Abstract
Biliary tract cancers (BTCs) are rare with poor prognosis. Due to the advent of genomic sequencing, new data have emerged regarding the molecular makeup of this disease. To add to the complexity, various subtypes also harbor a varied genetic composition. The commonly mutated genes associated with this cancer are KRAS, EGFR, IDH, FGFR and BAP1. Various clinical studies are looking at targeting these genetic mutations. Another therapeutic area of note is the potential for the use of immunotherapy in patients with BTC. Although BTC may be a result of chronic inflammation, this does not necessarily translate into increased immunogenicity. This literature review discusses the diverse molecular and immune-related pathways in patients with BTC and their potential therapeutic implications.
Introduction
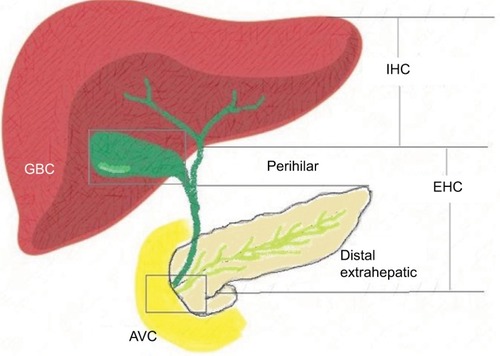
Biliary tract cancers (BTCs) constitute epithelial malignancies of the biliary tree and include the following: gallbladder cancer (GBC), ampulla of Vater cancer (AVC), (the extra-hepatic [EHC] and intra-hepatic [IHC] bile ducts). Historically, the term cholangiocarcinoma (CCA) encompasses EHC and IHC, excluding GBC and AVC.Citation1 The anatomical subtypes of BTC are depicted in .
Figure 1 Anatomical sub-variants of BTC.
Abbreviations: AVC, ampulla of Vater cancer; BTC, biliary tract cancer; EHC, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; IHC, intrahepatic cholangiocarcinoma.
BTC constitutes approximately 3% of all gastrointestinal malignanciesCitation2 and is the most common hepatobiliary cancer after hepatocellular carcinoma.Citation3 Unfortunately, the mortality rate (3.58 per 100,000) is very high. This is comparable to the incidence rate (3.64 per 100,000) in EnglandCitation4 and equates to a 5-year survival of 2% in the metastatic setting.Citation5,Citation6 The global prevalence of BTC has risen by a factor of 22%, and 150,000 patients were diagnosed with BTC in 2015.Citation7 Overall, there is a huge variation in incidence with certain areas depicting high prevalence (eg, Japan and South Korea). This can be accounted for by liver fluke (Opisthorchis viverrini [OV] and Clonorchiasis sinensis [CS]) infestation in zones (north-east Thailand and China), where CCA is more common.Citation8,Citation9 Areas with high prevalence of cholelithiasis correspond to a high prevalence of GBC, such as India and Chile.Citation10–Citation12 Geographical regions where the abovementioned risk factors are uncommon have less cases of BTC.Citation11
Apart from the abovementioned risk factors, primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC), cirrhosis due to other causes, hepatitis C and congenital malformations such as choledochal cysts and multiple biliary papillomatosis are also associated with an increased risk of developing BTC.Citation13–Citation15 Further to these, patients with germline mutations resulting in Lynch syndrome and BRCA1 and BRCA2 (breast cancer gene 1 and 2) genetic aberrations are also predisposed to BTC. There is a lifetime risk of 2% of developing BTC with Lynch syndrome and RR of 4.97% of developing CCA in carriers of BRCA2.Citation16,Citation17
Treatments for BTC are stratified according to the stage of the disease, where surgery remains the mainstay of cure in early stages, although this represents a small minority of patients (10%–40%).Citation18 Recent data from the BILCAP study support the use of adjuvant capecitabine with an improvement in median overall survival (OS) from 36 (observation alone after surgery) to 53 months (HR 0.75, P=0.028 in the sensitivity analysis).Citation19 For locoregional disease, treatments such as radio-embolization, chemoembolization and external beam radiotherapy can be considered, although due to preliminary evidence these techniques have not yet been adopted in standard practice. For the first-line treatment of advanced disease, the Phase III ABC-02 clinical trial confirmed the superiority of the combination of gemcitabine and cisplatin (GC) over single-agent gemcitabine. Reported median OS was 11.7 months vs 8.1 months, respectively (HR 0.64; 95% CI 0.52–0.80; P<0.001),Citation20 and henceforth this has become a global standard of care for late-stage BTC. Although the modest survival benefit gained from this regimen has not yet been surpassed in a randomized Phase III trial, the combination of gemcitabine with an oral fluoropyrimidine S-1, in a Phase III study, reported a median OS of 15.1 months for the gemcitabine and S-1 arm vs 13.4 months in the GC arm (HR 0.95; 90% CI 0.78–1.15; P=0.046 for non-inferiority).Citation21 This regimen may be considered as an alternative treatment for appropriate patients where comorbidities restrict the use of platinum agents. A Phase II clinical trial evaluating the combination of gemcitabine, cisplatin and nab-paclitaxel in the first-line setting in patients with advanced BTC has reported a superior median progression-free survival (PFS) than that associated historically with the standard GC regimen (11.4 months vs 8.0 months) in the preliminary results with a median OS of 19.2 months. This study (NCT02392637) is estimated to be completed in April 2019.Citation22,Citation23
There is no current defined standard-of-care regimen in the second-line setting in advanced BTC. The current ABC-06 randomized Phase III clinical study is analyzing the role of chemotherapy in this setting vs symptomatic management in patients who have received previous chemotherapy. This study is completed, and the results are expected (NCT01926236).Citation24
The advent of genomic sequencing has led to better understanding of pathogenesis of cancers. Studies in BTC have revealed not only germline and somatic mutations but also genetic aberrations exclusive to anatomical subtypes of BTC. These include KRASTP53ErbB2 in EHC; IDH1/2FGFR1/2 and BAP1 in IHC; and TP53, ErbB2, PIK3CAERrbB1/EGFR in GBC.Citation25–Citation28 These findings may potentiate the development of the use of personalized medicine in this disease group.
Further to the use of genomics and personalized medicine aiming at indubitable targets in this cancer, the in-depth analysis of the immune microenvironment may uncover potential targetable pathways, as BTC has been associated with chronic inflammatory pathology.Citation11
The aim of this review was to evaluate the various potential pathways implicated at the molecular level in the development and progression of BTC and also to address the immune microenvironment and its potential involvement. Localized therapy is beyond the remit of this review.
Methodology
A categorical review of electronic databases was performed, which included Embase, Medline, PubMed and clinicaltrails.gov. Full manuscripts as well as conference abstracts available in the English language and published up to July 2018 employing the following keywords were interrogated: biliary tract cancer, intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, gall bladder cancer, genome sequencing, KRAS, BRAF, FGF, IDH, VEGF, EGFR, BAP1, molecular targets and immunotherapy.
Significant potential targetable pathways in patients with BTC
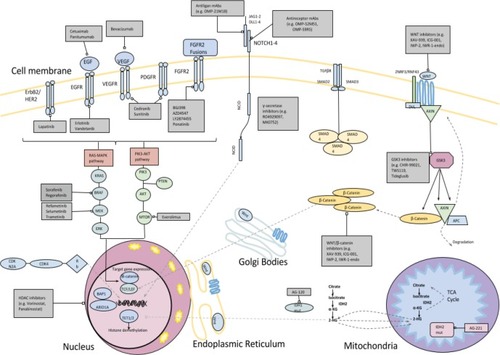
shows the important signaling pathways that may be targetable in patients with BTC.
Figure 2 Important signaling pathways of potential therapeutic significance in patients with BTC.
Abbreviations: AKT/PKB, protein kinase B; ARID1A, AT-rich interactive domain containing protein 1A; BAP1, BRCA1-associated protein 1; BRAF, V-Raf murine sarcoma viral oncogene homolog B; BTC, biliary tract cancer; Dvl, disheveled protein; ErbB1, erythroblastic leukemia viral oncogene 1; ErbB2, erythroblastic leukemia viral oncogene 2; FGFR, fibroblast growth factor receptor; FZD, frizzled family; HDAC, histone deacetylase; IDH, isocitrate dehydrogenase; KRAS, Kirsten rat sarcoma viral oncogene homolog; MAPK/ERK pathway, mitogen-activated protein kinase/extracellular signal-regulated kinase pathway; mTOR, mammalian target of rapamycin; NADP, nicotinamide adenine dinucleotide phosphate; NADPH (reduced), nicotinamide adenine dinucleotide phosphate; PDGFR, plasma-derived growth factor receptor; PI3KCA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit A; PKA, protein kinase; PORC, serine O-palmitoyltransferase porcupine protein; PTEN, phosphatase and tensin homolog; RNF43, ubiquitin E3 ligase ring finger 43; TCF, T cell factor; VEGFR, vascular endothelial growth factor receptor; Wnt, Wingless-related integration; ZNRF3, E3 ubiquitin ligase zinc and ring finger 3.
Ras–Raf–mitogen-activated protein kinase–extracellular signal-regulated kinase (Ras–Raf–MEK–ERK) pathway
The Ras–Raf–MEK–ERK pathway, as shown in , is one of the focal signaling pathways for the development of carcinogenesis of BTC,Citation29,Citation30 and one of its first links, KRAS, is a frequently mutated siteCitation31 in BTC. Studies in different geographical regions have shown a variance in the frequency of KRAS across the anatomical subtypes, demonstrating 67% in EHC, 45% in IHC and 84% in GBC by the Japanese group,Citation32 42% in EHC, 22% in IHC and 11% in GBC by the Cambridge and MD Anderson groupsCitation33 and 22% in EHC and 9% in IHC by the Mayo clinic group.Citation34 Further to differences in frequencies as per the anatomical variant, the KRAS mutation was evaluated in association with clinical outcomes in patients with BTC in different geographical regions by groups in Taiwan, India and USA.Citation32–Citation34
summarizes the association of OS with the mutation status for KRAS in the various anatomical subtypes of BTC. The frequency of KRAS mutations ranges from 0% to 41% in these studies, and its presence is associated with worse OS in all the different analyses.Citation27,Citation35–Citation38
Table 1 Association of survival and frequency of KRAS mutations in patients with BTC
Apart from KRAS, other links in this pathway have been evaluated. In Taiwan, the presence of mutations in EGFR, KRAS and BRAF genes was analyzed in relation to median OS. Although the rest of the mutations failed to reveal a significant association with OS, patients carrying EGFR mutations had a median OS of 6 months as compared to 16 months (P≤0.00001) in patients who did not have these mutations.Citation39 A German study evaluated 69 patients with CCA, reporting the presence of BRAF mutations in 22% of the cases, but OS was not significantly correlated with its presence.Citation40 Another study revealed the presence of BRAF mutations in 7.4% of patients with IHC with the OS for patients with wild-type (wt) tumors being 37.3 months as compared to 13.5 months in the population bearing the mutation.Citation41
Potential therapeutic targets for BTC within the Ras–Raf–MEK–ERK pathway
Different novel treatments for targeting the Ras–Raf– MEK–ERK pathway have been analyzed in various studies, including therapeutic agents such as sorafenib, selumetinib, refametinib, trametinib and pazopanib.
Sorafenib is a multi-kinase inhibitor and angiogenesis blocker, which after showing activity in vitroCitation42 was tested in patients with inoperable or advanced IHC in a pilot study reporting an OS of 5.7 months in these cases.Citation43 Further to this, a Phase II study described an increase in toxicities without any benefit in survival outcomes by adding sorafenib to cisplatin and gemcitabine in patients with advanced BTC.Citation44 Selumetinib is another molecule which targets MEK1/2 link by inhibition and has been tested in vitro and in xenografts, prepared from patients with CCA and GBC. It demonstrated activity through cell cycle arrest and delayed reinitiation of S-phase in the cell cycle.Citation45 A Phase II study of selumetinib in monotherapy in patients with predominantly pretreated advanced BTC reported a median OS of 9.8 months.Citation46 Another MEK inhibitor, refametinib and vemurafenib, which is a v-Raf murine sarcoma viral oncogene homolog B (BRAF) inhibitor, is being assessed in Phase II clinical studies (NCT01524978, NCT02346032).Citation47–Citation49 Trametinib, which is an MEK inhibitor, has been tested alongside a VEGFR TKI, pazopanib, resulting in dose-limiting toxicities in nearly all patients (96% [24/25]) in this study who had CCA, while the OS was only 6.4 months.Citation50 Another negative study was closed to accrual after interim analysis, where patients with pretreated advanced BTC who received trametinib failed to show any responses.Citation51
Relevance of the phosphatidylinositol-4,5-bisphosphate 3-kinase–AKT–mammalian target of rapamycin (PIK3–AKT–mTOR) pathway in patients with BTC
The PI3KA–AKT–mTOR pathway, as shown in , is also known to be a pivotal link in carcinogenesis.Citation52 Preclinical studies on human CCA cell lines, using MEK1/2 and PI3K inhibitors, showed that the CXC chemokine ligand-12/C-X-C motif chemokine receptor 4 (CXCL-12/CXC4) was blocked by these inhibitors. These receptors/ligands act as an activator for this pathway.Citation53 Another key element of this pathway, mTOR, was assessed via an mTOR inhibitor, everolimus, in vitro showing dose-dependent inhibition of cell proliferation by this inhibitor.Citation54 Further to this, a Phase II study investigated everolimus as a first-line treatment option in patients with advanced BTC, reporting a median OS of 9.5 months and was associated with everolimus resistance in patients with KRAS mutations (P=0.03), with a negative correlation seen between basal pAKT and tAKT with everolimus resistance (P=0.007), regardless of the KRAS status.Citation55
Relevance of the FGF pathway in BTC
The FGF pathway as shown in directly and indirectly upregulates the MAPK and PI3KA pathway, and some studies have shown that fusions in this pathway in BTC have a positive correlation with OS, specifically in the CCA cohort. These include FGFR2-BICCI, FGFR2-AHCYL1, FGFR2-MGEA5, FGFR2-TACC3, FGFR2-KIAA1598 and FGFR2-CREB5.Citation56–Citation59 The frequency of FGFR genetic aberrations varied from 8% to 25% with some exclusiveness for the IHC subtype, where most studies reported a range of 13%–14%. These include mutations, insertions, deletions, gene fusions, and translocations, etc.Citation56,Citation58–Citation62
summarizes the variance in FGFR genetic alterations in the anatomical subtypes and its association with survival in patients with BTC. These studies show a wide range of frequency of FGFR genetic aberrations from 0% to 100% with a positive correlation with survival in carriers of the genetic aberration and a more indolent course of disease, resulting in better outcomes.Citation26,Citation27,Citation56,Citation58–Citation62 Again, exclusiveness of the presence of FGFR genetic aberrations in the IHC subtype was noted.
Table 2 Frequency of FGF mutations across various anatomical subtypes of BTC and their association with survival
Preclinical studies used a multi-receptor inhibitor (including FGFR), pazopanib, to target cell lines with mutated FGFR2, which resulted in cell cycle arrestCitation63 after which ponatinib and/or pazopanib in two patients with IHC carrying the FGFR fusion gene who achieved partial responses.Citation57 Another highly selective pan-FGFR inhibitor, BGJ398, was assessed in an umbrella study which included patients with CCA and resulted in stable disease in patients with FGFR2 fusions and mutations, whereas one patient who had a KRAS mutation progressed rapidly on this study drug.Citation64 Recently, a Phase II study that analyzed the efficacy of BGJ398 in patients with advanced CCA harboring FGFR2 fusions or other FGFR molecular alterations that were refractory to standard-of-care chemotherapies was reported. It reported an overall response rate (ORR) of 14.8% and a disease control rate (DCR) of 75.4%; however, there was exclusiveness of response in the population harboring FGFR2 fusions only with a DCR of 83.3% in these patients. On the other hand, the patients (n=4) harboring FGFR3 amplifications did not show any response to BGJ398.Citation65 A Phase II basket study analyzed ARQ 087 which is a pan-FGFR inhibitor in patients with CCA, adrenocortical carcinomas and other solid tumors with FGFR1-3 or KIT/PDGFR genetic aberrations. Of the 80 patients analyzed, nearly one-half (n=7) of the 16 patients who exhibited durable response had a genetic alteration in the FGFR pathway, highlighting the response exclusivity to FGFR pathway alterations. In the IHC subgroup, three of five patients with FGFR2 fusions had response (partial response + stable disease) to treatment. All the five patients with IHC, but without FGFR fusions/amplifications, progressed on treatment. In other solid tumors, patients with FGFR amplifications also had a response.Citation66 The same agent, ARQ 087, was analyzed in another Phase I/II open-label study of patients with IHC who were carriers of FGFR2 genes, showing promising results, with a durable DCR in 67% in these patients, and nine patients were still having ongoing treatment at the time the study was published.Citation67 A Phase I study on an FGFR 1–4 inhibitor, TAS-120, in 45 patients with refractory CCA and FGFR2 gene fusions and FGF genetic aberrations has recently reported overall disease control rate (DCR) of 79% with good overall tolerability of the therapeutic agent. A Phase II has been initiated.Citation68 A Phase II study is evaluating an FGFR 1–3 inhibitor, INCB054828, in patients who are refractory to first line and have unresectable, advanced or metastatic CCA harboring the FGFR2 translocation, and it is estimated to be completed in December 2018.Citation69
Relevance of the isocitrate dehydrogenase (IDH) pathway in patients with BTC
IDH acts as a key enzyme for the citric acid cycle,Citation70 as shown in , and thus far mutations in this enzyme, which may result in oncogenesis,Citation71 have been exclusively linked to the IHC sub-variant of BTC.Citation72 Studies have reported a frequency of 19%–36% in patients with IHC,Citation61 where Borger et alCitation72 reported it as not only a mutation exclusive to IHC subtype but also the most frequently mutated gene in this disease subtype.
A preclinical study analyzed response to 122 Food and drug administration (FDA)-approved drugs to 17 BTC cell lines including two IHC cell lines with mutations in IDH1, employing high-throughput drug screening to produce a unique drug-sensitivity profile for each individual cell line. This study showed high sensitivity of the IDH-mutant IHC cell lines to dasatinib and saracatinib; both of which are inhibitors of Src family of tyrosine kinases, whereas dasat-inib also inhibits (segment of Abelson proto-oncogene and breakpoint cluster region) BCR/ABL family of tyrosine kinases. Interestingly, this response did not correlate with Src activity in the IDH mutant IHC cells, and neither cell lines with IDH mutation in other solid tumors showed such a striking sensitivity. Although this article provides helpful insight into this pathway, it needs to be verified in human studies.Citation73 Another preclinical study employed high-throughput screening to evaluate cell inhibition with 484 small molecular targeting compounds on cell lines and organoids derived from patients with IHC and EHC. This study reported pathways of resistance through micro-RNA 21 (MIR21) to heat shock protein 90 (HSP90) inhibitors. All cell lines, irrespective of mutations, were sensitive to HSP90 inhibitors, but high levels of MIR21 conferred resistance to these molecules. Not only this study was able to identify a potential therapeutic agent but also a biomarker for the efficacy of these agents warrants further evaluation in studies.Citation74
The IDH mutations in patients who had liver fluke infestation (OV and CV) leading to CCA were analyzed and found to be prevalent in cases of IHC which were not associated with OV. The non-OV-associated group had a higher prevalence of IDH mutations: 9.3% as compared to 2.8% in the OV-associated cases.Citation75
summarizes the frequency and correlation of survival in patients with BTC and IDH mutations.
Table 3 Association of survival and frequency of IDH mutations in patients with BTC
After the promising results of the Phase I study of an IDH1 inhibitor, AG-120, in patients with previously treated advanced BTC which showed stable disease in 56% of patientsCitation76 with IHC and EHC carrying the IDH1 mutation, a Phase III randomized clinical trial (RCT), “ClarIDHy”, has been developed, comparing AG-120 with a placebo in patients with CCA who carry the mutation in IDH1.Citation77 It is estimated to be completed in August 2020.
Relevance of the Wingless-related integration (Wnt) pathway in patients with BTC
The Wnt signaling cascade is a complex intracellular signaling pathway, as shown in , and its dysfunctionality can lead to stimulation of genes, such as c-myc, c-jun, VEGF and cyclin D.Citation78,Citation79 A preclinical study reported increased expression of Wnt and its components in human CCA and IHC cell lines, whereby the blockage of the Wnt pathway resulted in increased apoptosis and cell cycle arrest.Citation80 This pathway has also been studied in relation to liver fluke infestation and one of its components, ubiquitin E3 ligase ring finger 43 (RNF43), was found to be mutated in 9.3% of cases of CCA which were associated with OV, alongside a negative trend for survival in these patients (HR 7.775; P<0.001).Citation81 However, despite the abovementioned findings, apart from a preclinical study evaluating an inhibitor of the Wnt pathway, ie, Dickkopf-1 (DKK1) in cells lines from various tumor sites, including BTC, which suppressed cell invasion and growth, especially in cell lines which had a high expression of DKK1 gene.Citation82 Currently in this particular pathway there no current trails in BTC.
Relevance of the deoxyribonucleic acid damage response (DDR) pathway in patients with BTC
Functional BRCA1 and BRCA2 genes are essential for genomic stability and help the nuclei in resisting damage to deoxyribonucleic acid (DNA). These genes are one of the tumor suppressor genes, and defects in these have been associated with apoptosis and malignant cell transformation.Citation83 The breast cancer linkage consortium reports an RR of developing CCA in carriers of BRCA2 mutations to be 4.97%.Citation84 The combined data from six studiesCitation61,Citation85–Citation89 evaluating 142 patients with all four types of BTC stated a frequency of 2.41% for BRCA2 and 1.81% for BRCA1 genetic aberrations by the CCA Cancer Genome Atlas 2018,Citation90 reporting a median survival of approximately 24 months (all stages). A retrospective study evaluated 18 patients with CCA who either carried germline (five cases) or somatic (13 cases) mutations reporting a median OS of 25 months in advanced stages (III and IV) and 40.27 months in early disease (stage I and II). These patients were also evaluated for response to treatments, where a platinum-based chemotherapy agent was compared to a poly-ADP ribose polymerase inhibitor (PARPi). The later showed a better outcome, and patients who were treated with PARPi were reported to have an OS going up to 64.76 months.Citation16 A Phase I/II study, which is estimated to start in August 2018, is going to analyze the dose and side effects of liposomal irinotecan alongside a PARPi (rucaparib) in various cancers including BTC and is expected to be completed in 2021.Citation91 Another Phase II trial is analyzing the ORR with a PARPi (niraparib) in patients with BAP1 and other DNA damage response pathway-deficient cancers including CCA and is expected to be completed in 2021.Citation92
Clinical relevance of key targets identified in patients with BTC
After discussing individual potential implicated pathways, this review now evaluates some further mechanistic targets, including angiogenesis and their role in BTC.
Angiogenesis in tumors of patients with BTC
Although factors such as vascular endothelial growth factor (VEGF), FGF and EGF, which promote angiogenesis, have been identified in patients with BTC,Citation93,Citation94 BTCs are considered as hypovascularized tumors.Citation95 This fact is further supported in patients with IHC, by the presence of low microvessel density (MVD) in tumors, which denotes areas of neovascularization.Citation96 Another study reported a mean MVD of 30.5 vessels per ×200 optical field in a sample of 62 patients with GBC. Within this cohort, patients who had higher MVD had a worse median OS (2-year survival of 25%) as compared to patients with low MVD (2-year survival of 43%).Citation97 The same group evaluated MVD in another sample of 60 patients with GBC and revealed an MVD of 20 per ×200 optical field.Citation98 Another study on 118 patients with GBC confirmed a correlation of tumor stage and liver metastasis with MVD and classified MVD as an independent prognostic factor.Citation99
Role of VEGF in patients with BTC
VEGF, which has been reported as a pivotal angiogenesis factor, was found to be highly expressed at 75.6% in a study of 33 surgically resected cases of CCA.Citation100 A larger analysis of tumors from 236 patients for molecular profiling revealed the presence of the VEGF gene in 53.8% (n=57/106) of IHC and 59.2% (n=77/130) of EHC cases.Citation101 A study that assessed 60 cases of patients with GBC by immunohistochemistry revealed a high VEGF expression in 27 cases and a low VEGF expression in 33 cases,Citation98 where no significant association between VEGF expression and survival was found.Citation98
Among the various VEGF inhibitors, bevacizumab and cediranib have been assessed in patients with BTC. A Phase II study that evaluated the addition of bevacizumab to gem-citabine and oxaliplatin reported a median OS of 14.2 months in patients with advanced IHC (n=22) and 8.5 months in patients with advanced GBC (n=10), whereas median OS was not given for patients with EHC (n=3).Citation102 A Phase III study, ABC-03, evaluated the addition of cediranib (vs placebo) to GC chemotherapy in patients with advanced BTC. Although this study failed to reach its primary end point (improvement in PFS) or show a significant difference in OS, the response rate improved by 25% in the cediranib arm (P=0.0036).Citation103 A Phase II study comparing ramucirumab (VEGFR antibody) vs merestinib (MET inhibitor) vs placebo, in combination with GC in patients with advanced or metastatic BTC as a first-line treatment, has completed accrual and is awaiting results.Citation104
Role of EGFR in patients with BTC
The EGFR/HER2 receptor acts through targeting all the leading pathways including the Ras-Raf-MAP-ERK pathway, the PI3k-AKT-mTOR pathway, the phospholipase C, Ca2+/calmodulin-dependent kinase (CaMK/PKC), Janus-associated kinase (JAK) pathway and the STAT protein pathwayCitation105 which makes it a highly susceptible anti-tumorigenesis target. It was found to be present in 100% of IHC samples, 52.6% of EHC samples and 38.5% of GBC samples from treatment-naïve patients.Citation106
summaries the various EGFR antibodies such as erlotinib, cetuximab and panitumumab which have been analyzed in various combinations with gemcitabine in selective (KRAS wt) and nonselective patient groups with advanced (inoperable or metastatic) BTC in Phase II and III clinical studies. However, the largest Phase III study that analyzed samples from 268 patients who were diagnosed with all the four types of advanced BTC failed to show any difference in OS by the addition of erlotinib to gemcitabine and oxaliplatin.Citation107
Table 4 Potential treatments for targeting EGFR in patients with BTC
So far, with little or no clinical benefit, EGFR inhibitors are perhaps not the right therapeutic choice for patients with BTC until a predictive biomarker for EGFR inhibitors is developed, and trialing these targeted treatments in BTC is not advised. Although work in colorectal cancer examining the role of KRAS status in therapeutic decision-making has been validated,Citation108 the abovementioned evidence fails to confirm the role of KRAS status or EGFR expression in the therapeutic management of BTC.
Role of BAP1 in patients with BTC
Germline mutations in the BAP1 gene have been associated with cancers of the uvea, kidney, skin and mesothelium,Citation109 and it has been identified as a tumor suppressor gene.Citation110 A study of 64 patients has reported mutations in this gene in 20% of patients with IHC and 6% of patients with GBC.Citation61 A larger study reported the presence of BAP1 mutations in 26% of cases from a cohort of 211 patients with IHC.Citation111
This gene was also analyzed in 209 patients in association with OV-associated cases of CCA, and a 10.5% frequency of BAP1 genes in non-OV-associated cases was reported in comparison to 2.8% in OV-associated cases.Citation75
The presence of aberrations in the BAP1 gene was associated with short time to recurrence in postsurgical patients with CCA in a study that included 75 patients. It was also associated with shorter OS in patients with EHC (8.9 months vs 19.9 months, P=0.007), when compared to patients with EHC, who did not have the BAP1 gene mutation.Citation26 Another study that reviewed 22 patients with CCA who bore mutations in the BAP1 gene reported a mean time to progression of 3.8 months in these cases, and a patient who had undergone curative resection presented with recurrence 8 weeks after surgery, illustrating the aggressive nature of this disease.Citation112
Histone deacetylase inhibitors (HDACI) have been used to target BAP1 mutations and have shown preclinical activity with 30% inhibitory effect in the CCA cell linesCitation113 and in combination with cisplatin led to cytotoxicity, inhibition of growth and increased cell apoptosis in another preclinical study using CCA cell lines.Citation114
Differentiation of the proliferation and the inflammation class
Further to the abovementioned details, a multinational study has presented an interesting concept of defining two unique classes of BTC to help understand the tumor biology of BTC. This study assessed 149 samples (including all stages) of IHC from Milan, Barcelona and New York. The study analyzed genomic mutations using high-density single-nucleotide polymorphism array and gene expression profiles. It classified the samples into two broad categories: the “inflammation class” and the “proliferation class”. The first class, ie, “the inflammation class”, constituted 38% of the total samples and was found to have overexpression of cytokines and activation of STAT3. However, “the proliferation class”, accounting for 62% of the total samples, harbored activated oncogenic pathways with mutations expressed in MAPK, Ras pathways and KRAS, BRAF genes. A better median OS was associated with the inflammation class, ie, 47.2 months compared to 24.3 months in the proliferation class (P=0.048).Citation115 This innovative concept needs further exploration to assess whether this work can be used translationally.
summarizes the current trials in targeted treatments in BTC registered on clinicaltrials.gov (last accessed on August 25, 2018).
Table 5 Current trials involving molecular treatments in BTC registered on clinicaltrials.gov
Potential for the use of immunotherapy in patients with BTC
BTCs have been associated with chronic inflammatory conditions and viral infections; therefore, there may be a role for immunomodulatory agents in this disease group. Understanding the underlying immune environment may yield a successful strategy to target this poor prognostic disease group.
The concept of immunosurveillance and immune editing has been supported by Dunn et al.Citation116 They introduced the concept of elimination whereby the host immunity consisting of natural killer T cells (NKTC), natural killer cells (NKC), interferon γ (IFN γ; initiates immune reactions) led to cytotoxic death of cancer cells. This was followed by equilibrium whereby the immune environment and the cancer cells lived in harmony. Eventually, leading to escape, whereby the cancer cells that survived the equilibrium phase form tumorigenic growths. Research was conducted on the development of carcinoma in situ leading to fulminant tumors in a large sample size of 375 patients with BTC. This study reported an increase in the number of macrophages as the precancerous lesion developed into carcinoma, whereas B-lymphocytes, CD8+ T cells, CD4+ T cells, regulatory T cells, mast cells and NKC steadily declined as the cancer formed.Citation117 The same cells that reduced in number as the cancer formed were associated with better prognosis, highlighting a robust immunosurveil-lance mechanism led by these cells.
Inflammatory markers using neutrophil-to-lymphocyte ratio (NLR) and derived neutrophil-to-lymphocyte ratio (dNLR) have been evaluated as prognostic biomarkers in BTC. Higher values of NLR ≥3.0 in patients with BTC were associated with a poor OS of 12 months as compared to patients who had lower values of NLR <3.0 with an OS of 21.6 months (adjusted HR =1.26, P=0.01).Citation118 Similarly, high dNLR was associated with poor prognosis.Citation119
Although the role of cancer vaccines has been evaluated in Phase I clinical studies in BTC, the modest benefit has not sparked enough interest to lead to further trials.Citation120–Citation122 Another important component of the immune environment is the cytokines that were analyzed in a cohort of 54 patients with inoperable or advanced BTC who had stable disease after first-line chemotherapy followed by chemoradiation. This was followed by IL2 and retinoic acid (RA) infusion. Although only a small number completed treatment (seven patients), the median OS was not reached when the trial was reported (at 27.5 months).Citation123 Apart from vaccines, adoptive cell therapy (ACT) is also being evaluated in BTC, albeit in small case series or case reports. This involves using the patient’s own cells, which are adapted after extrapolating them from the host. The reformed cells are then again infused into the host after the depletion of lymph in patients. A single case study of a locally advanced patient with IHC treated with ACT was reported as being disease-free 3.5 years after surgery at the time of the case report.Citation124 In another case report, a patient with metastatic CCA who received ACT followed by IL2 was reported to have stable disease for 13 months.Citation125 A case series that reported on the combination of the use of vaccine and ACT in the adjuvant setting reported a better OS of 31.9 months in the patients who received this adjuvant treatment strategy as compared to a median OS of 17.4 months in patients (P=0.022) who underwent surgery alone.Citation126
Immune check point inhibitors are currently being used across various poor prognostic tumor groups with good results; however, there is some association of programmed cell death protein ligand-1 (PD-L1), expression and effectiveness of these treatments.Citation127,Citation128 Expression of PD-L1 in BTC shows a wide range from 29% to 100%,Citation129,Citation130 and the full analysis of the KEYNOTE-028 study is still awaited. This Phase Ib trial is evaluating the effects of treatment with a monoclonal antibody against human immune cell check point programmed death 1 (PD-1), pembrolizumab in patients with previously pretreated advanced BTC who have PD-L1 expression.Citation131 BTCs are infrequently associated with Lynch syndrome, a genetic disorder thatpredisposes to mic-rosatellite instability (MSI) and mismatch repair deficiency (MMR).Citation17 With the food and drug association (FDA) approval of pembrolizumabCitation132 for MSI- and MMR-deficient tumors, in patients who have BTC associated with Lynch syndrome, there may be an option for treating them with immune check point inhibitors, where available..Citation133
summarizes the current immune-mediated trials in BTC registered on clinicaltrials.gov (last accessed on August 25, 2018).
Table 6 Current trials involving the immune system in BTC registered on clinicaltrials.gov
Discussion
BTCs are rare cancers which are poorly understood and have few treatment options, low response rates and bad prognosis. The rarity and difficulty in getting good diagnostic samples pose hurdles to effective development of translational research. The various complex issues that govern this disease group include the following: identification of a driver mutation, heterogeneity that exists within the tumor, difficulty in getting repeat samples on recurrence and difference in the behavior of cell lines from real-life patients.
Available information on genomic and somatic mutations in patients with BTC has expanded, but this comes with its own limitations. Various techniques used for molecular profiling yield different results, and there is a lack of global stan-dardization. Much work is needed to reduce the variance in the results obtained across the use of different methodologies.
Intra-tumoral heterogeneity has been an area of debate from as early as 1976, further supplemented by work done in clonal evolution in 1990 by Fearon and Vogelstein.Citation134,Citation135 Further research has been completed recently in this area, where central and peripheral samples from the same tumori-genic mass, from four patients with surgically resected IHC, were evaluated for private and common mutations. Therefore, private mutations were defined as exclusive mutations found in only one region of one tumor for one patient. In contrast, common mutations were, as the name suggests, the mutations which were found in most of the patients. Overall, 75% of patients exhibited private mutations in the center as well as the periphery, whereas one patient had a high percentage (58%) of private mutations in the periphery. The average mean percentage of private mutations was 12% across all samples in all patients.Citation136 Although exciting, this heterogeneity limits the use of personalized medicine in everyday clinical practice. In lung adenocarcinomas, these private mutations or “neoantigens” have been shown to increase sensitivity to immune check point inhibitors, such as anti-cytotoxic T-lymphocyte-associated antigen 4 (anti-CTLA4) and PD-1 inhibitors with resulting improved outcomes.Citation137
Another important facet of this cancer where limited research has been performed is the “tumor microenvironment” (TME). The dense collagenous stroma constitutes TME and contains important components such as cancer-associated fibroblasts (CAFs), α-smooth muscle actin (α−SMA+), which probably originate from activated hepatic stellate cells or hepatic portal fibroblasts.Citation138 In a mouse study, it was reported that there was intrahepatic accumulation of extracellular matrix components, type III collagen and activated fibroblasts, which then resulted in CCA genesis and progression, in mice that were treated with carbon tetrachloride (CCL4).Citation139 Indeed, studies in pancreatic ductal adenocarcinoma have previously shown a role of the stroma in tumor growth.Citation140
Another point to note is that most of the molecular profiling studies include the analysis of surgical samples and therefore represent early-stage disease, which may not be a true representation of the patients who are seen in clinics.
The role of immunomodulating treatments in BTC is still an area of exploration, and none of the current immune investigational drugs have been approved in this disease group. The expression of PD-L1 is a predictive biomarker in other tumor sites, such as non-small-cell lung cancer (NSCLC), for the efficacy of these immunotherapies. Apart from this biomarker, another recently emerging predictor of response is the human microbiome, where certain bacterial species are associated with clinical efficacy of immunotherapies.Citation141
The use of monotherapy vs combination treatment in advanced BTC is also an issue. Historically, clinical studies have used both novel agents as monotherapy as well as in combination with cytotoxic treatments. However, further research in combining treatments that potentiate cytotoxic effects and are at the same time tolerable is necessary. There is also a niche for developing prognostic and predictive bio-markers in BTC to better inform treatment choice.
Currently, there are still gaps in the understanding of the whole process that governs carcinogenesis and resistance to treatments in BTC, and future studies may be able to address this dilemma. In time, prospective studies may further identify novel therapies targeting this disease and lead to improvements in survival outcome.
Disclosure
Noor-ul-Ain Tariq received honoraria for lecturers, participation in writing guidelines and travel reimbursements in 2014 from Boehringer Ingelheim and received funding from the Timpson fellowship. Boehringer Ingelheim or Timpson have no influence over the contents of this review. Mairéad G McNamara was advisory board member of Ipsen, SHIRE, Celgene and Sirtex, received research support from NuCana BioMed Ltd. and SHIRE, received honoraria for participation in Speaker’s Bureau from Pfizer and Ipsen and received travel expenses from Bayer. Juan W Valle received travel grants from Celgene, Ipsen, Novartis, NuCana for more than 5 years, received honoraria for participation in Speakers’ Bureau for Abbott, Celgene, Ipsen, Novartis, Pfizer and Sir-tex and provided consulting or advisory role for for Abbott, Agios, AstraZeneca, Baxalta, Bioven, Celgene, Delcath, Genoscience Pharma, Incyte, Ipsen, Keocyt, Lilly, Merck, MidaTech, Mundipharma, Novartis, NuCana, PCI Biotech, Pfizer, Pieris Pharmaceuticals and QED Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
- de GroenPCGoresGJLarussoNFGundersonLLNagorneyDMBiliary tract cancersN Engl J Med Overseas Ed19993411813681378
- CharbelHAl-KawasFHCholangiocarcinoma: epidemiology, risk factors, pathogenesis, and diagnosisCurr Gastroenterol Rep201113218218721271364
- HennedigeTPNeoWTVenkateshSKImaging of malignancies of the biliary tract-an updateCancer Imaging201414114707330
- England Public HealthNational Cancer Intelligence Network: Rare and Less Common Cancers, Incidence and Mortality in EnglandLondon2015
- Cancer.govSurveillance epidemiology and end results program2015 Seer Data Available from: https://seer.cancer.gov/statfacts/html/livibd.htmlAccessed February 20, 2019
- Seer Data%U Available from: https://seer.cancer.gov/csr/1975_2014/results_merged/sect_1901_overview.pdf#search=biliary+tract+cancer+incidence%~Seer.Cancer.gov
- VosTAllenCAroraMGlobal, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015Lancet2016388100531545160227733282
- ParkinDMSrivatanakulPKhlatMLiver cancer in Thailand I. A case-control study of cholangiocarcinomaInt J Cancer19914833233281645697
- KhanSAToledanoMBTaylor-RobinsonSDEpidemiology, risk factors, and pathogenesis of cholangiocarcinomaHPB2008102778218773060
- RandiGMalvezziMLeviFEpidemiology of biliary tract cancers: an updateAnn Oncol200920114615918667395
- KhanZRNeugutAIAhsanHChabotJARisk factors for biliary tract cancersAm J Gastroenterol19999411491529934746
- KirsteinMMVogelAEpidemiology and risk factors of cholangio-carcinomaVisc Med201632639540028229073
- KhanSAToledanoMBTaylor-RobinsonSDEpidemiology, risk factors, and pathogenesis of cholangiocarcinomaHPB2008102778218773060
- LeeSSKimMHLeeSKClinicopathologic review of 58 patients with biliary papillomatosisCancer2004100478379314770435
- ChapmanRWRisk factors for biliary tract carcinogenesisAnn Oncol199910Suppl 4S308S311
- GolanTRaitses-GurevichMKelleyRKOverall survival and clinical characteristics of BRCA-associated cholangiocarcinoma: a multicenter retrospective studyOncologist201722780481028487467
- ShigeyasuKTanakayaKNagasakaTEarly detection of meta-chronous bile duct cancer in Lynch syndrome: report of a caseSurg Today201444101975198123896635
- CidonEUResectable cholangiocarcinoma: reviewing the role of adjuvant strategiesClin Med Insights Oncol201610CMO.S32821
- PrimroseJNFoxRPalmerDHAdjuvant capecitabine for biliary tract cancer: The BILCAP randomized study.American Society of Clinical Oncology35201740064183
- ValleJWasanHPalmerDHCisplatin plus gemcitabine versus gemcitabine for biliary tract cancerN Engl J Med2010362141273128120375404
- MorizaneCOkusakaTMizusawaJRandomized phase III study of gemcitabine plus S-1 combination therapy versus gemcitabine plus cisplatin combination therapy in advanced biliary tract cancer: a Japan Clinical Oncology Group study (JCOG1113, FUGA-BT)J Clin Oncol2018364_suppl205
- ShroffRTBoradMJXiaoLA phase II trial of gemcitabine (G), cisplatin (C), and nab-paclitaxel (N) in advanced biliary tract cancers (aBTCs)J Clin Oncol20173515_suppl4018
- ShroffRTXiaoLKasebAOA phase II trial of gemcitabine (G), cispla-tin (C), and nab-paclitaxel (N) in advanced biliary tract cancers (aBTCs): Updated survival analysisJ Clin Oncol2018364_suppl35029215955
- The Christie NHS Foundation Trust, UK Cancer Research [homepage on the Internet]Active symptom control alone or with mFOLFOX chemotherapy for locally advanced/metastatic biliary tract cancers2014 Available from: https://ClinicalTrials.gov/show/NCT01926236Accessed January 3, 2019
- JainAJavleMMolecular profiling of biliary tract cancer: a target rich disease molecular profiling of biliary tract cancer: a target rich diseaseJ Gastrointest Cancer201675797803
- ChuriCRShroffRWangYMutation profiling in cholan-giocarcinoma: prognostic and therapeutic implicationsPLoS One2014912e11538311620325536104
- JavleMBekaii-SaabTJainABiliary cancer: utility of next-generation sequencing for clinical managementCancer2016122243838384727622582
- NakamuraHAraiYTotokiYGenomic spectra of biliary tract cancerNat Genet20154791003101026258846
- SohalDPShrotriyaSAbazeedMCruiseMKhoranaAMolecular characteristics of biliary tract cancerCrit Rev Oncol Hematol201610711111827823638
- HezelAFDeshpandeVZhuAXGenetics of biliary tract cancers and emerging targeted therapiesJ Clin Oncol201028213531354020547994
- BridgewaterJAGoodmanKAKalyanAMulcahyMFBiliary tract cancer: epidemiology, radiotherapy and molecular profilingAm Soc Clin Oncol Educ Book201635e19420327249723
- OhashiKTstsumiMNakajimaYNakanoHKonishiYKi-ras point mutations and proliferation activity in biliary tract carcinomasBr J Cancer19967469309358826860
- RossJSWangKCatenacciDVTComprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and genomic alterationsJ of Clin Oncol2015333 Suppl231
- VossJSHoltegaardLMKerrSEMolecular profiling of cholan-giocarcinoma shows potential for targeted therapy treatment decisionsHum Pathol20134471216122223391413
- MalatsNPortaMPiñolJLCorominasJMRifàJRealFXKi-ras mutations as a prognostic factor in extrahepatic bile system cancer. PANK-ras I project investigatorsJ Clin Oncol1995137167916867602358
- ChenT-CJanY-YYehT-SK-ras mutation is strongly associated with perineural invasion and represents an independent prognostic factor of intrahepatic cholangiocarcinoma after hepatectomyAnn Surg Oncol201219S3675681
- KazmiHRChandraANigamJNoushifMParmarDGuptaVPrognostic significance of K- ras codon 12 mutation in patients with resected gallbladder cancerDig Surg201330323323923838952
- MafficiniAAmatoECataldoIAmpulla of vater carcinoma: sequencing analysis identifies TP53 status as a novel independent prognostic factor and potentially actionable ERBB, PI3K, and WNT pathways gene mutationsAnn Surg2018267114915627611608
- ChangYTChangMCHuangKWTungCCHsuCWongJMClinicopathological and prognostic significances of EGFR, KRAS and BRAF mutations in biliary tract carcinomas in TaiwanJ Gastroenterol Hepatol20142951119112524372748
- TannapfelASommererFBenickeMMutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinomaGut200352570671212692057
- RobertsonSHyderODodsonRThe frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcomeHum Pathol201344122768277324139215
- HuetherAHöpfnerMBaradariVSchuppanDScherüblHSorafenib alone or as combination therapy for growth control of cholangiocar-cinomaBiochem Pharmacol20077391308131717266941
- LuoXJiaWHuangZEffectiveness and safety of sorafenib in the treatment of unresectable and advanced intrahepatic cholangio-carcinoma: a pilot studyOncotarget20178101724627783997
- LeeJKCapanuMO’ReillyEMA phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adeno-carcinomasBr J Cancer2013109491591923900219
- XuJKnoxJJIbrahimovESequence dependence of MEK inhibitor AZD6244 combined with gemcitabine for the treatment of biliary cancerClin Cancer Res201319111812723091117
- Bekaii-SaabTPhelpsMALiXMulti-institutional phase II study of selumetinib in patients with metastatic biliary cancersJ Clin Oncol201129172357236321519026
- Roche H-L [homepage on the Internet]A Study of Zelboraf (Vemu-rafenib) in Patients with BRAF V600 mutation-positive cancers2012 Available from: https://ClinicalTrials.gov/show/NCT01524978Accessed January 3, 2019
- University Health Network, Toronto [homepage on the Internet]A study of different dosing schedules of selumetinib with Cisplatin/Gemcitabine (CIS/GEM) versus CIS/GEM alone in biliary cancer2014 Available from: https://ClinicalTrials.gov/show/NCT02151084Accessed January 3, 2019
- Samsung Medical Center [homepage on the Internet]Phase II study of Refametinib, a MEK inhibitor, as second-line treatment in advanced biliary tract adenocarcinoma2015 Available from: https://ClinicalTri-als.gov/show/NCT02346032Accessed January 3, 2019
- ShroffRTYarchoanMO’ConnorAThe oral VEGF receptor tyrosine kinase inhibitor pazopanib in combination with the MEK inhibitor trametinib in advanced cholangiocarcinomaBr J Cancer2017116111402140728441383
- KimRDMcDonoughSLEl-KhoueiryABSWOG S1310: Randomized phase II trial of single agent MEK inhibitor trametinib vs. 5-fluorouracil or capecitabine in refractory advanced biliary cancerJ Clinl Oncol20173515_suppl4016
- CullyMYouHLevineAJMakTWBeyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesisNat Rev Cancer20066318419216453012
- LeelawatKLeelawatSNarongSHongengSRoles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasionWorld J Gastroenterol20071310156117461449
- MoolthiyaPTohtongRKeeratichamroenSLeelawatKRole of mTOR inhibitor in cholangiocarcinoma cell progressionOncol Lett20147385486024527093
- YeungYHChionhFJMPriceTJPhase II study of everolimus monotherapy as first-line treatment in advanced biliary tract cancer: RADicholJ Clinl Oncol20143215_suppl4101
- AraiYTotokiYHosodaFFibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholan-giocarcinomaHepatology20145941427143424122810
- BoradMJChampionMDEganJBIntegrated genomic charac-terization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinomaPLoS Genet2014102e100413524550739
- WuYMSuFKalyana-SundaramSIdentification of targetable FGFR gene fusions in diverse cancersCancer Discov20133663664723558953
- ZhengZLiebersMZhelyazkovaBAnchored multiplex PCR for targeted next-generation sequencingNat Med201420121479148425384085
- GrahamRPBarr FritcherEGPestovaEFibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinomaHum Pathol20144581630163824837095
- JiaoYPawlikTMAndersRAExome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intra-hepatic cholangiocarcinomasNat Genet201345121470147324185509
- RossJSWangKGayLNew routes to targeted therapy of intra-hepatic cholangiocarcinomas revealed by next-generation sequencingThe Oncologist201419323524224563076
- KimSTJangHLLeeSJPazopanib, a novel multitargeted kinase inhibitor, shows potent in vitro antitumor activity in gastric cancer cell lines with FGFR2 amplificationMol Cancer Ther201413112527253625249557
- NogovaLSequistLVPerez GarciaJMEvaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: Results of a global phase I, dose-escalation and dose-expansion studyJ Clin Oncol201735215716527870574
- JavleMLoweryMShroffRTPhase II study of BGJ398 in patients With FGFR-altered advanced cholangiocarcinomaJ Clin Oncol201836327628229182496
- PapadopoulosKPEl-RayesBFTolcherAWA Phase 1 study of ARQ 087, an oral pan-FGFR inhibitor in patients with advanced solid tumoursBr J Cancer2017117111592159928972963
- MazzaferroVEl-RayesBFCotsoglouCARQ 087, an oral pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced intrahepatic cholangiocarcinoma (iCCA) with FGFR2 genetic aberrationsAm Soc Clin Oncol20173515_suppl4017
- Meric-BernstamFArkenauHTranBO-001Efficacy of TAS-120, an irreversible fibroblast growth factor receptor (FGFR) inhibitor, in cholangiocarcinoma patients with FGFR pathway alterations who were previously treated with chemotherapy and other FGFR inhibitorsAnn Oncol201829suppl_5mdy149
- BoradMJDavisSLLoweryMALihouCFAbou-AlfaGKPhaseA-AGKPhase 2, open-label, multicenter study of the efficacy and safety of INCB054828 in patients (pts) with advanced, metastatic, or surgically unresectable cholangiocarcinoma (CCA) with inadequate response to prior therapyJ Clin Oncol20173515_supplTPS4145
- SulkowskiPLCorsoCDRobinsonND2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivitySci Transl Med20179375eaal246328148839
- SahaSKParachoniakCAGhantaKSMutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancerNature2014513751611011425043045
- BorgerDRTanabeKKFanKCFrequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotypingOncologist2012171727922180306
- SahaSKGordanJDKleinstiverBPIsocitrate dehydrogenase mutations confer dasatinib hypersensitivity and SRC dependence in intrahepatic cholangiocarcinomaCancer Discov20166772773927231123
- LampisACarotenutoPVlachogiannisGMIR21 drives resistance to heat shock protein 90 inhibition in cholangiocarcinomaGastroenterology20181544e1065.10661079
- Chan-OnWNairismägiMLOngCKExome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancersNat Genet201345121474147824185513
- LoweryMAAbou-AlfaGKBurrisHAPhase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholan-giocarcinoma dose escalation and expansion cohortsJ Clin Oncol20173515_suppl4015
- LoweryMAAbou-AlfaGKValleJWClarIDHy: A phase 3, multicenter, randomized, double-blind study of AG-120 vs placebo in patients with an advanced cholangiocarcinoma with an IDH1 mutationJ Clin Oncol20173515_supplTPS4142
- HaoH-XJiangXCongFControl of Wnt receptor turnover by R-spondin-ZNRF3/RNF43 signaling Module and its dysregulation in cancerCancers20168654
- LiuJPanSHsiehMHTargeting Wnt-driven cancer through the inhibition of Porcupine by LGK974Proc Natl Acad Sci U S A201311050202242022924277854
- ZhangKSZhouQWangYFLiangLJInhibition of Wnt signaling induces cell apoptosis and suppresses cell proliferation in cholangio-carcinoma cellsOncol Rep20133031430143823799613
- OngCKSubimerbCPairojkulCExome sequencing of liver fluke-associated cholangiocarcinomaNat Genet201244669069322561520
- SatoNYamabukiTTakanoAWnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapyCancer Res201070135326533620551066
- LiuYWestSCDistinct functions of BRCA1 and BRCA2 in double-strand break repairBreast Cancer Res200241911879553
- Breast Cancer Linkage ConsortiumCancer risks in BRCA2 mutation carriersJ Natl Cancer Inst199991151310131610433620
- OngCKSubimerbCPairojkulCExome sequencing of liver fluke-associated cholangiocarcinomaNat Genet201244669069322561520
- National Cancer Institute; The Cancer Genome Atlas [webpage on the Internet]2018 Contains harmonized cancer genomic data sets2018 Available from: https://tcga-data.nci.nih.gov/docs/publications/tcga/Accessed August 25, 2018
- National Cancer Institute PanCanAltas Publications [webpage on the Internet]2018 initiative aims to answer big, overarching questions about cancer by examining the full set of tumors characterized in the robust TCGA dataset2018 Available from: https://gdc.cancer.gov/about-data/publications/pancanatlasAccessed January 3, 2019
- Chan-OnWNairismägiM-LOngCKExome sequencing identifies distinct mutational patterns in liver fluke–related and non-infection-related bile duct cancersNature Genetics201345121474147824185513
- LiMZhangZLiXWhole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathwayNat Genet201446887287624997986
- Oncology CfM; cBioPortal for Cancer genomic [webpage on the Internet] Combined study contains data from 6 studies (Cholan-giocarcinoma (National University of Singapore, Nat Genet 2012) Cholangiocarcinoma (TCGA, PanCancer Atlas) Cholangiocarcinoma (TCGA, Provisional) Cholangiocarcinoma (National Cancer Centre of Singapore, Nat Genet 2013) Intrahepatic Cholangiocarcinoma (Johns Hopkins University Nat Genet 2013) Gallbladder Carcinoma (Shanghai, Nat Genet 2014)2018 Available from: http://www.cbioportal.org/study?id=chol_nccs_2013%2Cchol_nus_2012%2Cchol_tcga_pa_can_atlas_2018%2Cchol_tcga%2Cchol_jhu_2013%2Cgbc_shanghai_2014#summaryAccessed August 25, 2018
- Academic, Community Cancer Research United; National Cancer InstituteLiposomal Irinotecan, Fluorouracil, Leucovorin Calcium, and Rucaparib in Treating Patients With Metastatic Pancreatic, Colorectal, Gastroesophageal, or Biliary Cancer2021 Available from: https://clinicaltrials.gov/ct2/show/NCT03337087Accessed February 20, 2019
- A Trial of Niraparib in BAP1 and Other DNA Damage Response (DDR) Deficient Neoplasms (UF-STO-ETI-001) Available from: https://clinicaltrials.gov/ct2/show/NCT03207347Accessed February 20 019
- Leyva-IlladesDMcMillinMQuinnMDemorrowSCholangio-carcinoma pathogenesis: role of the tumor microenvironmentTransl Gastrointest Cancer2012117123002431
- MoeiniASiaDBardeesyNMazzaferroVLlovetJMMolecular pathogenesis and targeted therapies for intrahepatic cholangiocarci-noma.Clin Cancer Res20151078143225520391
- KawaharaNOnoMTaguchiKEnhanced expression of thrombospondin-1 and hypovascularity in human cholangiocarcinomaHepatology1998286151215179828214
- BunsiripaiboonPSornmayuraPWilasrusmeeCLertsithichaiPThe prognostic significance of microvessel density in intrahepatic cholangiocarcinomaJ Med Assoc Thai20109316620196413
- GiatromanolakiASivridisEKoukourakisMIPolychronidisASimopoulosCPrognostic role of angiogenesis in operable carcinoma of the gallbladderAm J Clin Oncol2002251384111823693
- GiatromanolakiAKoukourakisMISimopoulosCPolychroni-disASivridisEVascular endothelial growth factor (VEGF) expression in operable gallbladder carcinomasEur J Surg Oncol2003291087988314624781
- ChenYChenYYuGDingHLymphangiogenic and angiogenic microvessel density in gallbladder carcinomaHepatogastroenterol-ogy2011581052025
- TangDNaganoHYamamotoHAngiogenesis in cholangiocel-lular carcinoma: Expression of vascular endothelial growth factor, angiopoietin-1/2, thrombospondin-1 and clinicopathological significanceOncology Reports200615352510215251335
- YoshikawaDOjimaHIwasakiMClinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinomaBr J Cancer200898241842518087285
- ZhuAXMeyerhardtJABlaszkowskyLSEfficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 studyLancet Oncol2010111481470542045
- ValleJWWasanHLopesACediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trialThe Lancet Oncol201516896797826179201
- SamaARDenlingerCSVogelAGemcitabine and cisplatin plus ramucirumab or merestinib or placebo in first-line treatment for advanced or metastatic biliary tract cancer: a double-blind, randomized phase II trialJ Clin Oncol2017354_supplTPS509
- YardenYSliwkowskiMXUntangling the ErbB signalling networkNat Rev Mol Cell Biol20012212713711252954
- PignochinoYSarottoIPeraldo-NeiaCTargeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomasBMC Cancer201010163114716312407
- LeeJParkSHChangHMGemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 studyLancet Oncol201213218118822192731
- AmadoRGWolfMPeetersMWild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancerJ Clin Oncol200826101626163418316791
- MuraliRWiesnerTScolyerRATumours associated with BAP1 mutationsPathology201345211612623277170
- BattagliaAThe importance of multidisciplinary approach in early detection of BAP1 tumor predisposition syndrome: clinical management and risk assessmentClin Med Insights Oncol20148374724855403
- AndriciJGoeppertBSiosonLLoss of BAP1 expression occurs frequently in intrahepatic cholangiocarcinomaMedicine2016952e249126765459
- Al-ShamsiHOAnandDShroffRTBRCA-associated protein 1 mutant cholangiocarcinoma: an aggressive disease subtypeJ Gas-trointest Oncol201674556561
- IwahashiSIshibashiHUtsunomiyaTEffect of histone deacetylase inhibitor in combination with 5-fluorouracil on pancreas cancer and cholangiocarcinoma cell linesJ Med Investig2011581,210610921372494
- AsgarMDSenawongGSripaBSenawongTSynergistic anticancer effects of cisplatin and histone deacetylase inhibitors (SAHA and TSA) on cholangiocarcinoma cell linesInt J Oncol201648140942026575528
- SiaDTovarVMoeiniALlovetJMIntrahepatic cholangiocarci-noma: pathogenesis and rationale for molecular therapiesOncogene201332414861487023318457
- DunnGPBruceATIkedaHOldLJSchreiberRDCancer immu-noediting: from immunosurveillance to tumor escapeNat Immunol200231199199812407406
- GoeppertBFrauenschuhLZucknickMPrognostic impact of tumour-infiltrating immune cells on biliary tract cancerBr J Cancer2013109102665267424136146
- McnamaraMGTempletonAJMagantiMNeutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancerEur J Cancer20145091581158924630393
- GrenaderTNashSPlotkinYDerived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC-02 and BT-22 studiesAnn Oncol20152691910191626037798
- KaidaMMorita-HoshiYSoedaAPhase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancerJ Immunother2011341929921150717
- HigashiMYonezawaSHoJJExpression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinomaHepatology19993061347135510573510
- YamamotoKUenoTKawaokaTMUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancerAnticancer Res20052553575357916101182
- RecchiaFSicaGCandeloroGMulticenter phase II study of sequential chemotherapy, radiotherapy, and immunotherapy in locally advanced pancreatic (Pa) and biliary tree (Bt) adenocarcinoma (ADK)J Clin Oncol20092715S30473047
- HiguchiRYamamotoMHatoriTShimizuKImaiKTakasakiKIntrahepatic cholangiocarcinoma with lymph node metastasis successfully treated by immunotherapy with CD3-activated T cells and dendritic cells after surgery: report of a caseSurg Today200636655956216715430
- TranETurcotteSGrosACancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancerScience2014344618464164524812403
- ShimizuKKoteraYArugaATakeshitaNTakasakiKYamamotoMClinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinomaJ Hepatobiliary Pancreat Sci201219217117821874278
- ThompsonRHGillettMDChevilleJCCostimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic targetProc Natl Acad Sci U S A200410149171741717915569934
- OkazakiTHonjoTPD-1 and PD-1 ligands: from discovery to clinical applicationInt Immunol200719781382417606980
- SabbatinoFVillaniVYearleyJHPD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinomaClin Cancer Res201622247047826373575
- YeYZhouLXieXJiangGXieHZhengSInteraction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasionJ Surg Oncol2009100650050419697355
- BangYJDoiTBraudFD525 Safety and efficacy of pembroli-zumab (MK-3475) in patients (pts) with advanced biliary tract cancer: Interim results of KEYNOTE-028Eur J Cancer201551S112
- NAEP Release [webpage on the Internet]FDA approves first cancer treatment for any solid tumor with a specific genetic feature; 2017. FDA approval of cancer treatment(pembrolizumab) for solid tumour with specific genetic feature;2018 Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm560167.htmAccessed January 3, 2019
- FDA News Release [webpage on the Internet]FDA approval of cancer treatment for solid tumour with specific genetic feature Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm560167.htmAccessed January 3, 2019
- NowellPCThe clonal evolution of tumor cell populationsScience197619442602328959840
- FearonERVogelsteinBA genetic model for colorectal tumorigenesisCell19906157597672188735
- WalterDDöringCFeldhahnMIntratumoral heterogeneity of intrahepatic cholangiocarcinomaOncotarget2017891495728146430
- McgranahanNFurnessAJRosenthalRClonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockadeScience201635162801463146926940869
- SiricaAECampbellDJDumurCICancer-associated fibroblasts in intrahepatic cholangiocarcinomaCurr Opin Gastroenterol201127327628421297470
- FaraziPAZeisbergMGlickmanJZhangYKalluriRDepinhoRAChronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient miceCancer Res200666136622662716818635
- OliveKPJacobetzMADavidsonCJInhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancerScience200932459331457146119460966
- MatsonVFesslerJBaoRThe commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patientsScience2018359637110410829302014
- JavleMBekaii-SaabTJainABiliary cancer: utility of next-generation sequencing for clinical managementCancer2016122243838384727622582
- GoyalLGovindanAShethRAPrognosis and clinicopathologic features of patients with advanced stage isocitrate dehydrogenase (IDH) mutant and IDH wild-type intrahepatic cholangiocarcinomaOncologist20152091019102726245674
- WangPDongQZhangCMutations in isocitrate dehydroge-nase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomasOncogene201332253091310022824796
- PhilipPAMahoneyMRAllmerCPhase II study of erlotinib in patients with advanced biliary cancerJ Clin Oncol200624193069307416809731
- RubovszkyGLángIGanofszkyECetuximab, gemcitabine and capecitabine in patients with inoperable biliary tract cancer: a phase 2 studyEur J Cancer201349183806381224007821
- ChenJSHsuCChiangNJA KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancerAnn Oncol201526594394925632066
- MalkaDCerveraPFoulonSGemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trialLancet Oncol201415881982824852116
- JensenLHLindebjergJPloenJHansenTFJakobsenAPhase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancerAnn Oncol20122392341234622367707
- VogelAKasperSWeichertWPanitumumab in combination with gemcitabine/cisplatin (GemCis) for patients with advanced kRAS WT biliary tract cancer: A randomized phase II trial of the Arbeitsge-meinschaft Internistische Onkologie (AIO). I American Society of Clinical Oncology: 0732 183X2015
- HezelAFNoelMSAllenJNPhase II study of gemcitabine, oxaliplatin in combination with panitumumab in KRAS wild-type unresectable or metastatic biliary tract and gallbladder cancerBr J Cancer2014111343043624960403
- Sun Yat-sen University; Jiangsu HengRui Medicine Co. Ltd [homepage on the Internet]Apatinib as Second Line Therapy in Patients With Advanced Refractory Biliary Tract Cancers2017 Available from: https://ClinicalTrials.gov/show/NCT03144856Accessed January 3, 2019
- Eisai Co Ltd.; Eisai Inc [homepage on the Internet]Study of Len-vatinib (E7080) in Unresectable Biliary Tract Cancer Who Failed Gemcitabine-based Combination Chemotherapy2015 Available from: https://ClinicalTrials.gov/show/NCT02579616Accessed Janu-ary 3 2019
- Seoul National University Hospital [homepage on the Internet]MEK162 in Combination With Capecitabine in Advanced Biliary Tract Cancer2016 Available from: https://ClinicalTrials.gov/show/NCT02773459Accessed January 3, 2019
- Aslan Pharmaceuticals [homepage on the Internet]Varlitinib in Combination With Capecitabine for Advanced or Metastatic Biliary Tract Cancer2017 Available from: https://ClinicalTrials.gov/show/NCT03093870Accessed January 3, 2019
- Aslan Pharmaceuticals [homepage on the Internet]Study of Varlitinib Plus Capecitabine in Patients With Advanced or Metastatic Biliary Tract Cancer2017 Available from: https://ClinicalTrials.gov/show/NCT03129074Accessed January 3, 2019
- Samsung Medical Center [homepage on the Internet]Pemetrexed in combination with Erlotinib as a salvage treatment in patients with metastatic biliary tract cancer (BTC) who failed gemcitabine containing chemotherapy: a phase II single arm prospective study2017 Available from: https://ClinicalTrials.gov/show/NCT03110484Accessed January 3, 2019
- Aslan Pharmaceuticals [homepage on the Internet]Varlitinib in combination with gemcitabine and cisplatin for treatment naïve advanced or metastatic BTC2016 Available from: https://ClinicalTrials.gov/show/NCT02992340
- Eli Lilly and Company [homepage on the Internet]A Study of Ramucirumab (LY3009806) or Merestinib (LY2801653) in Advanced or Metastatic Biliary Tract Cancer2016 Available from: https://ClinicalTrials.gov/show/NCT02711553
- Hutchison Medipharma Limited [homepage on the Internet]Study of Sulfatinib as Second-line Treatment in Patients With Biliary Tract Carcinoma2016 Available from: https://ClinicalTrials.gov/show/NCT02966821Accessed January 3, 2019
- AndersonMDCancer Center; Eli Lilly and Company [homepage on the Internet]Ramucirumab for advanced pre-treated biliary cancers2015 Available from: https://ClinicalTrials.gov/show/NCT02520141Accessed January 3, 2019
- Shanghai Jiao Tong University School of Medicine; Xinhua Hospital, Shanghai Jiao Tong University School of Medicine; Ruijin Hospital, RenJi Hospital, Eastern Hepatobiliary Surgery Hospital, Huashan Hospital [homepage on the Internet]Molecularly target therapy with GEMOX in advanced or recurrent extrahepatic cholangiocarcinoma and gallbladder carcinoma2016 Available from: https://ClinicalTrials.gov/show/NCT02836847AccessedJanuary 3, 2019
- Eli Lilly and Company, Merck Sharp & Dohme Corp [homepage on the Internet]A Study of Ramucirumab Plus Pembrolizumab in Participants With Gastric or GEJ Adenocarcinoma, NSCLC, Transitional Cell Carcinoma of the Urothelium, or Biliary Tract Cancer2015 Available from: https://ClinicalTrials.gov/show/NCT02443324Accessed January 3, 2019
- Institut du Cancer de Montpellier – Val d’Aurelle [homepage on the Internet]Activity of Regorafenib in combination with chemotherapy in patients with advanced biliary tract cancer2014 Available from: https://ClinicalTrials.gov/show/NCT02386397Accessed January 3, 2019
- University of Nebraska; National Cancer Institute (NCI); Adherex Technologies, Inc [homepage on the Internet]ADH-1, Gemcitabine hydrochloride and cisplatin in treating patients with metastatic pancreatic or biliary tract cancer that cannot be removed by surgery2013 Available from: https://ClinicalTrials.gov/show/NCT01825603Accessed January 3, 2019
- Asian Pharmaceuticals [homepage on the Internet]A study of varli-tinib in Japanese subjects with advanced or metastatic solid tumours2017 Available from: https://ClinicalTrials.gov/show/NCT03082053Accessed January 3, 2019
- Abramson Cancer Center of the University of Pennsylvania Study of gemcitabine, irinotecan and panitumumab in patients with advanced and metastatic biliary tract adenocarcinoma2009 Available from: https://ClinicalTrials.gov/show/NCT00948935Accessed January 3, 2019
- Mayo Clinic National Cancer Institute (NCI) [homepage on the Internet]Ponatinib hydrochloride in treating patients with advanced biliary cancer with FGFR2 Fusions2014 Available from: https://ClinicalTrials.gov/show/NCT02265341Accessed January 3, 2019
- University of Washington; National Cancer Institute (NCI) [homepage on the Internet]Afatinib Dimaleate and Capecitabine in Treating Patients With Advanced Refractory Solid Tumors, Pancreatic Cancer or Biliary Cancer2015 Available from: https://ClinicalTrials.gov/show/NCT02451553Accessed January 3, 2019
- RenJi Hospital [homepage on the Internet]The Effect of Individual-ized Precision Therapy Programs in Patients With BTC2016 Available from: https://ClinicalTrials.gov/show/NCT02943031Accessed January 3, 2019
- ShenLinPeking University [homepage on the Internet]Anti-HER2 Therapy in Patients of HER2 Positive Metastatic Carcinoma of Digestive System2017 Available from: https://ClinicalTrials.gov/show/NCT03185988Accessed January 3, 2019
- National Cancer Institute (NCI) [homepage on the Internet]Trametinib or Combination Chemotherapy in Treating Patients With Refractory or Advanced Biliary or Gallbladder Cancer or That Cannot Be Removed by Surgery2014 Available from: https://ClinicalTrials.gov/show/NCT02042443Accessed January 3, 2019
- AstraZeneca [homepage on the Internet]A study to assess the safety, tolerability and anti-tumour activity of ascending doses of selumetinib in combination with MEDI4736 and selumetinib in combination with MEDI4736 and tremelimumab in patients with advanced solid tumours2015 Available from: https://ClinicalTrials.gov/show/NCT02586987Accessed January 3, 2019
- Senhwa Biosciences, Inc [homepage on the Internet]Study of CX-4945 in combination with gemcitabine and cisplatin for frontline treatment of cholangiocarcinoma2014 Available from: https://Clini-calTrials.gov/show/NCT02128282Accessed January 3, 2019
- University of Pittsburgh Bayer [homepage on the Internet]A phase 2 trial of regorafenib as a single agent in advanced and metastatic biliary tract carcinoma/cholangiocarcinoma patients who have failed first-line chemotherapy2014 Available from: https://ClinicalTrials.gov/show/NCT02053376Accessed January 3, 2019
- Peking Union Medical College Hospital; 3D Medicines [homepage on the Internet]Precise Treatment in Hepatobiliary Cancers (PTHBC)2015 Available from: https://ClinicalTrials.gov/show/NCT02715089Accessed January 3, 2019
- MazzaferroVEl-RayesBFCotsoglouCARQ 087, an oral pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced intrahepatic cholangiocarcinoma (iCCA) with FGFR2 genetic aberrations%! ARQ 4087, an oral pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced intrahepatic cholangiocarcinoma (iCCA) with FGFR4012 genetic aberrationsJ Clin Oncol20173515_suppl40174017
- Hellenic Cooperative Oncology Group; GlaxoSmithKline [homepage on the Internet]Clinical Trial to Investigate the Efficacy of Treatment With Gemcitabine/Pazopanib in Patients With Biliary Tree Cancer2013 Available from: https://ClinicalTrials.gov/show/NCT01855724Accessed January 3, 2019
- Lee MoffittHCancer Center and Research Institute; Institute R Bayer [homepage on the Internet]Copanlisib (BAY 80-6946) in Combination With Gemcitabine and Cisplatin in Advanced Cholangiocarcinoma2016 Available from: https://ClinicalTrials.gov/show/NCT02631590Accessed January 3, 2019
- Loxo Oncology, Inc [homepage on the Internet]Study of LOXO-101 (Larotrectinib) in Subjects With NTRK Fusion Positive Solid Tumors (NAVIGATE)2015 Available from: https://ClinicalTrials.gov/show/NCT02576431Accessed January 3, 2019
- Eli Lilly and Company [homepage on the Internet]A Study of Merestinib (LY2801653) in Japanese Participants With Advanced or Metastatic Cancer2017 Available from: https://ClinicalTrials.gov/show/NCT03027284Accessed January 3, 2019
- Wake Forest University Health Sciences; National Cancer Institute (NCI) [homepage on the Internet]CPI-613 in Treating Patients With Advanced or Metastatic Bile Duct Cancer That Cannot Be Removed By Surgery2013 Available from: https://ClinicalTrials.gov/show/NCT01766219Accessed January 3, 2019
- University of Southern California National Cancer Institute (NCI); Vasgene Therapeutics, Inc [homepage on the Internet]Recombinant EphB4-HSA Fusion Protein With Standard Chemotherapy Regimens in Treating Patients With Advanced or Metastatic Solid Tumors2015 Available from: https://ClinicalTrials.gov/show/NCT02495896Accessed January 3, 2019
- Seoul National University Hospital; Merck Serono International SAStudy of TH-302 Monotherapy as Second-line Treatment in Advanced Biliary Tract Cancer2016 Available from: https://ichgcp.net/clinical-trials-registry/NCT02433639Accessed February 20, 2019
- LinShenPeking UniversityAnti-HER2 Therapy in Patients of HER2 Positive Metastatic Carcinoma of Digestive System2021 Available from: https://clinicaltrials.gov/ct2/show/NCT03185988Accessed February 20, 2019
- Lee MoffittHCancer Center and Research Institute; Research I BSingle Agent Regorafenib in Refractory Advanced Biliary Cancers2019 Available from: https://clinicaltrials.gov/ct2/show/NCT02115542Accessed February 20, 2019
- Aslan PharmaceuticalsVarlitinib in Combination With Capecitabine for Advanced or Metastatic Biliary Tract Cancer2019 Available from: https://clinicaltrials.gov/ct2/show/NCT03093870Accessed February 20, 2019
- Peking Union Medical College Hospital; 3D MedicinesPrecise Treatment in Hepatobiliary Cancers (PTHBC)2017
- Lee MoffittHCancer Center and Research Institute; Research I B. Copanlisib (BAY 80 6946) in Combination With Gemcitabine and Cisplatin in Advanced Cholangiocarcinoma2019 Available from: https://clinicaltrials.gov/ct2/show/NCT02631590Accessed February 20, 2019
- Lee MoffittHCancer Center and Research Institute; Bristol-Myers Squibb [homepage on the Internet]Study of Nivolumab in Patients With Advanced Refractory Biliary Tract Cancers2016 Available from: https://ClinicalTrials.gov/show/NCT02829918Accessed January 3 2019
- Samsung Medical Center [homepage on the Internet]Study of Pembrolizumab in Metastatic Biliary Tract Cancer as Second-line Treatment After Failing to at Least One Cytotoxic Chemotherapy Regimen: Integration of Genomic Analysis to Identify Predictive Molecular Subtypes2017 Available from: https://ClinicalTrials.gov/show/NCT03110328Accessed January 3, 2019
- Seoul National University Hospital [homepage on the Internet]Durvalumab(MEDI4736)/Tremelimumab in Combination With Gemcitabine/Cisplatin in Chemotherapy-naïve Biliary Tract Cancer2017 Available from: https://ClinicalTrials.gov/show/NCT03046862Accessed January 3, 2019
- University of Michigan Cancer Center [homepage on the Internet]Study of Nivolumab in Combination With Gemcitabine/Cisplatin or Ipilimumab for Patients With Advanced Unresectable Biliary Tract Cancer2017 Available from: https://ClinicalTrials.gov/show/NCT03101566Accessed January 3, 2019
- Second Military Medical University [homepage on the Internet]Immunotherapy Using Precision T Cells Specific to Personalized Neo-antigen for the Treatment of Advanced Malignant Tumor of Biliary Tract2015 Available from: https://ClinicalTrials.gov/show/NCT02632019Accessed January 3, 2019
- AstraZeneca [homepage on the Internet]A Phase I, Open-Label, Multicentre Study to Evaluate the Safety, Tolerability and Phar-macokinetics of MEDI4736 in Patients With Advanced Solid Tumours2013 Available from: https://ClinicalTrials.gov/show/NCT01938612Accessed January 3, 2019
- Merck Sharp & Dohme Corp. [homepage on the Internet]Study of Pembrolizumab (MK-3475) in Participants With Advanced Solid Tumors (MK-3475-158/KEYNOTE-158)2015 Available from: https://ClinicalTrials.gov/show/NCT02628067Accessed January 3, 2019
- National Cancer Institute (NCI); National Institutes of Health Clinical Center [homepage on the Internet]A Pilot Study of Combined Immune Checkpoint Inhibition in Combination With Ablative Therapies in Subjects With Hepatocellular Carcinoma (HCC) or Biliary Tract Carcinomas (BTC)2016 Available from: https://ClinicalTrials.gov/show/NCT02821754Accessed January 3, 2019
- National Cancer Institute (NCI); National Institutes of Health Clinical Center [homepage on the Internet]Tremelimumab With Chemoem-bolization or Ablation for Liver Cancer2013 Available from: https://ClinicalTrials.gov/show/NCT01853618Accessed January 3, 2019
- The First Affiliated Hospital with Nanjing Medical University; Miao Y; Abingen Ltd, Nanjing [homepage on the Internet]T Cell Mediated Adaptive Therapy for Her2-positive Neoplasms of Digestive System2016 Available from: https://ClinicalTrials.gov/show/NCT02662348Accessed January 3, 2019
- National Cancer Institute (NCI); National Institutes of Health Clinical CenterPembrolizumab, a monoclonal antibody against PD-1, in combination with capecitabine and oxaliplatin (CAPOX) in people with advanced biliary tract carcinoma (BTC)2019 Availabel from: https://clinicaltrials.gov/ct2/show/NCT03111732Accessed February 20, 2019
- EORTCPembrolizumab in Biliary Tract Cancer. NCT03260712, ed; 2020 Available from: https://clinicaltrials.gov/ct2/show/NCT03260712Accessed February 20, 2019
- Yonsei UniversityPhase 1 clinical trial to evaluate the safety of allo-geneic NK Cell (“SMT-NK”) cell therapy in advanced biliary tract cancer2018 Available from: https://ichgcp.net/clinical-trials-registry/NCT03358849Accessed February 20, 2019
- TariqNUVogelAMcNamaraMGValleJWBiliary tract cancer: implicated immune-mediated pathways and their associated potential targetsOncol Res Treat201841529830429705791
