formation after irradiation than non-mutation carriers.
Different cell types, such as normal fibroblastsCitation14 and ovarian and breast epithelial cellsCitation15 from heterozygous BRCA1 mutation carriers, display a different gene expression profile than controls in response to DNA damage, again suggesting that BRCA heterozygosity itself contributes to breast cancer initiation. Moreover, it was found that losing a single allele of BRCA1 contributes specifically to alteration in the expression of genes having a role in cellular differentiation.Citation16 This finding supports the hypothesis that single copy loss of BRCA1 may cause variations in cell differentiation and, eventually, causes cells to undergo malignant processes.Citation16 Our previous study demonstrated the differences in gene expression in lymphocytes subject to ionizing radiation derived from BRCA mutation carriers as compared with controls, confirming a measurable heterozygous effect.Citation17 Finally, it has been shown using fluorescence lifetime (FLT) imaging microscopy, a method used to differentiate between distinct cell populations, that lymphocytes derived from control or BRCA1 mutation carriers differ in their FLT values from lymphocytes derived from BRCA2 mutation carrier individuals. This study demonstrates that BRCA1 and BRCA2 heterozygous lymphocytes have innate differences independent of irradiation. In addition, it suggests that these heterozygous mutations can lead to changes in the physical properties of the cells themselves, such as pH, temperature, viscosity, and oxygen concentration.Citation18
In the current study, we wished to extend our previous research and examine whether there is a difference in gene expression in untreated cells from BRCA carriers and controls. The spleen tyrosine kinase (SYK) gene was identified as differently expressed in BRCA1 mutation carriers. The results of this study will allow for a more accurate characterization of intrinsic differences between healthy cells harboring a mutation in BRCA1/2 genes and normal cells. Moreover, our observations demonstrate that SYK could prove to be a good candidate for more effective diagnosis, treatment, and prevention of BRCA1 mutation-associated breast cancer.
Materials and methods
Sample information
Our cohort consisted of 50 healthy women aged between 25 and 50 years, of whom 24 were individuals with no personal history of cancer but with BRCA1 or BRCA2 heterozygous mutations and 26 women had no BRCA mutations. Note that some of the subjects in the various analyses (RNA-Seq and real-time quantitative polymerase chain reaction [qPCR]) in this study overlap. The mutation carriers were diagnosed in the BRCA1 and BRCA2 predictive testing program of the Oncogenetic Clinic of Hadassah University Medical Center (Jerusalem, Israel).
The women in the study who served as controls needed to fulfill two conditions. The first one was the absence of any personal or familial history of breast or ovarian cancer in the participants. Second, they needed to be non-carriers of known BRCA1 or BRCA2 mutations.
The local Ethics Committee of Hadassah Medical Center approved the study protocol, and written informed consent was obtained from all participating individuals before inclusion in the study.
Lymphocyte extraction and preparation
Lymphocyte extraction and treatment were conducted as previously described.Citation17 In summary, primary lymphocyte cells were obtained from peripheral blood using LymphoPrep (Sigma) and short-term cultured for 6 days in RPMI-1640 with l-glutamine (Biological Industries), supplemented with interleukin-2, 15% fetal calf serum, 1% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 1% penicillin/streptomycin. One day following lymphocyte extraction, half of each sample was γ-irradiated with 8 Gy at a high-dose rate (0.86 Gy/min, Orthovoltage X-ray machine), as previously described.Citation19 Although this dose exceeds the portion used in classical radiotherapy or screening radiation, the DNA damage it causes presumably mimics the additive effect of repeated radiation exposure, which usually occurs in the clinic.Citation9 Additionally, we chose this dose based on our previous study showing differences in gene expression in BRCA mutation carriers after ionizing radiation.Citation17
Gene expression analysis
Poly(A)-selected RNA was sequenced using the Illumina TruSeq protocol on the HiSeq 2500 sequencing machine. Quality control checks on the raw sequence data were carried out using the FastQC tool (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Then, the Trim Galore! tool (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/), which is based on cutadapt,Citation20 was used for adapter trimming and for removing low-quality bases from the ends of reads. Clean reads were mapped to the human genome (hg38) using tophat2.Citation21 Next, the number of reads mapping each human gene (as annotated in Ensembl release 77) was counted using the “union” mode of HTseq-count script.Citation22 Differential expression analysis was performed using the edgeR and Limma packages from the Bioconductor framework.Citation23 Briefly, features with <1 read per million in at least five samples were removed. The remaining gene counts were normalized using the trimmed mean of M values method, followed by voom transformation.Citation24,Citation25 Linear models, as implemented by the Limma package,Citation23 were used to find differentially expressed genes. Since FDR application was too stringent for this data set, genes with a P-value <0.001 were considered as differentially expressed. Gene set enrichment and pathway analysis were done using GeneAnalytics.Citation26
RNA extraction and real-time qPCR
Total RNA was extracted from primary lymphocyte cells using a Direct-zol™ RNA MiniPrep kit (Zymo Research), according to the protocol recommended by the manufacturer. Complementary DNA was obtained by reverse transcription of 850 ng of total RNA in a final reaction volume of 20 μL containing 4 μL qScript Reaction Mix and 1 μL qScript Reverse Transcriptase (Quantabio). Quantitative real-time PCR assays containing the primers (Table S1) and probe mix for SYK, TSPAN18, DPEPT, and OTUB2 were purchased from Biosearch Technologies and utilized according to the manufacturer’s instructions. PCR was carried out in a final reaction volume of 10 μL containing 20 ng of complementary DNA template, 5 μL of PerfeCTa SYBR Green FastMix, ROX (Quantabio), and 1 μL of primers mix. All reactions were run in triplicate, and the housekeeping genes, GAPDH (Figure S1) and beta-actin (Figure S2), were amplified in a parallel reaction for normalization.
Analysis of publicly available ICGC and GTEx expression data
The expression values of BRCA1, BRCA2, and SYK genes across the breast tissues taken from healthy donors and patients with breast cancer (available from the GTExCitation27 and ICGCCitation28 databases, respectively) were visualized using the UCSC Xena genome browser.Citation29 In each database, samples were divided into two groups based on the expression of BRCA1 or BRCA2 genes using the median value as the cutoff point. The chart view option in Xena was used to view the distribution of SYK expression across the two groups and to calculate the Welch’s t-test. Mean centered values of the normalized SYK expression were downloaded and box plots were generated using R language.
Results
Characterizing the mRNA expression profile in BRCA1/2 mutation carriers versus non-carrier controls
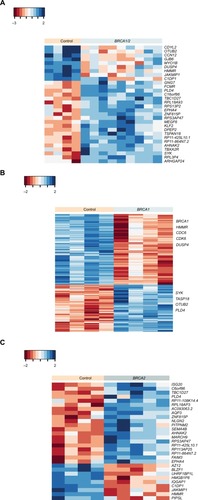
In a previous study, we tested whether BRCA1 or BRCA2 mutation carriers could be distinguished from control mutation-negative individuals based on gene expression changes triggered by the activation of BRCA1/2 genes following irradiation.Citation17 As opposed to the previous study, herein, we tested for differences in basal gene expression in these groups using non-irradiated lymphocytes. We used an RNA-Seq analysis on lymphocyte-derived RNA to evaluate the gene expression in BRCA1 and BRCA2 heterozygous mutants and mutation-free individuals. The mutation types identified in carriers used in this study are summarized in . Notably, all the mutations are known to be functional and contribute to the risk of developing breast cancer. When comparing the control group to BRCA1/2 together, 30 genes were significantly differentially expressed (P<0.001; ; Table S2). Furthermore, when we did a separate statistical analysis on BRCA1 and BRCA2, as compared with the control group, we were able to identify 203 genes in BRCA1 and 29 genes in BRCA2 that were differentially expressed (; Table S2).
Table 1 Mutation data of patients enrolled in the study
Figure 1 Basal gene expression profile in BRCA1 and/or BRCA2 mutation carriers versus non-carriers.
Notes: Heat map of hierarchical cluster analysis showing 30 DE genes (P<0.001, fold change ≥2) between BRCA1/2 mutation carriers and control samples (n=4–5/group) (A), 203 DE genes (P<0.001) between BRCA1 mutation carriers and controls (B), and 29 DE genes (P<0.001) between BRCA2 carriers versus non-carriers (C). Red indicates a decrease in gene expression, while blue indicates an increase. Euclidean distance was used as the distance method and complete linkage as the agglomeration method.
Abbreviation: DE, differentially expressed.
Differently expressed genes that may predict BRCA1 status, but not BRCA2, indicate defects in DNA repair
To further understand the underlying biology leading to the classification schemes (), we used the GeneAnalytics applicationCitation26 to enable us to examine the networks formed from the differentially regulated genes in BRCA1 mutation carriers.
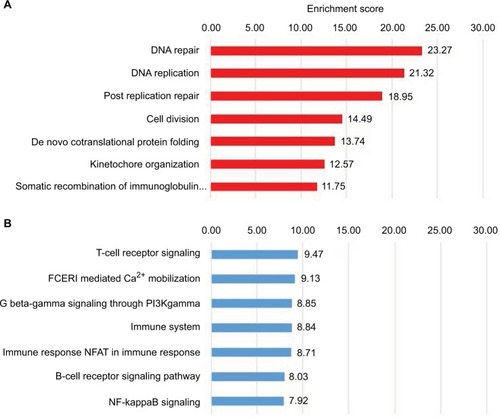
Genes differentially expressed in BRCA1 mutation carriers versus control () are involved in tumorigenic-related pathways (). Most of these genes (122) are downregulated and are involved in critical cellular functions, including cell division, DNA replication, and DNA repair (). Analysis of 81 upregulated genes revealed over-representation of genes involved in the T-cell receptor signaling, immune system, and B-cell receptor signaling pathway (). Surprisingly, although the BRCA2 gene has been implicated in a diverse array of cellular functions, including DNA repair, differential gene expression among BRCA2 mutation carriers and controls revealed only 29 differently expressed genes (), none of which indicated any significant defects in DNA repair or other cellular signaling (data not shown).
Figure 2 Analysis of genes predicting BRCA1 status.
Notes: GeneAnalytics analysis of the 203 genes differentially regulated in cells derived from BRCA1 mutation carriers in the RNA-Seq data. One hundred and twenty-two downregulated (A) and 81 upregulated (B) genes were subjected to enrichment analysis of Gene Ontologies–Biological Processes.
Abbreviations: NFAT, nuclear factors of activated T-cells; FCERI, Fc epsilon receptor.
Validation of selected RNA-Seq transcripts by real-time qPCR
To ensure technical reproducibility and to validate our observation, we conducted a real-time qPCR analysis of those transcripts identified as significantly differently expressed for BRCA1/2 mutation carriers, and their expression levels in samples were relatively high. Lymphocytes were obtained from 12 control mutation-negative individuals and 14 BRCA1/2 mutation carriers (). RNA was prepared and subjected to the SYBR Green assay for the indicated target genes. As presented in , although the expression of TSPAN18, SYK, and DPEPT showed consistency between RNA-Seq and real-time qPCR-based approaches in terms of overall average expression values across multiple samples, these differences were statistically significant only for TSPAN18. No variations in OTUB2 gene expression were observed among the different groups. Comparing the control mutation-negative individuals to BRCA1 mutation carriers only ( and ) revealed significant differences in the expression of TSPAN18 and SYK.
Figure 3 Predictive transcripts for BRCA1/2 mutation carriers.
Notes: Distribution of OTUB2, TSPAN18, SYK, and DPEPT expression levels in lymphocytes of 12 control mutation-negative individuals and 14 BRCA1/2 mutation carriers, as determined by real-time qPCR (A). Distribution of TSPAN18 and SYK expression levels across lymphocytes of mutation-negative individuals (n=12) and BRCA1 mutation carriers (n=5) (B). GAPDH was used for normalization (Figure S1). *P<0.05, calculated using Student’s t-test.
Abbreviations: qPCR, quantitative polymerase chain reaction; SYK, spleen tyrosine kinase.
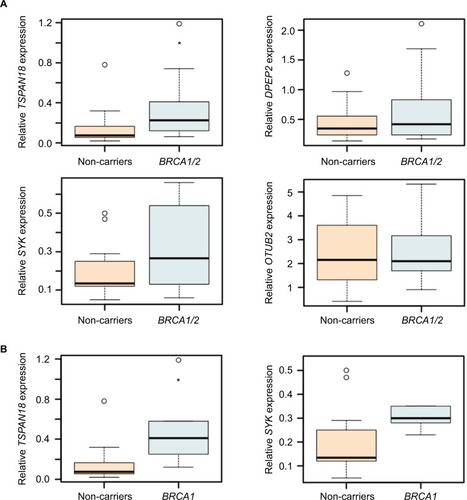
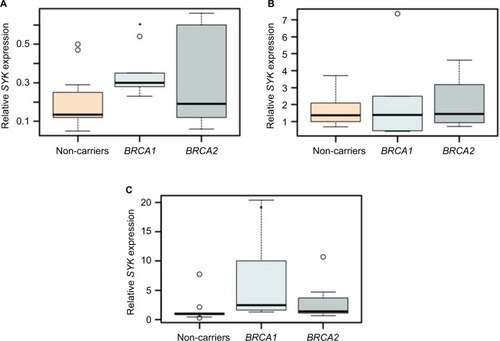
Figure 4 SYK expression levels may distinguish control from BRCA1-mutated lymphocytes.
Notes: The box plots represent the expression of SYK in lymphocytes of BRCA1/2 mutation carriers and controls without irradiation (A) and at 1 hour (B) and 6 days (C) following irradiation, as determined by real-time qPCR. GAPDH was used for normalization. (n=4–12/group). For all experiments, *P<0.05, calculated using Student’s t-test.
Abbreviations: qPCR, quantitative polymerase chain reaction; SYK, spleen tyrosine kinase.
SYK expression levels may distinguish control from BRCA1-mutated lymphocytes
Oxidative stress of B-cell precursor acute lymphoblastic leukemia cells following ionizing radiation leads to the activation of PLK1 protein kinase, which then turns on the SYK protein.Citation30,Citation31 Since accumulating evidence suggests that BRCA1 and BRCA2 regulate ionizing radiation-induced oxidative stress,Citation32 we hypothesized that the mutation status of BRCA1 and BRCA2 genes directly affects the SYK expression profile following irradiation. We tested this hypothesis by examining the levels of SYK mRNA in control mutation-negative individuals and BRCA1 or BRCA2 mutation carriers using irradiated human lymphocytes.
For this purpose, primary lymphocytes obtained from peripheral blood were γ-irradiated with 8 Gy. RNA was extracted from the lymphocytes of BRCA1/2 mutation carriers and controls 1 hour following irradiation (10 and 11 subjects per group, respectively) and from lymphocytes of BRCA1/2 mutation carriers and controls, 6 days following irradiation (17 and 10 subjects per group, respectively), and a real-time qPCR analysis was conducted.
Our results demonstrate that, 6 days post-ionizing irradiation, the expression of SYK was, indeed, significantly upregulated in the lymphocytes of BRCA1 heterozygote carriers, as compared with controls. In contrast, 1 hour following irradiation, there were no differences in the expression levels of the gene among the different groups ( and S2). Notably, even without irradiation, the expression of SYK was significantly upregulated in short-term cultured lymphocytes of BRCA1 mutation carriers, as compared with BRCA2 ( and ).
Expression pattern of BRCA1/2 and SYK in patients with breast cancer
SYK is also expressed in the breast tissue.Citation33 Considerable evidence demonstrates that SYK functions as a tumor suppressor in this tissue. Taking into account our results with lymphocytes, which provide evidence for a link between SYK expression and heterozygous BRCA1/2 mutations, and the known fact that SYK plays a critical role in breast cancer, an effort was made to identify correlations between the expression pattern of BRCA1/2 and SYK genes in breast cancer.
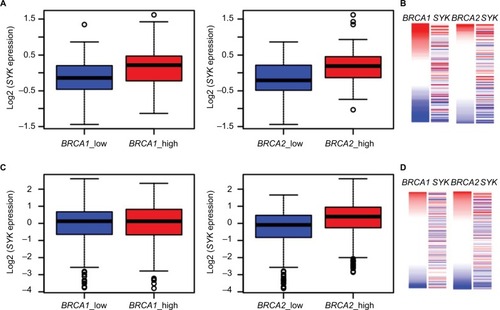
To accomplish this aim, we first analyzed the published GTEx dataset to gain a view on BRCA1, BRCA2, and SYK gene expression in normal breast tissues. As shown in , we found that samples having low BRCA1 or BRCA2 expression exhibit lower SYK expression and vice versa. Next, we analyzed the ICGC datasets of breast tumors. This analysis showed that, in contrast to normal tissues, the expression levels of the BRCA1 and SYK genes were not intercorrelated in breast samples from patients with ductal or lobular breast cancer (). Conversely, the expression levels of BRCA2 in these tissues were positively correlated with those of SYK, which was similar to the expression pattern of these genes in healthy breast tissues (). Taken together, our observations suggest that determining the expression levels of both BRCA1 and SYK in the same tumor may be clinically significant.
Figure 5 Association between BRCA2 and SYK expression in normal and cancerous breast tissues.
Notes: Healthy breast tissues (n=214) from the GTEx database (A, B) and breast cancer samples (n=943) from the ICGC database (C, D) were divided into two groups based on the expression of BRCA1 (A, C) and BRCA2 (B, D) genes, using the median as the cutoff point. The box plots display the expression distribution of SYK across these groups. Higher SYK expression was detected in healthy breast tissues exhibiting an increased level of BRCA1 (A) P=2.391×10−6 and BRCA2 expression (B) P=5.122×10−7. In cancerous breast tissues, this association is preserved for BRCA2 (D) P=6.198×10−15, but not for BRCA1 (C) P=1.000.
Abbreviation: SYK, spleen tyrosine kinase.
Discussion
Our RNA-Seq analysis demonstrates that when a group of BRCA1 and BRCA2 mutation carriers are compared to BRCA mutation-free controls, genes are differentially expressed (). However, larger and more significant differences were found in the comparison between BRCA1 mutation carriers and control groups, and these include many functionally crucial genes, such as SLF1, MSH2, MSH6, CLSPN, TOPBP1, UBE2A, BRCA1, and others involved in DNA repair and replication pathways. Previously, Vuillaume et alCitation34 attempted to identify BRCA1 mutation carriers based on the gene expression pattern in peripheral blood lymphocytes. Unlike our results, they found no significant difference in gene expression patterns between BRCA1 mutation carriers and controls. We assume that we were able to distinguish BRCA1 mutation carriers from non-carriers because we used RNA-Seq technology, which has superior benefits over microarray analyses in transcriptome profiling.Citation35 Furthermore, in our study, short-term lymphocyte cultures were established for 6 days after obtaining the cells, instead of immediate RNA isolation. By doing so, lymphocyte populations became less heterogeneous between samples obtained from different individuals, and the results were affected mainly by BRCA carrier status rather than other factors associated with the donor of the lymphocytes.
It is already known that BRCA1 and BRCA2 have distinct functions in DNA repair following ionizing radiation-induced DNA damage and are key players at different stages of homologous recombination, which provides a mechanism to precisely repair damage.Citation36 Therefore, it is not surprising that, after irradiation, the gene expression profile differs, based on which of the two BRCA genes is mutated.Citation17 However, here, we demonstrate, for the first time, that even without irradiation, BRCA1- and BRCA2-mutated cells differ in their gene expression profiles. These results corroborate our previous findings, in which cells carrying the BRCA1 mutation showed higher FLT values than those carrying BRCA2, suggesting an intrinsic difference between the carriers of the two mutations.Citation18
Overall, greater differences in gene expression, in terms of the number and type of genes, were observed when comparing BRCA1, but not BRCA2, mutation carriers to the control group. These differences may be explained because the BRCA1 gene itself is downregulated in BRCA1 mutation carriers compared with controls, while the BRCA2 gene is not altered in BRCA2 mutation carriers ().
The available methods for identifying BRCA1 and BRCA2 mutation carriers are based on sequencing approaches and do not detect all functional mutations (e.g., carriers with regulatory mutations, which are found in regulatory sequences of the genes). Previously, we suggested a functional diagnostic tool for identifying BRCA mutation carriers by using fresh lymphocytes.Citation17 However, this approach cannot be easily applied since it requires irradiation of fresh lymphocytes by an X-ray machine, which is not available in most medical centers. Here, we demonstrate the potential of using real-time qPCR analysis in setting up a functional screening tool for BRCA1 mutation carriers in untreated accessible blood lymphocytes. Further studies in larger cohorts are needed to identify a set of specific genes to be used by this tool and verify its clinical efficacy. As presented in , the SYK gene is a major candidate to be used by this tool. SYK is primarily expressed in hematopoietic cells. In lymphoid cells, this protein tyrosine kinase regulates several signaling pathways and is of great importance in B-cell receptor signaling, functioning as the main downstream effector protein. It has been shown that ionizing radiation in B-cell precursor acute lymphoblastic leukemia cells leads to oxidative stress and the activation of PLK1 protein kinase, which induces SYK enzymatic activity.Citation30,Citation31 In addition to our findings showing that SYK expression levels can distinguish controls from BRCA1-mutated lymphocytes, the study of gene activity in BRCA carriers may have other clinical implications, especially as SYK functions as a tumor suppressor.Citation33,Citation37 As shown in , SYK expression level was significantly higher in lymphocytes of BRCA1 heterozygote carriers, as compared with controls, regardless of irradiation. Six days following the ionizing irradiation, the differences were even greater and more significant. A similar phenomenon can also be seen in normal breast tissue where samples with low BRCA1 expression exhibit lower SYK expression and vice versa. In contrast, in cancerous breast tissues, the situation is different; no link was found between the expression levels of the two genes, BRCA1 and SYK (). We assume that, in healthy lymphocytes, the oxidative stress due to a mutation in BRCA1 increases, especially after ionizing irradiation, but a corresponding increased level of SYK expression and its suppressor function help maintain normal cell activity. Furthermore, in normal breast tissue, since both SYK and BRCA1 are tumor suppressors, we are not surprised that their expression pattern is similar. However, in cancerous tissues, such as breast tumors, even though BRCA1 is lowly expressed, the lack of corresponding increase in SYK expression supports the disruption of normal cellular activity, thus contributing to tumor formation and progression. In addition to research indicating a clear link between mutations in BRCA1 and the development of breast cancer,Citation2 this hypothesis is supported by various studies demonstrating that SYK is expressed in normal human breast tissue, and in benign breast lesions and low-tumorigenic breast cancer cell lines.Citation37,Citation40 However, in the case of invasive breast carcinoma tissues and cell lines, the expression of SYK mRNA and protein is low compared to normal expression. This reduced SYK expression increases the risk of distant metastasis and also causes a poorer prognosis in a few tumor types, including breast carcinoma, hepatocellular carcinoma, oral squamous cell carcinoma, and melanoma.Citation38
From a clinical perspective, the prognostic usefulness of reduced SYK levels in BRCA1 mutation and/or lowly expressed BRCA1-associated breast cancer should be investigated.
In normal breast tissue, when the expression levels of BRCA2 are low, the expression levels of SYK are correspondingly low and vice versa. Unlike BRCA1, in breast tumors, BRCA2 and SYK behave similarly – when the expression level of one is high, the other one is highly expressed and vice versa (). These results again support the notion that both proteins, BRCA1 and BRCA2, have distinct functions in DNA repair and cell cycle progression.
Finally, poly(ADP-ribose) polymerase inhibitors represent a promising strategy for treating BRCA-associated cancers harboring specific DNA repair defects.Citation39 However, we still need to investigate and understand the proper use of these agents. Some of the challenges that we face include identifying predictive biomarkers of response, which may be used for selecting a subset of patients who would maximally benefit from treatment with this novel agent; determining optimal dose and schedule; and establishing efficacy as a single agent or in combination with other chemotherapeutics. Since SYK expression increases due to oxidative stress and DNA damage, we assume that patients expressing high levels of SYK mRNA will respond better to treatment with poly(ADP-ribose) polymerase inhibitors.
Conclusion
Collectively, our observations demonstrate that SYK may be a good candidate for better diagnosis, treatment, and prevention of BRCA1 mutation-associated breast cancer. However, more detailed studies are needed to gain a clearer picture regarding the role of SYK in the pathogenesis of BRCA mutation-associated tumors.
Acknowledgments
This work was supported by the Israel Science Foundation (grant number 1702/12) and the Israel Cancer Association Fund (grant number ICA 20130179) and the Ariel Center for Applied Cancer Research, Ariel University.
Disclosure
The authors report no conflicts of interest in this work.
References
- Stoppa-LyonnetDThe biological effects and clinical implications of BRCA mutations: where do we go from here?Eur J Hum Genet201624Suppl 1S3S927514841
- ChenSParmigianiGMeta-analysis of BRCA1 and BRCA2 penetranceJ Clin Oncol200725111329133317416853
- ThompsonDEastonDThe genetic epidemiology of breast cancer genesJ Mammary Gland Biol Neoplasia20049322123615557796
- MerschJJacksonMAParkMCancers associated with BRCA1 and BRCA2 mutations other than breast and ovarianCancer2015121226927525224030
- MoynahanMEChiuJWKollerBHJasinMBrca1 controls homology-directed DNA repairMol Cell19994451151810549283
- TuttABertwistleDValentineJMutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequencesEMBO J200120174704471611532935
- StaritaLMMachidaYSankaranSBRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome numberMol Cell Biol200424198457846615367667
- Kote-JaraiZMatthewsLOsorioAAccurate prediction of BRCA1 and BRCA2 heterozygous genotype using expression profiling after induced DNA damageClin Cancer Res200612133896390116818684
- Kote-JaraiZSalmonAMengitsuTIncreased level of chromosomal damage after irradiation of lymphocytes from BRCA1 mutation carriersBr J Cancer200694230831016404418
- ErnestosBNikolaosPKoulisGIncreased chromosomal radiosensitivity in women carrying BRCA1/BRCA2 mutations assessed with the G2 assayInt J Radiat Oncol Biol Phys20107641199120520206018
- BoultonSJCellular functions of the BRCA tumour-suppressor proteinsBiochem Soc Trans200634Pt 563364517052168
- KnudsonAGMutation and cancer: statistical study of retinoblastomaProc Natl Acad Sci USA19716848208235279523
- JengYMCai-NgSLiABrca1 heterozygous mice have shortened life span and are prone to ovarian tumorigenesis with haploinsufficiency upon ionizing irradiationOncogene200726426160616617420720
- Kote-JaraiZWilliamsRDCattiniNGene expression profiling after radiation-induced DNA damage is strongly predictive of BRCA1 mutation carrier statusClin Cancer Res200410395896314871973
- BellacosaAGodwinAKPeriSAltered gene expression in morphologically normal epithelial cells from heterozygous carriers of BRCA1 or BRCA2 mutationsCancer Prev Res (Phila)201031486120051372
- FeilotterHEMichelCUyPBathurstLDaveySBRCA1 haploinsufficiency leads to altered expression of genes involved in cellular proliferation and developmentPLoS One201496e10006824950059
- SalmonAYSalmon-DivonMZahaviTDetermination of molecular markers for BRCA1 and BRCA2 heterozygosity using gene expression profilingCancer Prev Res (Phila)201362829023341570
- ZahaviTYahavGShimshonYUtilizing fluorescent life time imaging microscopy technology for identify carriers of BRCA2 mutationBiochem Biophys Res Commun20164801364127721065
- BarwellJPangonLGeorgiouALymphocyte radiosensitivity in BRCA1 and BRCA2 mutation carriers and implications for breast cancer susceptibilityInt J Cancer200712171631163617582599
- MartinMCutadapt removes adapter sequences from high-throughput sequencing readsEMBnet J North Am20111711012
- KimDPerteaGTrapnellCPimentelHKelleyRSalzbergSLTopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusionsGenome Biol2013144R3623618408
- AndersSPylPTHuberWHTSeq – a Python framework to work with high-throughput sequencing dataBioinformatics201431216616925260700
- SmythGKLimma: linear models for microarray dataGentlemanRCareyVJHuberWIrizarryRADudoitSBioinformatics and Computational Biology Solutions Using R and BioconductorNew York, NYSpringer2005397420
- RobinsonMDOshlackAA scaling normalization method for differential expression analysis of RNA-seq dataGenome Biol2010113R2520196867
- LawCWChenYShiWSmythGKvoom: precision weights unlock linear model analysis tools for RNA-seq read countsGenome Biol2014152R2924485249
- Ben-Ari FuchsSLiederIStelzerGGeneanalytics: an integrative gene set analysis tool for next generation sequencing, RNAseq and microarray dataOmi A J Integr Biol2016203139151
- KeenJCMooreHMThe genotype-tissue expression (GTEx) project: linking clinical data with molecular analysis to advance personalized medicineJ Pers Med201551222925809799
- RamsayAJMartínez-TrillosAJaresPRodríguezDKwarciakAQuesadaVNext-generation sequencing reveals the secrets of the chronic lymphocytic leukemia genomeClin Transl Oncol20131513822911550
- SpeirMLZweigASRosenbloomKRThe UCSC Genome Browser database: 2016 updateNucleic Acids Res201644D1D717D72526590259
- RepanaKPapazisisKFoukasPExpression of Syk in invasive breast cancer: correlation to proliferation and invasivenessAnticancer Res266C4949495417214368
- BarrPMWeiCRogerJSyk inhibition with fostamatinib leads to transitional B lymphocyte depletionClin Immunol2012142323724222284392
- FridlichRAnnamalaiDRoyRBernheimGPowellSNBRCA1 and BRCA2 protect against oxidative DNA damage converted into double-strand breaks during DNA replicationDNA Repair (Amst)201530112025836596
- FlückMZürcherGAndresACZiemieckiAMolecular characterization of the murine syk protein tyrosine kinase cDNA, transcripts and proteinBiochem Biophys Res Commun199521312732817639745
- VuillaumeM-LUhrhammerNVidalVUse of gene expression profiles of peripheral blood lymphocytes to distinguish BRCA1 mutation carriers in high risk breast cancer familiesCancer Inform20097415619352458
- ZhaoSFung-LeungW-PBittnerANgoKLiuXComparison of RNA-Seq and microarray in transcriptome profiling of activated T cellsPLoS One201491e7864424454679
- PrakashRZhangYFengWJasinMHomologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteinsCold Spring Harb Perspect Biol201574a01660025833843
- CoopmanPJDoMTBarthMThe Syk tyrosine kinase suppresses malignant growth of human breast cancer cellsNature2000406679774274710963601
- CoopmanPJMuellerSCThe Syk tyrosine kinase: a new negative regulator in tumor growth and progressionCancer Lett2006241215917316442709
- SonnenblickAde AzambujaEAzimHAPiccartMAn update on PARP inhibitors – moving to the adjuvant settingNat Rev Clin Oncol2015121274125286972
- NaldiALariveRMCzerwinskaUReconstruction and signal propagation analysis of the Syk signaling network in breast cancer cellsPLoS Comput Biol2017133e100543228306714
